Back to Journals » OncoTargets and Therapy » Volume 16
Primary Diffuse Large B-Cell Lymphoma of the Penis: A Case and Literature Review
Authors Yu T, Zou L, Wang Y, Luo C, Yu L
Received 11 February 2023
Accepted for publication 12 May 2023
Published 25 July 2023 Volume 2023:16 Pages 631—638
DOI https://doi.org/10.2147/OTT.S408195
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Arseniy Yuzhalin
Tiantian Yu,1 Lingyu Zou,2 Ya Wang,1 Cancan Luo,1 Li Yu1
1Department of Hematology, The Second Affiliate Hospital of Nanchang University, Nanchang, People’s Republic of China; 2Department of Pathology, The Second Affiliate Hospital of Nanchang University, Nanchang, People’s Republic of China
Correspondence: Li Yu, The Second Affiliate Hospital of Nanchang University, 1 Minde Road, Donghu District, Nanchang, 330025, People’s Republic of China, Tel +86-791-86300947, Email [email protected]; [email protected]
Abstract: Primary diffuse large B-cell lymphoma (DLBCL) of the penis is an exceptionally rare malignant disorder, and less than 20 cases have been previously reported. The diagnosis can be difficult, and the standard treatment has not been established yet. We reported an 86-year-old male patient with DLBCL of the penis with an annular penile ulcer, which was not sensitive to the classic R-C(H)OP regimen for three circles; then underwent surgical resection and achieved complete remission for 73 months until now. Including our patient, we collected the clinical characteristics of 20 patients with primary DLBCL of the penis. The median age was 69 years, and most patients manifested mass, diffuse swelling, non-healing ulcer in the penis, and difficulty with urination. Chemo-immunology and radiography were used as first-line therapy, and surgery still plays an essential role in refractory or recurrence. Due to its anatomical independence and physiological particularity, there is still no standard for diagnosing and treating primary DLBCL of the penis. Systemic chemotherapy and radiography were considered first-line therapy to induce remission and preserve the structure and function of the penile; however, surgery still plays a vital role in the refractory or recurrence of single extranodal lymphoma.
Keywords: diffuse large B-cell lymphoma, primary extra nodal lymphoma, penis
Background
Diffuse large B cell lymphoma (DLBCL) is the most common B lymphoid malignancy disorder, representing more than 30% of non-Hodgkin’s lymphoma.1 Although typical morphology is characterized by the diffuse or sheet-like proliferation of mature large B cells, DLBCL is a class of highly heterogeneous diseases exhibiting varying clinical and biological performances.2 DLBCL typically originates from the lymph nodes, bone marrow and immune-privileged tissue, and 20–40% of all cases of DLBCL initially have extranodal involvement.3 In the last decade, intensive research indicated that extranodal DLBCLs should be considered distinct entities with strikingly different molecular pathogenesis, prognosis and clinical presentation. Primary extranodal DLBCL commonly presents in the stomach, mediastinum, central nervous system, and testicle, among which the penis remains rare.4 Most of the reported primary lymph malignant of the penis sited in the penile shaft and/or the glans penis without specific symptoms,5 exhibiting penile masses, nodules, diffuse swelling, refractory erosions and ulcers,5–14 and dysuria occurs when lymphoma masses compress the urethra.15–18 There are also cases of penile erectile dysfunction, penis swelling and other clinical manifestations.19 Diagnosing is very difficult, and patients often are misdiagnosed, leading to delayed treatment. In the meantime, therapeutic options for primary DLBCL of the penis have been controversial due to the limited number of patients and treatment-related sequelae.
While DLBCL has been studied extensively, less is known about the primary DLBCL of the penis due to the small number of cases reported. We described the case of an 86-year-old man with primary DLBCL of the penis identified in our hospital and performed a review of the literature on the character of treatment and prognosis.
Case
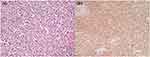
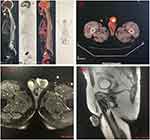
An 86-year-old man was referred to our hospital due to excessive foreskin and 9 months of penile head erosion in December 2016. Physical examination showed a circle of ulceration on the penis head about 1.0 cm wide, with mild tenderness and no obvious secretion. There was no weight loss, fever, night sweats, chills, fatigue or shortness of breath. Routine laboratory tests showed normal red blood cells, white blood cells and platelet counts. Prepuce circumcision under local anesthesia and penis biopsy was performed after primary consideration of penile mass and prepuce glans inflammation. Under the pathological examination of the squamous epithelium and necrotic tissue after the biopsy, the tumor cells were ovoid, with large nuclei, obvious nucleoli, sparse cytoplasm and patchy, diffuse infiltrating growth (Figure 1). Immunohistochemistry presented diffuse positivity for CD20 and PAX-5 in large lymphoid cells, while c-myc, BCL-2, BCL-6 and MUM-1 were partly positivity. The tumor cells were negative for CD5, CD10, Cyclin D1, CD56, CD23, CK, F8, P16, P40, CD34 and CD31. The Ki-67 index was positive in 50% of the tumor cells. Negative for in situ hybridization EBER were noted. FISH analysis showed no rearrangement of BCL6, BCL2 or c-MYC genes. Pathological revealed diffuse large B cell lymphoma (non-germinal center type subtype). A positron emission tomography computerized tomography revealed the remarkable focal hypermetabolism (max SUV 12.6) on the head position at the penis, with a size of about 31 × 30 mm (Figure 2a and b). He had bladder cancer before and was treated with electric resection of bladder cancer combined with chemotherapy, sustained remission during the 6-year follow-up. According to ESMO guidelines,3 this patient was comprehensively diagnosed as primary DLBCL of the penis, non-germinal center type (Ann Arbor stage IE stage A group, IPI score 1 point, low-risk group).
The patient has managed with R-COP (Rituximab Cyclophosphamide, Vincristine and Prednisolone) chemotherapy for two cycles and one R-miniCHOP (R-COP plus liposome doxorubicin). After 3 cycles of chemotherapy, the mass of the glans penis was revalued by MRI with a size of 23 × 33 × 26 mm (Figure 2c and d), revealing partly remission. Considering the insensitive to chemotherapy, subtotal penectomy was performed in the urology department. Postoperative pathological examination showed diffuse distribution and invasive growth of heterotypic lymphoid cells under the squamous epithelium. Pathological diagnosis (glans penis) showed DLBCL with ulceration formation, tumor tissue involved in the spongy body and urethral mucosa, and no involvement in the cutting margin, same as the first. No chemotherapy or radiotherapy was proceeding. The patient achieved complete remission (CR) after surgery and sustained remission until now.
Discussion
Primary DLBCL of the penis is a very rare entity with no specific clinical presentation, standard treatment and predictable outcome. The pathophysiology is not fully understood, as the pathogenesis and gene background might keep similar to other extranodal lymphomas or not that is unknown. The definitive diagnosis primarily depends on the pathological because of the absence of specific clinical manifestations and radiological features. Moreover, the treatment cannot be standardized, and the prognosis cannot be revalued due to the inadequate cases reported. In our case, we observed a gradual change of mucosa; surgery subtotal penectomy was performed to get remission after chemotherapy failed. To our knowledge, few similar cases have been reported in the literature.
The first case of primary DLBCL of the penis was reported in 1988,6 and until now, only 19 cases of primary DLBCL of the penis have been reported in the literature (Table 1). The main clinical characteristics were summarized in Table 1 and Table 2, including our case. The median age was 69 years (range: 42–86 years), and only 3 patients were younger than 60, which suggests such cases were predominantly in the elderly. Among them, stage IE patients accounted for 63.2% of the total and stage IV was not reported, indicating most DLBCL originated from the penis remain the early stage. Two patients had a history of hematologic malignancies, chronic myelogenous leukemia and monoclonal gammopathy of undetermined significance (MGUS), respectively.10,17 Chronic myelogenous leukemia achieved CR after treatment, while MGUS still existed in this course. Most patients manifested mass, diffuse swelling, non-healing ulcer in the penis, difficulty with urination, etc.5–14 Remarkably, painless mass was observed as the first symptom in 36.8% of cases, which could be explained as a reason for the delay in diagnosis. Additional studies are needed on molecular mechanisms and specific indicators, which should give clinicians comprehensive awareness and understanding of the early-stage symptoms of this disease and avoid misdiagnosis.
 |
Table 1 Summary of Cases Primary Diffuse Large B-Cell Lymphoma of the Penis in the Literature, Including Our Case |
 |
Table 2 Clinical Characteristics, Treatment and Outcome of 18 Patients with Primary Diffuse Large B-Cell Lymphoma of Penis, Including Our Case |
Multiple therapy options, such as surgery, radiotherapy and chemotherapy, are used for treating extranodal DLBCL.4 Due to the specificity of the penile tissue and the integrity of its function, 18 (90%) cases chose chemotherapy or chemotherapy combination with radiography as the initial treatment. Only 1 patient received radiography alone,20 and 1 received surgery. The initial treatment response was satisfactory, and the rate of CR was 90%. This data suggests that patients will likely receive effective treatment responses whatever therapeutic options are pursued. Three patients relapsed one year later after initial treatment,8,12,15 and 1 patient succumbed to the central nervous system infiltration of lymphoma.12 Once recurrence, the chemotherapy effect was poor, and the disease rapidly progressed within months after treatment. The patient we reported displayed insufficient responses to R-C(H)OP, then received surgical resection. Surprisingly, the patient survived disease-free during the follow-up of 73 months at the time of the report. Of the 19 patients treated with these multiple options above, 17 reported a long follow-up duration. The median follow-up duration was 13 months (range: 2–73 months). Twelve-month progressive free survival was 76.5%. Compared with DLBCL in older age, primary DLBCL of the penis was sensitive to chemotherapy and associated with a better PFS rate.
DLBCL in this location acts as an indolent disease that is different from other extranodal DLBCL, and the mechanisms remain unclear. It is interesting that one patient developed DLBCL at the injection site during the treatment of prostatitis.7 The injection treatment of prostaglandin E, papaverine, and phentolamine may induce chronic stimulation leading to immune dysfunction. Prostaglandin E is known to be a suppressor of B-cell proliferation.23 This case points to potential relationships between the therapeutic use of prostaglandins might contribute to lymphomagenesis.
Here is still no standard for its treatment. Similar relevant reports are rare. A multidisciplinary approach is often necessary, and current treatment modalities include chemo-immunotherapy, local radiotherapy, radiotherapy and chemotherapy combinations, surgical resection, and postoperative chemotherapy. The controversy lies in whether or how to use and combinate chemotherapy, radiotherapy, or surgery to eradicate the tumor. For younger patients, the structure and functionality of the penis are strongly associated with quality of life. Thus, the anatomy and physiology of the penis must be preserved whenever possible. At the same time, chemotherapy is not the only option for stage IE patients, while radiotherapy and surgery are still effective options. Chemotherapy should still be the preferred treatment. Surgery is a worthwhile option when chemotherapy is not practical. Due to the small sample size, this data does not reflect the overall efficacy of chemotherapy for primary penile lymphoma. However, as extranodal lymphoma, primary DLBCL of the penis still has a high risk of central nervous system invasions, and intrathecal injection is recommended to prevent invasion simultaneously. In conclusion, investigating the molecular mechanism and developing the diagnosis and prognosis biomarkers are valuable for recognizing this disease and maximizing its clinical management. Identifying an optimal treatment for such rare patients needs more evidence from further clinical studies.
Conclusion
In summary, the primary DLBCL of the penis is unique in treatment compared with DLBCL due to its anatomical independence and physiological particularity. Systemic chemotherapy and radiography were considered first-line therapy to achieve total alleviation and preservation of penile function and shape. However, surgery still plays an important role in the refractory or recurrence of single extranodal lymphoma. Further investigation is needed to shed light on the underlying molecular mechanisms and unique microenvironment, as it is difficult to draw solid conclusions.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Funding
This research is supported by National Natural Science Foundation of China 81460030, 81770221 and 82260043, and Leading Talent Foundation of Jiangxi Province 20225BCJ22001.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sehn LH, Salles G. Diffuse large B-cell lymphoma. N Engl J Med. 2021;384(9):842–858. doi:10.1056/NEJMra2027612
2. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the world health organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi:10.1182/blood-2016-01-643569
3. Tilly H, Gomes DSM, Vitolo U, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v116–v125. doi:10.1093/annonc/mdv304
4. Vitolo U, Seymour JF, Martelli M, et al. Extranodal diffuse large B-cell lymphoma (DLBCL) and primary mediastinal B-cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v91–v102. doi:10.1093/annonc/mdw175
5. Fenech M, Pisani D, Camilleri DJ. Primary high-grade diffuse large B-cell lymphoma of the glans penis. BMJ Case Rep. 2021;14(11):e243844. doi:10.1136/bcr-2021-243844
6. Marks D, Crosthwaite A, Varigos G, et al. Therapy of primary diffuse large cell lymphoma of the penis with preservation of function. J Urol. 1988;139(5):1057–1058. doi:10.1016/S0022-5347(17)42771-6
7. Beal K, Mears JG. Short report: penile lymphoma following local injections for erectile dysfunction. Leuk Lymphoma. 2001;42(1–2):247–249.
8. Kuwahara Y, Kubota Y, Hibi H, et al. Malignant lymphoma of the penis: report of two cases. Hinyokika Kiyo. 1997;43(5):371–374.
9. Arena F, Di Stefano C, Peracchia G, et al. Primary lymphoma of the penis: diagnosis and treatment. Eur Urol. 2001;39(2):232–235. doi:10.1159/000052441
10. Gallardo F, Pujol RM, Barranco C, et al. Progressive painless swelling of glans penis: uncommon clinical manifestation of systemic non-Hodgkin’s lymphoma. Urology. 2009;73(4):923–929. doi:10.1016/j.urology.2008.04.059
11. Stamatiou K, Pierris N. Lymphoma presenting as cancer of the glans penis: a case report. Case Rep Pathol. 2012;2012:948352.
12. Chu L, Mao W, Curran VK, et al. Primary malignant lymphoma of the glans penis: a rare case report and review of the literature. Asian J Androl. 2013;15(4):571–572. doi:10.1038/aja.2013.21
13. Karki K, Mohsin R, Mubarak M, et al. Primary non-hodgkin’s lymphoma of penis masquerading as a non-healing ulcer in the penile shaft. Nephrourol Mon. 2013;5(3):840–842. doi:10.5812/numonthly.6885
14. Diao L, Yang S, Shang P, et al. Report of penis lymphoma and review of the literature. Asian J Surg. 2022;45(11):2528–2529. doi:10.1016/j.asjsur.2022.05.136
15. El-Sharkawi A, Murphy J. Primary penile lymphoma: the case for combined modality therapy. Clin Oncol. 1996;8(5):334–335.
16. Kim HY, Oh SY, Lee S, et al. Primary penile diffuse large B cell lymphoma treated by local excision followed by rituximab-containing chemotherapy. Acta Haematol. 2008;120(3):150–152. doi:10.1159/000178146
17. Hamamoto S, Tozawa K, Nishio H, et al. Successful treatment of primary malignant lymphoma of the penis by organ-preserving rituximab-containing chemotherapy. Int J Clin Oncol. 2012;17(2):181–184.
18. Karunanithi S, Sharma P, Naswa N, et al. Primary penile lymphoma: the use of PET-CT for accurate staging and response monitoring. Diagn Interv Radiol. 2013;19(2):130–133.
19. Guo Y, Bai RJ, Gao S. FDG PET/CT detects malignant lymphoma invading the penis. Clin Nucl Med. 2011;36(12):e204–e206. doi:10.1097/RLU.0b013e31823361e1
20. Hashine K, Akiyama M, Sumiyoshi Y. Primary diffuse large cell lymphoma of the penis. Int J Urol. 1994;1(2):189–190. doi:10.1111/j.1442-2042.1994.tb00035.x
21. Ibarz SL, Arzoz FM, Ruiz DJ, et al. Primary lymphoma of penis. Actas Urol Esp. 2009;33(7):826–829.
22. Wang GC, Peng B, Zheng JH. Primary penile malignant lymphoma: report of a rare case. Can Urol Assoc J. 2012;6(6):E277–E279. doi:10.5489/cuaj.136
23. Murn J, Alibert O, Wu N, et al. Prostaglandin E2 regulates B cell proliferation through a candidate tumor suppressor, Ptger4. J Exp Med. 2008;205(13):3091–3103.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.