Back to Journals » Infection and Drug Resistance » Volume 16
Primary Cutaneous Aspergillosis Due to Aspergillus fumigatus in an Immunocompetent Patient with Diabetes Mellitus After Tattooing: A Case Report and Review of Literature
Authors Zhang R, Zhang Y , Xu W, Han X, Zhao J
Received 23 November 2022
Accepted for publication 31 January 2023
Published 5 February 2023 Volume 2023:16 Pages 791—797
DOI https://doi.org/10.2147/IDR.S398858
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Ruina Zhang,1,* Yizhen Zhang,1,* Wenjing Xu,2 Xiaomin Han,2 Junying Zhao1
1Department of Dermatology, Beijing Friendship Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Key Laboratory of Food Safety Risk Assessment, Ministry of Health, China National Center for Food Safety Risk Assessment, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaomin Han, Key Laboratory of Food Safety Risk Assessment, Ministry of Health, China National Center for Food Safety Risk Assessment, Beijing, People’s Republic of China, Tel +8613488894538, Email [email protected] Junying Zhao, Department of dermatology, Beijing friendship hospital, capital medical university, 95 Yongan Road, Xicheng District, Beijing, People’s Republic of China, Tel +8613621098570, Email [email protected]
Background: Aspergillosis is an uncommon fungal infection in which primary cutaneous sites are very rare, and most cases occur in patients with immunocompromised status. Although primary cutaneous aspergillosis is usually encountered in immunocompromised patients, it also occurs in immunocompetent individuals.
Case Presentation: We report a case of primary cutaneous aspergillosis in a 46-year-old immunocompetent woman with diabetes mellitus after tattooing. She presented with erythematous papules, papulopustules and a plaque on the right lower limb of more than two years duration which had failed to respond to antihistamine treatment. Histological examination of a skin biopsy sample showed oval spores in the corneous layer, a slightly thickened epidermis, and infiltrating lymphocytes and neutrophils around the blood vessels in the superficial dermis. Aspergillus fumigatus was isolated and identified in cultures. Clinical and biological examinations did not reveal any systemic localization of aspergillosis, ruling out a hypothesis of blood dissemination. Lesions resolved completely after systemic antifungal treatment with itraconazole.
Conclusion: Clinical lesions of primary cutaneous aspergillosis are nonspecific and usually present as a variety of lesions, including macules, papules, nodules, plaques, purpura, blood blisters, and pustules. The nonspecific features and variety of lesions can lead to misdiagnosis and delayed treatment. Direct microscopy, microbiological culture, and histopathological examination are helpful for diagnosing primary cutaneous aspergillosis. Moreover, the physicians should be aware of the possibility of Aspergillus infection in tattooed cases.
Keywords: Aspergillosis, primary cutaneous aspergillosis, Aspergillus fumigatus
Introduction
Aspergillus fumigatus (A. fumigatus), one of the most common opportunistic pathogenic fungi in the genus Aspergillus, is widely distributed and has an optimal growth temperature of 25°C to 30°C.1 A. fumigatus reproduces and spreads through the release of spores. A normal human immune defence system can remove spores, preventing A. fumigatus infection. However, immunocompromised individuals cannot remove spores and may develop A. fumigatus infections. The incidence of fungal infections is increasing with the use of immunosuppressive treatments, and infections are not only systemic but also with primary and secondary skin involvement.2 Although primary cutaneous aspergillosis (PCA) is usually encountered in immunocompromised patients, PCA also occurs less frequently in immunocompetent individuals. In healthy people, PCA usually associated with trauma, surgery, foreign body, catheter use and excessive Aspergillus exposure, these patients were mainly farmers, gardeners and tile manufacturers.3,4
PCA in patients with immunocompromised status has been well described in extensive investigations. However, in immunocompetent hosts, PCA occurs rarely and therefore remains poorly characterized. Herein, we present the case of an immunocompetent patient with PCA who presented with papules and an erythematous plaques covered with pustular eruption, which can help more doctors recognise this disease.
Case Presentation
A 46-year-old woman presented with erythematous papules, papulopustules, and a plaque on the right lower limb of more than two years duration without other symptoms. Before her presentation here, she had visited multiple other hospitals. After the diagnoses of dermatitis and eczema, she was treated orally with antihistamines such as cetirizine, loratadine, and ebastine, and topical glucocorticoid ointments, such as mometasone furoate and halometasone. However, the lesions did not respond to treatment, and the rash gradually increased. The patient had a history of type 2 diabetes mellitus for more than 10 years and a tattoo on her right lower limb acquired two years before the lesion onset. After her diagnosis of diabetes mellitus, she was taking metformin orally (0.5g three times a day). In the first half of the year, the glycemia control was satisfactory, generally at 6–7 mmol/L (fasting) and 9–10 mmol/L (postprandial). However, the patient failed to regularly monitor her glycemia levels for a long period because she had not experienced any symptoms. As the patient remembered, her postprandial glycemia were generally 20–30 mmol/L around tattooing and 13–15 mmol/L around lesion onset (see Figure 1). She denied any use of immunosuppressive agents, or immunodeficient disorders.
 |
Figure 1 Medical history flow chart. |
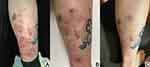
The physical examination revealed only the presence of a cutaneous lesion on the right lower limb and red and blue-black tattoos (Figure 2A). The human immunodeficiency virus test was negative. Results of the laboratory tests for routine blood, T and B lymphocyte subpopulations, renal and hepatic functions, Treponema pallidum hemagglutination, rapid plasma reaction, and immune indices were the normal range. Specifically, the immune index test measures levels of anti-nuclear antigen and anti-extractable nuclear antibodies, immunoglobulins, and complement.
A KOH (potassium hydroxide) examination revealed visible fungal hyphae under the microscope (Figure 3A). A lesion biopsy specimen was cultured on Sabouraud dextrose agar at 25°C, and numerous and identical fungal colonies grew that were dense, and grey-black in appearance (Figure 3B). Microscopically, the colonies were characterized by septate and hyaline hyphae with columnar conidia 2.5 to 3 µm in diameter, produced in chains basipetally from single palisade-like layer phialides that were borne directly on broadly clavate vesicles (Figure 3C). The pathogen was identified as A. fumigatus based on these characteristics and matrix-assisted laser desorption/ionisation-time of flight mass spectrometry.
The histopathological examination of the skin biopsy revealed a reticulate corneous layer, oval spores in the corneous layer, a slightly thickened epidermis, and in the superficial dermis a few infiltrating lymphocytes and neutrophils around the blood vessels in the superficial dermis (Figure 4A). Periodic acid-Schiff staining was positive, and spores were observed in the cuticle (Figure 4B). Therefore, the patient was given a diagnosis of PCA based on the clinical manifestation, histopathology, and culture, and the exclusion of infection in other systems.
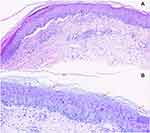 |
Figure 4 Histopathological examination of the skin biopsy. (A) Hematoxylin and eosin, 200×. (B) Periodic acid-Schiff staining shows spores (200×). |
Treatment included oral itraconazole (200 mg/d) and local wound care with ciclopirox olamine 1% ointment. One month after initiating treatment there had been gradual improvement to a certain extent in most lesions (Figure 2B). Complete resolution of lesions was achieved three months after initiating treatment (Figure 2C). There was no report of any adverse event during the treatment period. Until now, no recurrence has been observed after 6 months of follow-up.
Discussion
PCA is a local skin infection caused by direct colonisation of Aspergillus in the skin under certain circumstances. It does not rely on blood spread and deep infection and does not affect other systems beyond the skin.5 PCA is most often diagnosed in immunodeficient patients. Risk factors include the following: hematologic malignancies; human immunodeficiency virus/acquired immunodeficiency syndrome; solid organ transplantation; burns; corticosteroid use; diabetes mellitus; chronic granulomatous disease; trauma; and premature birth.6 PCA is mainly caused by the species A. fumigatus, A. flavus, A. niger, A. terreus, and A. ustus. In a study reviewing PCA, A. fumigatus, A. flavus, and A. niger were the three most common causative agents, with rates of 43.3%, 35.1%, and 10.8%, respectively.3
PCA rarely affects immunocompetent individuals. Data concerning the prevalence, diagnostic approaches, and management strategies for PCA in the immunocompetent host are limited, due to the few numbers of case reports and case series. Of more than 130 cases of PCA overall reported to date, PCA occurs in approximately 11.5% of patients with immunocompetent patients status.3
The specific mechanism of primary aspergillosis in immunocompetent patients is not clear. Zhang et al5 reported a patient with PCA who harbored a mutation in CARD9 (caspase recruitment domain-containing protein 9). CARD9 encodes caspase recruitment domain-containing protein 9, and the authors speculated that a deficiency of the protein increased the susceptibility to A. fumigatus. Further investigation is warranted to elucidate the mechanism underlying PCA in immunocompetent patients.
PCA in immunocompetent patients is primarily associated with excessive exposure to Aspergillus and chronic, repeated skin injuries. Infections occur most often in individuals who work as farmers, gardeners, brick manufacturers, or welders, and mostly on the hands, chest, feet, trunk, and face.7 Serious Aspergillus dermatitis infections have also been reported in health dietitians investigating Aspergillus contaminants.8 These lines of evidence reveal that excessive exposure to Aspergillus is a risk factor for PCA.
PubMed was searched for relevant papers in the last 30 years using the following terms: PCA, immunocompetent hosts and A. fumigatus. As summarized in Table 1, all nine patients2,7,9–15 had normal immune functions and were infected by A. fumigatus. The present patient who was diagnosed with PCA caused by A. fumigatus, had no history of immunodeficiency or immunosuppressant use, and one of the potentially relevant medical histories was diabetes mellitus. The patient’s blood glucose level was not well controlled and was generally maintained between 13 and 15 mmol/L before and after tattooing. In patients with diabetes mellitus, damage to microvessels and peripheral nerves due to high blood glucose is associated with chronic dehydration, hypoxia, and malnutrition.16 Compared with individuals without diabetes mellitus, those with diabetes mellitus are more susceptible to dry skin surface, elasticity loss, thinning skin, reduced regenerative capacity, and reduced anti-infection barrier function.17 Furthermore, the phagocytes’ chemotactic, phagocytic, and bactericidal functions are weaker and associated with hyperglycaemia in these patients, who are prone to various infectious skin diseases.18 Therefore, multiple factors can lead to a great reduction in the skin’s regenerative ability and to resist the invasion of external pathogenic microorganisms. After exposure, fungi, bacteria, and viruses stay on the skin surface for a prolonged time, and invasion through the weakened tissue on the skin surface to the underlying endothelial and muscle tissue results in infection.
 |
Table 1 Studies on Primary Cutaneous Aspergillosis Caused by Aspergillus fumigatus, in Order of Publication and First Author |
The patient had acquired a tattoo on the right lower limb 2 years before presenting the lesions manifestations at our hospital. Tattoo needle penetration of the skin barrier may introduce various microorganisms into individuals, and microorganisms may find their way into the body during the healing phase of a tattoo, resulting in a range of infections such as bacterial, fungal, viral, parasitic, and even spirochetal infections.19 A study showed that approximately 10% of new inks were contaminated with bacteria that are pathogenic to humans and that label “Sterile” on a product was not reliable.20 Primary A. fumigatus infection due to unclean tattoos was reported previously,11 and only one case related to a tattoo has been reported so far.
The duration of the tattoo, from inoculation until the patient became aware of the dermatophyte lesion within the tattoo, varied from 6 days to nearly 6 years.21 The possible causes of early fungal infection include skin injury from the tattoo needle, non-sterile instruments, or contaminated ink, and/or contact with a human or animal dermatophyte source. While tattoo ink-related factors (presence of nanoparticles, polycyclic aromatic hydrocarbons, and cytokine-enhancement) and/or the creation of an immunocompromised cutaneous region are potential causes of late fungal infections.21 In the current case, the symptom started 2 years after the tattooing, hyphae were not detected in the deep tissues and only spores have been observed in the cuticle, so we suspect that it may be associated with the patient’s strong immune system, which may also be one possible reason for the delay between tattooing and the appearance of symptoms. Moreover, the lesions just appeared on her right lower limb nearby the tattoo site, not on other parts of the left limb or other body parts. Thus, according to the lesion distribution, we speculate tattoo and diabetes mellitus are potentially relevant factors for the patient to suffer from A. fumigatus infection.
Our patient was administered oral itraconazole (200 mg/d) for three months, and the rash completely resolved. The patient experienced no adverse reactions during the treatment. Preferred first-line treatment regimens have not been established for PCA, and the treatment course duration varies depending on the immune status and disease severity. In our literature review, most patients with normal immune function responded to treatment with itraconazole, terbinafine, and amphotericin (Table 1), combined with debridement surgery, if necessary.
Conclusion
The diagnosis of PCA in patients with normal immune function is challenging. First, PCA lesions are nonspecific and usually present as a variety of lesions, including macules, papules, nodules, plaques, purpura, blood blisters, and pustules. In addition, PCA occurs most often in immunodeficient patients. Thus, a diagnosis of PCA might be easily missed in individuals with normal immune function. Healthy hosts can develop dermal aspergillosis after exposure to high spore counts in surgical wounds, traumatic inoculations, or occupations such as farming, which should be considered in evaluating suspicious cases. Although the infection in the current patient could not be definitively proven to be caused by tattooing, physicians must evaluate patients who have tattoos to be aware of the possibility of Aspergillus infection or other kinds of fungal infection originating within the tattoo. Direct microscopy, microbiological culture, and histopathological examination are helpful for diagnosing PCA and invasive A. fumigatus.
Ethics Approval and Informed Consent
The authors certify that the patient consent form has been obtained. Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal. The ethics Committee of Beijing Friendship Hospital approved to publish the case details.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; have drafted, revised or critically reviewed the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
Ruina Zhang and Yizhen Zhang are co-first authors for this study. The authors report no conflicts of interest in this work.
References
1. van de Veerdonk FL, Gresnigt MS, Romani L, et al. Aspergillus fumigatus morphology and dynamic host interactions. Nat Rev Microbiol. 2017;15(11):661–674. doi:10.1038/nrmicro.2017.90
2. Liu X, Yang J, Ma W. Primary cutaneous aspergillosis caused by Aspergillus.fumigatus in an immunocompetent patient: a case report. Medicine. 2017;96(48):e8916. doi:10.1097/MD.0000000000008916
3. Tatara AM, Mikos AG, Kontoyiannis DP. Factors affecting patient outcome in primary cutaneous aspergillosis. Medicine. 2016;95(26):e3747. doi:10.1097/MD.0000000000003747
4. He B, Li XF, Liu WD. Primary cutaneous aspergillosis. Chin J Mycol. 2015;10(6):373–376.
5. Zhang Y, Huang C, Song Y, et al. Primary cutaneous aspergillosis in a patient with CARD9 deficiency and aspergillus susceptibility of card9 knockout mice. J Clin Immunol. 2021;41(2):427–440. doi:10.1007/s10875-020-00909-0
6. Shields BE, Rosenbach M, Brown-Joel Z, et al. Angioinvasive fungal infections impacting the skin: background, epidemiology, and clinical presentation. J Am Acad Dermatol. 2019;80(4):869–880.e5. doi:10.1016/j.jaad.2018.04.059
7. Sharma S, Yenigalla BM, Naidu SK, et al. Primary cutaneous aspergillosis due to Aspergillus tamarii in an immunocompetent host. BMJ Case Rep. 2013:010128. doi:10.1136/bcr-2013-010128
8. Tahir C, Garbati M, Nggada HA, et al. Primary cutaneous aspergillosis in an immunocompetent patient. J Surg Tech Case Rep. 2011;3(2):94–96. doi:10.4103/2006-8808.92802
9. Mowad CM, Nguyen TV, Jaworsky C, et al. Primary cutaneous aspergillosis in an immunocompetent child. J Am Acad Dermatol. 1995;33(1):136–137. doi:10.1016/0190-9622(95)90041-1
10. Camus M, Anyfantakis V, Dammak A, et al. Primary cutaneous aspergillosis in an immunocompetent farmworker. Ann Dermatol Venereol. 2010;137(5):373–376. doi:10.1016/j.annder.2010.03.006
11. Kluger N, Saarinen K. Aspergillus fumigatus infection on a home-made tattoo. Dermatol. 2014;170(6):1373–1375. doi:10.1111/bjd.12859
12. Rocha PD, Pinto RG, Rodrigues S, et al. Cytodiagnosis of primary cutaneous aspergillosis in an immunocompetent host. J Cytol. 2016;33(1):59–60. doi:10.4103/0970-9371.175532
13. Chaturvedi R, Kolhe A, Pardeshi K, et al. Primary cutaneous aspergillosis, mimicking malignancy, a rare presentation in an immunocompetent patient. Diagn Cytopathol. 2018;46(5):434–437. doi:10.1002/dc.23869
14. Mada PK, Saldaña Koppel DA, Al Shaarani M, et al. Primary cutaneous Aspergillus fumigatus infection in immunocompetent host. BMJ Case Rep. 2020;13(2):e233020. doi:10.1136/bcr-2019-233020
15. Fan X, Qi Y, Liu YQ, et al. An immunocompetent patient with primary cutaneous aspergillosis caused by Aspergillus fumigatus. Chin J Clin Infect Dis. 2021;14(5):389–391. doi:10.3760/cma.j.issn.1674-2397.2021.05.011
16. Todd DOB. Impaired dermal microvascular reactivity and implications for diabetic wound formation and healing: an evidence review. J Wound Care. 2020;29(Sup9):S21–S28. doi:10.12968/jowc.2020.29.Sup9.S21
17. Yao ZC, Li M, Ni T. Effect of external substance P on the Rac⁃1/JNK pathway of fibroblasts and wound healing in diabetic mice. Anat Res. 2021;43(1):54–58.
18. Alves C, Casqueiro J, Casqueiro J. Infections in patients with diabetes mellitus: a review of pathogenesis. Indian J Endocrinol Metab. 2012;16(Suppl 1):S27–S36. doi:10.4103/2230-8210.94253
19. Serup J, Carlsen KH, Sepehri M. Tattoo complaints and complications: diagnosis and clinical spectrum. Curr Probl Dermatol. 2015;48:48–60. doi:10.1159/000369645
20. Høgsberg T, Saunte DM, Frimodt- Møller N, et al. Microbial status and product labelling of 58 original tattoo inks. J Eur Acad Dermatol Venereol. 2013;27(1):73–80. doi:10.1111/j.1468-3083.2011.04359.x
21. Cohen PR, Crowley CS, Erickson CP, et al. Tinea and tattoo: a man who developed tattoo-associated tinea corporis and a review of dermatophyte and systemic fungal infections occurring within a tattoo. Cureus. 2022;14(1):e21210. doi:10.7759/cureus.21210
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.