Back to Journals » Infection and Drug Resistance » Volume 17
Prevalence, Transmission and Genetic Diversity of Pyrazinamide Resistance Among Multidrug-Resistant Mycobacterium tuberculosis Isolates in Hunan, China
Authors Liu B, Su P, Hu P, Yan M, Li W, Yi S, Chen Z, Zhang X, Guo J, Wan X, Wang J, Gong D, Bai H, Wan K, Liu H , Li G, Tan Y
Received 14 September 2023
Accepted for publication 15 January 2024
Published 1 February 2024 Volume 2024:17 Pages 403—416
DOI https://doi.org/10.2147/IDR.S436161
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Zhi Ruan
Binbin Liu,1,* Pan Su,1,* Peilei Hu,1,* Mi Yan,1 Wenbin Li,1 Songlin Yi,1 Zhenhua Chen,1 Xiaoping Zhang,1 Jingwei Guo,1 Xiaojie Wan,1 Jue Wang,1 Daofang Gong,1 Hua Bai,1 Kanglin Wan,2 Haican Liu,2 Guilian Li,2 Yunhong Tan1
1Clinical Laboratory, Hunan Chest Hospital, Changsha, People’s Republic of China; 2National Key Laboratory of Intelligent Tracking and Forecasting for Infectious Diseases, National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yunhong Tan, Clinical Laboratory, Hunan Chest Hospital, Changsha, People’s Republic of China, Tel +86-13874947876, Email [email protected] Guilian Li, National Key Laboratory of Intelligent Tracking and Forecasting for Infectious Diseases, National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, People’s Republic of China, Tel +86-18519886112, Email [email protected]
Background: China is a country with a burden of high rates of both TB and multidrug-resistant TB (MDR-TB). However, published data on pyrazinamide (PZA) resistance are still limited in Hunan province, China. This study investigated the prevalence, transmission, and genetic diversity of PZA resistance among multidrug-resistant Mycobacterium tuberculosis isolates in Hunan province.
Methods: Drug susceptibility testing (DST) with the Bactec MGIT 960 PZA kit and pyrazinamidase (PZase) testing were conducted on all 298 MDR clinical isolates. Moreover, 24-locus MIRU-VNTR and DNA sequencing of pncA, rpsA, and panD genes were conducted on 180 PZA-resistant (PZA-R) isolates.
Results: The prevalence of PZA resistance among MDR-TB strains reached 60.4%. Newly diagnosed PZA-R TB patients and clustered isolates with identical pncA, rpsA, and panD mutations showed that transmission of PZA-R isolates played a significant role in the formation of PZA-R TB. Ninety-eight mutation patterns were observed in the pncA among 180 PZA-R isolates, and seventy-one (72.4%) were point mutations. Twenty-four of these mutations are new, including 2 base substitutions (V93G and T153S) and 22 nucleotide deletions or insertions. The W119C was found in PZA-S isolates, on the other hand, F94L and V155A mutations were found in both PZA resistant and susceptible isolates with positive PZase activity, indicating that they were not associated with PZA resistance. This is not entirely in line with the WHO catalogue. Ten novel rpsA mutations were found in 10 PZA-R isolates, which all combined with mutations in pncA. Thus, it is unpredictable whether these mutations in rpsA can impact PZA resistance. No panD mutation was found in all PZA-R isolates.
Conclusion: DNA sequencing of pncA and PZase activity testing have great potential in predicting PZA resistance.
Keywords: tuberculosis, pyrazinamide, transmission, pncA mutations, rpsA mutations, panD mutations, China
Introduction
Drug-resistant tuberculosis (TB), especially multidrug-resistant tuberculosis (MDR-TB, defined as resistance to isoniazid (INH) and rifampicin (RFP)), is still a severe public health threat. Globally in 2021,1 there were approximately 5.8 million notified new TB cases, 71% (2.4/3.4 million) of people diagnosed with bacteriologically confirmed pulmonary TB were tested for rifampicin resistance (RR), the same level of coverage as in 2020 (2.1/3.0 million) and up from in 2019 (2.2/3.6 million). Among these, 141,953 cases of MDR/RR-TB and 25,038 cases of pre-extensively drug-resistant TB (pre-XDR-TB, defined as TB that is resistant to RFP and any fluoroquinolone) and XDR-TB (resistant to RFP, any fluoroquinolone and at least one of bedaquiline or linezolid) were detected, for a combined total of 166,991. China remains the second highest MDR-TB burden country. A national survey of drug-resistant TB in China showed that 5.7% and 25.6% of new cases and retreated cases respectively had MDR-TB, nearly twice the global average MDR-TB,2 including pre-XDR-TB and XDR-TB, poses a significant challenge to TB therapy and control programs.3
Pyrazinamide (PZA) is a critical first-line antituberculosis drug with effectiveness in targeting intracellular and semi-dormant bacilli living in an acidic environment inside macrophages.4 In clinic, PZA is used at the initial intensive phase of chemotherapy in combination with other first-line drugs, including INH, RFP, and ethambutol (EMB). PZA can shorten the treatment regimen for drug-susceptible TB and can also increase the success rate of the MDR-TB treatment.5 Despite the important role of PZA in TB treatment, PZA resistance in MTB has increased in TB cases. The estimated global burden of new PZA-resistant TB cases annually is 1.4 million cases, of which 270,000 cases occur in MDR-TB patients.6
Currently, the automated Bactec MGIT 960 system is the commonly used phenotypic assay for PZA susceptibility testing. However, false resistance to PZA by this method has been often reported by previously studies, which demonstrated that high M. tuberculosis inoculum may cause loss of PZA activity by increasing the pH of the medium.7,8 In addition, pyrazinamidase (PZase) activity test, known as Wayne’s method, can also be used as a screening method for indirect PZA drug susceptibility testing (DST). This method uses orange-red color change of pyrazinoic acid (POA, the active form of PZA catalyzed by the enzyme PZase9) to react with ferrous ammonium sulfate.10 Subsequently, modified Wayne’s methods that indirectly measure pyrazinamidase activity via pyrazinoic acid in a liquid medium was reported.11 However, PZase activity test requires a significant number of clinical M. tuberculosis cultures and its sensitivity is lower than that of Bactec MGIT 960 system.12
Molecular diagnostic approaches are increasingly being used for prediction of PZA resistance in M. tuberculosis. Recently, a novel line probe assay, the Genoscholar PZA-TB II assay (NIPRO Corporation, Japan), has been endorsed by WHO for the detection of PZA resistance in clinical M. tuberculosis complex isolates.13 This assay encompasses the PZase coding gene, pncA, and 18 nucleotides upstream. Mutations in pncA can affect its enzyme activity and are considered as the major mechanism of PZA resistance. Previous studies showed that the prevalence of pncA mutation in PZA-R M. tuberculosis complex isolates varied from 24 to 100%.14–18 In addition, ribosomal protein S1 (RpsA) is critical in protein translation and ribosome-sparing process of trans-translation,19 and has been reported as a target of POA. However, one recent study found that RpsA interacts with single-strand RNA, but not with POA.20 Some studies showed that mutations in rpsA that coded ribosomal protein S1 was associated with PZA resistance in isolates expressing wild-type pncA,21–23 whilst some studies showed that the frequency of rpsA mutations showed no statistical difference between PZA-R and PZA-susceptible (PZA-S) isolates.24,25 Another gene panD, encoding aspartate alpha-decarboxylase, is considered to be a new target of POA. PanD involves the synthesis of β-alanine, which is a precursor for pantothenate and coenzyme biosynthesis. However, previous studies found that POA is a weak PanD enzyme inhibitor.26,27 The roles of mutations in panD in PZA resistance were uncertain. None of mutations in panD were detected in a study from Pakistan,23 whilst only four out of 161 and one out of 11 PZA-R isolates carried mutations in panD without that in pncA in two studies from China.21,28 These findings suggest that the mechanism of PZA resistance in M. tuberculosis is complex and remains to be investigated further.
On the other hand, molecular epidemiological methods that combine epidemiological investigations and genotyping of M. tuberculosis strains provide the means to assess the transmission of TB.29,30 Due to the critical role of PZA in TB therapy, understanding the transmission patterns of PZA-R TB is crucial to take effective public health measures in controlling such disease. Previous studies have investigated the transmission patterns of PZA-R TB among MDR-TB patients in the mainland China.21,25,28,31 However, no data is available in Hunan province, which remains a high TB burden province in China.32
In this study, we investigated the prevalence of PZA resistance, mutation characteristics of pncA, rpsA and panD genes in PZA-R isolates, and transmission of PZA-R isolates among MDR-TB in Hunan province. The association between the activity of PZase and pncA mutation was also analyzed in this study.
Materials and Methods
Study Population and M. tuberculosis Complex Isolates
Our study was conducted in Hunan Chest Hospital, the province-level and largest specialized hospital for TB treatment in Hunan province, China. A total of 737 patients diagnosed as MDR-TB between January 2016 and December 2018 were reviewed (Figure 1). Information including gender, age, treatment history, and results of the phenotypic DST for first-line including isoniazid (INH), rifampicin (RFP), streptomycin (STM), ethambutol (EMB) and PZA, and second-line anti-tuberculosis drugs, was collected. DST for the first-line antituberculosis drugs was carried out using Bactec MGIT 960 SIRE and PZA kits according to the manufacturer’s instructions (Becton, Dickinson and Company, Sparks, MD, USA), while DST for the second-line drugs including kanamycin (KM), amikacin (AK), capreomycin (CPM), and levofloxacin (LFX) was performed with the proportion method on Lowenstein-Jensen (L-J) medium. The critical concentrations of first-line and second-line drugs for DST were as follows: STM, 1.0 μg/mL; INH, 0.1μg/mL; RFP, 1.0 μg/mL; EMB, 5.0 μg/mL; PZA, 100.0 μg/mL; KM, 30.0 μg/mL; AK, 30.0 μg/mL; CPM, 40.0 μg/mL; LFX, 2.0 μg/mL.33,34
 |
Figure 1 Study outline. Abbreviation: PZA-R, pyrazinamide-resistant; PZA-S, pyrazinamide-susceptible; PZase, pyrazinamidase. |
We randomly selected 315 cases from 737 MDR-TB patients for further analysis. There were no statistical differences between the selected and unselected cases, in terms of patients’ gender, age and TB treatment history. All 315 patients’ isolates were preserved at −80°C before use. Finally, excluding 17 isolates failed to grow on L-J solid medium, a total of 298 isolates were included in the study.
MGIT960 Pyrazinamide Susceptibility Testing, Pyrazinamidase Activity Detection and Pyrazinamide MIC Test
PZA susceptibility testing using Bactec MGIT 960 PZA kits was repeated on the recovered isolates to confirm PZA resistance. The inoculum was prepared from bacterial growth on Lowenstein-Jensen solid medium. After incubating at 36 ± 1°C for 21 days, the fresh colonies were scraped as many as possible using a sterile 10 µL loop, then were added to a sterile screw cap bottle containing glass beads moistened by 100 µL 0.05% Tween 80. Bacterial clumps were dispersed by vortexing for 15-20 seconds and then were suspended in 3 mL saline solution. Thereafter, the suspension was left to settle by standing for 10-15 minutes and the upper 0.5 mL suspension was transferred to a new tube for bacterial concentration measurement. The bacterial concentration was adjusted to a McFarland turbidity of 0.5 and then diluted in sterile saline (1:5 dilution). This diluted inoculum was used for the Bactec MGIT 960 PZA DST, which were performed according to the manufacturer’s instructions (Becton, Dickinson and Company, Sparks, MD). The critical concentration for PZA was 100.0 µg/mL. M. tuberculosis H37Rv (ATCC 27924) was used as PZA susceptible control strain. If the first two rounds of the PZA susceptibility testing results were discordant, the assay was performed again to confirm the phenotype of PZA susceptibility.
The PZase activity testing was carried out through the Wayne’s screening method, with slight modifications.35 Briefly, 7H10 agar (9.5 g) (Difco) was dissolved in 450 mL water, then 2 mL glycerol and PZA (Sigma) at a final concentration of 400 ug/mL were added to make the PZase agar medium, followed by autoclaved at 121 °C for 10 min. Then 5 mL aliquots of this autoclaved PZase agar were distributed in sterilized plastic tubes. Then a heavy loopful of actively growing culture was carefully inoculated on the surface of the PZase agar medium and incubated at 37°C for 4 days. One milliliter of ferrous ammonium sulfate (1%) was added to each tube after incubation and observed for an initial 4 h for the appearance of a pink band (positive) in the subsurface agar. The PZA-susceptible M. tuberculosis H37RV was used as a positive control strain and PZA-resistant isolates of M. tuberculosis confirmed to be negative by the PZase activity testing earlier were used as negative controls. The PZase assay results were recorded independently by two observers who were not aware of the phenotype of PZA susceptibility results.
For the PZA susceptible isolates that harbored pncA mutations classified as linked to PZA resistance36, the PZA MICs were determined using the BD EpiCenter system by dissolving PZA powder to 25, 50, 100, and 200 μg/mL in MGIT culture medium.
DNA Extraction, PCR Amplification and Sequencing
Fresh cultured bacteria on L-J solid medium were scraped and resuspended in 500 μL of 0.9% saline water. After heat inactivation at 85 °C for 20 min, the cell suspension was incubated in an ultrasonic bath for 15 min at 95 °C. Cells were then centrifuged at 13,000 × g for 5 min, and the supernatant containing DNA was transferred to a fresh tube and stored at −20 °C for further use. The fragments containing pncA were amplified using primers according to a previous study:24 pncA-F (5’-TGCCACTCGCCGGTAACCGG-3’ (nt 321 to 340 downstream of pncA)) and pncA-R (5’-GGTGGCCGCCGCTCAGCTGG-3’ (nt −119 to −100 of pncA)). The fragments containing rpsA were amplified using two primer sets as follows: rpsA-f1 (5’-GGAGGTGTCGGTGGGCTA-3’), rpsA-r1 (5’-CTTCCTGAGTCGCCTTGAGT-3’); rpsA-f2 (5’-TCGTCAACTTCGGCGCGTTC-3’), rpsA-r2 (5’-GACCACTTCACGCGCCAACA-3’). The fragments containing panD were amplified using primers as follows: panD-F (5’-GGCTGCTGGACAACATTGC-3’) and panD-R (5’-GATCGTCAGTGCCAGTTCGT-3’). All PCRs were conducted under the following conditions: 94 °C denaturation for 5 min, followed by 35 cycles of 30 s at 94 °C, 1 min at 60 °C for pncA and rpsA, or 57 °C for panD, and 1 min at 72 °C, with a final extension of 7 min at 72 °C. All the PCR products were sent to Beijing Ribio Biotech Co, Ltd for DNA sequencing using the Applied Biosystems 3730XL DNA sequencer. The sequences were analyzed using GeneDoc (Version 3.2) by comparing with the M. tuberculosis H37Rv sequence (GenBank accession no.NC_000962.3).
Mycobacterial Interspersed Repetitive Units-Variable-Number Tandem Repeat Typing and Data Analysis
The 24-locus MIRU-VNTR typing method was carried out using previously reported primers.37 PCRs for all MIRU-VNTR loci were performed in a reaction volume of 20 μL containing 10 μL 2 × Taq mixture, 2 μL genomic DNA template, and 0.5 μmol of each primer set. The PCR amplification program was 95 °C for 15 min, followed by 40 cycles at 94 °C for 1 min, 59 °C for 30 s, and 72 °C for 1.5 min, with a final extension at 72 °C for 10 min. Five microliters of the amplicon was run on a 2.0% agarose gel, with a 100 bp DNA ladder being run every ten lanes. The size of the PCR fragments was defined using the Bio-Rad Quantity One software, version 4.6.2 (BIO-RAD Laboratories, Hercules, CA, USA). The data from 24-locus MIRU-VNTR genotypes were analyzed through BioNumerics software, version 8.0 (Applied Maths, Sint-Martens-Latem, Belgium).
Statistical Analysis
IBM SPSS (version 16.0) (IBM Corporation, Armonk, NY, USA) was used to analyze the data. We used a chi-square test to examine the correlations between demographic characteristics, clinical variables and PZA resistance. Furthermore, Pearson correlation coefficient was used to determine the correlation between PZase activity, PZA susceptibility and pncA mutation. A two-sided P < 0.05 is considered statistically significant.
Results
Pyrazinamide Resistance Among Multidrug Resistant Isolates
The detailed susceptibility profiles of the 737 MDR isolates to nine drugs (INH, RFP, STM, EMB, PZA, LFX, KM, CPM, and AK) are shown in Table 1. In order to assure the accuracy of PZA resistance, PZA susceptibility testing was repeated on 298 MDR isolates. In total, 45 isolates had discordant PZA susceptibility results. Considering the results of the third round of PZA susceptibility testing, 39 were classified as PZA-susceptible, while the remaining six were classified as PZA-resistant. In total, 180 out of 298 (60.4%) were resistant to PZA.
 |
Table 1 Drug Susceptibility Profiles of 737 MDR Isolates |
Factors Associated with Pyrazinamide Resistance
The risk factors associated with PZA resistance are summarized in Table 2. MDR isolates from patients aged 30 to 59 were less likely to produce PZA resistance (OR: 0.424, 95% CI: 0.223~0.806) than those from patients who were less than 30 years old. MDR-TB patients with STM resistance, EMB resistance, or LFX resistance demonstrated a significantly increased risk for developing PZA resistance, with odds ratios (95% CI) of 2.408 (1.452~3.991), 2.558 (1.584~4.130) and 2.245 (1.370~3.680), respectively. Other factors such as gender, treatment history and resistance to other drugs were not statistically significant among patients infected with PZA-resistant and PZA-susceptible MDR-TB isolates (P>0.05).
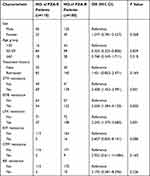 |
Table 2 Factors Associated with PZA Resistance in MDR-TB Patients |
Mutations in the pncA Gene
Among 180 PZA-R isolates, 169 were successfully sequenced. The results showed that 162 (95.9%) harbored mutations in the pncA gene as well as its promoter region (Supplementary Table 1), including 129 isolates with nucleotide substitutions in the promoter or the coding region of pncA, and 33 isolates with insertions or deletions. It is of note that ten isolates carried multiple mutations, including six isolates with double mutations and four isolates with triple mutations. Overall, 98 mutation patterns were found here (Supplementary Table 1). The most frequent mutation pattern was the nucleotide substitution from A to G at position −11 (n=13) in the promoter region of pncA, followed by V7G in the coding region of pncA (n=11). Based on the WHO catalogue,36 of the 71 base substitution types in the pncA gene, 39 (54.9%) were classified as “associated with resistance”, followed by 12 (16.9%) as “associated with resistance-interim” and 2 (2.8%) as “not associated with resistance” (Supplementary Table 1). Significantly, 8 (11.3%) base substitution types in the pncA gene were regarded as “uncertain significance” (I5T, L35P, F58V, G78D, T100P, D136G, T142P, and V155A). The remaining 10 types (23.2%) were not included in the WHO catalogue, including eight (C14Y, G24R, L27M, V93G, C138Y, T153S, L156R, and A178P) described previously and two (V9S and A28N) detected for the first time (Table 3). In addition, the WHO catalogue omitted 26 nucleotide deletions or insertions with various lengths of nucleotides in pncA, of which 4 were previously reported and 22 were newly reported (Table 3). It is worth noting that seven PZA-resistant isolates harbored wild-type pncA.
 |
Table 3 pncA Mutations Not Included in WHO Catalogue36 and Classified as “Uncertain Significance” by WHO in PZA-Resistant MDR Strains and Their Pyrazinamidase Activities |
In contrast, 17 of the 118 PZA-susceptible isolates harbored non-synonymous mutations in the pncA gene (Supplementary Table 2), of which four carried mutations (P62T, F94L, W119C, and V180F) were regarded as PZA resistance-associated by WHO.36 Nonetheless, two isolates with F94L and W119C mutations did show PZA susceptibility since their MIC values ≤100.0 ug/mL in our study and they possessed positive Pzase activity (Table 4).
 |
Table 4 pncA Mutation Patterns, Pyrazinamidase Activity and Pyrazinamide MIC Results in Four Pyrazinamide-Susceptible Strains |
Correlation of pncA Mutations to Pyrazinamidase Activity and PZA Susceptibility
PZase activity testing showed that eight PZA-R isolates harboring pncA mutations including L35P, P62L, D63A, G78D, V93G, F94L, and V155A showed positive PZase activity (Supplementary Table 1). We also found six PZA-susceptible isolates that were PZase-negative (Supplementary Table 2). Interestingly, the F94L and V155A mutations were found both in PZA resistant and susceptible isolates with positive PZase activity. The correlation coefficient between PZase activity and pncA mutation was 0.828 (P<0.05), while the correlation coefficient between pncA mutation and PZA susceptibility was 0.808 (P<0.05).
Mutations in the rpsA and panD Genes
Ten PZA-resistant isolates harbored novel non-synonymous mutations in the rpsA gene which combined with mutations in pncA, while four PZA-susceptible isolates carried non-synonymous mutations (Table 5). In addition, a synonymous mutation rpsA mutation (A636C) was found in 147 PZA-resistant and 76 PZA-susceptible isolates. In terms of the panD gene, non-synonymous mutations were found exclusively in two PZA-susceptible isolates, but not in 180 PZA-resistant isolates. However, seven PZA-resistant isolates did not harbor any non-synonymous mutations in the pncA, rpsA or panD genes.
 |
Table 5 rpsA and panD Mutation Patterns of PZA-Resistant and PZA-Susceptible MDR Strains |
Prediction of PZA Resistance by DNA Sequencing and Pyrazinamide Activity Testing
Using MGIT 960 PZA susceptibility testing as the reference method, the detection of mutations in pncA showed a sensitivity of 95.9% (95% CI, 91.7%~98.0%) and a specificity of 84.7% (95% CI, 76.8%~90.2%) (Table 6). The sensitivities of sequencing rpsA and panD were very low, indicating little diagnostic value. Sequencing pncA together with rpsA or panD mutations did not increase the sensitivity of 95.9% (95% CI, 91.7%~98.0%) but the specificity decreased to 74.5% (95% CI, 65.5% ~81.9%). PZase activity testing reached a sensitivity of 96.1% (95% CI, 92.2%~98.1%) and specificity of 94.9% (95% CI, 89.4% ~97.7%).
 |
Table 6 The Evaluation of the Drug Susceptibility and DNA Sequencing, PZase Activity |
Transmission of PZA-R Isolates
Drug resistance in newly diagnosed TB patients is generally considered as primary drug resistance, while drug-resistant TB patients with clustered isolates indicate recent transmission. Among the 180 PZA-R cases, 40 (28.6%) were newly diagnosed TB patients, while 140 (71.4%) were retreated. To identify potential recent transmission of PZA-R isolates during the research period, 180 PZA-R isolates were analyzed by 24-locus MIRU-VNTR typing. The results showed 141 genotypes: 124 (68.9%) isolates had a unique MIRU-VNTR profile and 56 (31.1%) isolates from eight newly diagnosed TB patients and 48 retreated patients were involved in 17 clusters. We further found that seven clusters respectively had at least two isolates with identical pncA, rpsA and panD mutations. Additionally, the residence information of patients showed that two PZA-R patients in Cluster 2, respectively four and two in Cluster 11 and three in Cluster 13 lived in the same village (Figure 2). These clues suggest the recent transmission of PZA-R M. tuberculosis.
 |
Figure 2 The clustered isolates with pncA, rpsA and panD mutations. 24 loci VNTR based dendrogram and isolates profiles of 17 clusters in which pncA, rpsA and panD mutant isolates were involved. |
Discussion
It is necessary to better understand the transmission of PZA-R tuberculosis and genetic basis of PZA resistance to achieve the goal of ending TB. To the best of our knowledge, this was the first study on the transmission and genetic basis of PZA-resistant M. tuberculosis in Hunan province, China. To minimize errors in PZA drug susceptibility testing, we repeated MGIT 960 PZA susceptibility testing on recovered strains to validate the phenotype of PZA resistance. The results showed that 180 out of 298 isolates had true PZA resistance, while the remaining 118 isolates were susceptible to PZA. We further investigated the 180 isolates with PZA resistance.
Our results showed that the proportion of PZA resistance in MDR isolates in Hunan, China was 60.4%, which was at the medium level compared with other regions in Mainland China (47% - 69.02%),21,25,28,31,38,39 and was higher than those from other countries, including Myanmar (58.9%),40 Peru41 (47.7%), and South Korea (31.5%).42 This suggests that rapid detection of PZA resistance is an urgent need for the control of MDR-TB in the whole of China including Hunan province. We also analyzed the correlations between PZA resistance and demographic characteristics. The results showed that MDR-TB patients aged 30 to 59 were less likely to produce PZA resistance than those aged less than 30. Furthermore, we also found that PZA resistance was associated with resistance to STM, EMB, and LFX. Previous studies have established the correlation between the resistance of PZA and other anti-tuberculosis drugs.25,28,38,39 A study from Alame-Emane found that PZA resistance in M. tuberculosis arose after RFP and fluoroquinolone resistance,43 which partly supported our findings on the link between PZA and LFX resistance. Given the correlations of resistance between PZA and other drugs, PZA susceptibility testing is crucial for the proper management of MDR-TB patients with regimens containing PZA.
Our study also showed that 28.6% (40/180) PZA-R MDR isolates were from newly diagnosed TB patients and 31.1% (56/180) PZA-R MDR isolates were clustered, indicating the transmission of drug resistance. In addition, identical types of mutation in pncA, rpsA, and panD were also detected in the same clustered isolates, and patients whose isolates in the same clusters were from the same living regions. This further indicated recent transmission of PZA-R MDR-TB.
We further investigated the mutations in pncA, rpsA, and panD genes among 169 PZA-R isolates that were successfully sequenced. Of the 71 types of base substitution in the pncA gene, 8 (11.3%) (I5T, L35P, F58V, G78D, T100P, D136G, T142P, and V155A) were classified as “uncertain significance” by the WHO,36 while 10 types (23.2%) were not included in the WHO catalogue,36 including two novel mutation types (V9S and A28N) and eight that had been previously reported (C14Y, G24R, L27M, V93G, C138Y, T153S, L156R, and A178P).16,17,44–49 Base substitutions of C14Y, L27M, C138Y, and L156R were found in the PZA-R resistant isolates in previous studies, and the connection between I5T, G24R, F58V, G78D, T100P, D136G, T142P, V155A, and A178P with PZA resistance is contentious based on findings from previous studies.13,14,44–49 For substitution mutations in pncA classified as “Uncertain significance” and those not included in the WHO catalogue, PZA resistance was predicted using the SUSPECT-PZA webserver.50 The results showed that pncA mutations T100P, T153S, and A178P were predicted as “susceptible”, while other mutations were predicted as “resistant”. Our study detected 24 pncA mutations in PZA-R isolates for the first time, and most of them (91.7%) were deletions or insertions with various lengths of nucleotides.
Unexpectedly, PZase activity testing showed that eight PZA-R isolates harboring pncA mutations including L35P, P62L, D63A, G78D, V93G, F94L, and V155A without mutations in rpsA and/or panD, showed positive PZase activity. This suggests that these mutations may not affect the PZase activity, and other PZA resistance mechanisms may be responsible for these PZA-R isolates. On the other hand, there were 17 PZA-susceptible isolates harboring pncA non-synonymous mutations. We further determined the MICs of four PZA-susceptible strains carrying pncA mutations (P62T, F94L, W119C, and, V180F) that had been regarded as PZA resistance associated by the WHO.36 We also discovered that two isolates with mutations of F94L and W119C did show PZA susceptibility (MIC values ≤ 100 ug/mL) and possessed positive activity. F94L and V155A mutations with positive PZase activity were found both in PZA resistant and susceptible isolates. This is not completely consistent with the WHO catalogue36 and suggests that the F94L, W119C, and V155A mutations do not appear to be associated with PZA resistance. It also indicated that drug resistance resulted from interactions of multiple macromolecules in organisms, including genes, transcripts and proteins and so on.51
Non-synonymous rpsA mutations were detected in four PZA-S isolates, and 10 PZA-R isolates which combined with mutations in pncA, thus it is uncertain whether these mutations in rpsA can affect PZA resistance. A recent study found that RpsA is not the binding target of POA, the active form of PZA, and concluded that RpsA may not be involved in the mechanism of PZA action in M. tuberculosis.52 While previous studies showed that aspartate decarboxylase PanD emerged as a target of POA27,53, no non-synonymous mutations were detected in the panD of all PZA-R isolates in our study. This means that panD mutation may not be associated with PZA resistance. In addition, seven PZA-R isolates did not harbor any mutations in pncA, rpsA, and panD, indicating that additional unknown mechanisms may be involved in PZA resistance.
The WHO target product profiles for new molecular assays for M. tuberculosis require more than 90% sensitivity and 95% specificity.54 Using MGIT 960 PZA susceptibility testing as reference, pncA sequencing for PZA resistance prediction showed sensitivity exceeded 90% and a moderate specificity of 84.7%. However, the mutations found in rspA or panD did not improve the sensitivity, but reduced the specificity by 10.2%. Although the specificity of pncA sequencing was slightly lower in our study, the sensitivity was higher than that in other studies conducted in Mainland China, such as 90.0% (Hangzhou21), 77.97% (Zhejiang25), 89.52% (Henan28), 83.1% (Ningbo31), 79.3% (Beijing39). Therefore, it is necessary to detect pncA mutations to improve PZA resistance detection in clinical practice. We also evaluated the prediction performance of PZase activity testing. Compared to MGIT 960 PZA drug susceptibility testing, PZase activity testing reached a sensitivity of 96.1% and specificity of 94.9%. The PZase activity testing can be completed within four days, and the equipment and reagents involved in this experiment are affordable and easily accessible. Hence, PZase activity testing can be used as an alternative DST method to predict PZA resistance, particularly for low-income countries.
Limitation of the Study
Our research has several limitations. Firstly, we did not conduct whole-genome sequencing (WGS) on PZA-R isolates. Compared to MIRU-VNTR, WGS has a higher resolution in genotyping and can provide information on the lineage of the clinical MTB isolates, which would enable us to analyze the linkage between PZA resistance and specific strain lineages. Additionally, the mechanism of PZA resistance is complex and involves multiple genes such as pncA, rpsA and panD. Our study used Sanger sequencing to individually sequence each gene, while WGS can provide information on genes related to PZA resistance at the whole-genome level. Recently, WHO evaluated the application prospects of targeted next-generation sequencing (tNGS) in the diagnosis of drug-resistant tuberculosis. They consider that the available evidence supports the use of targeted NGS to detect drug resistance after TB diagnosis, to guide clinical decision-making for drug-resistant TB treatment. Therefore, we believe that the use of tNGS for the detection of PZA-R isolates has good application prospects in the future.
Conclusion
Given the important role of transmission in the incidence of PZA-R TB, targeted interventions such as PZA-R TB cases management and source of infection finding are urgently needed to prevent further transmission of PZA-R TB in Hunan province. DNA sequencing of pncA and PZase activity testing have great potential in predicting PZA resistance. In addition, sequencing data of rpsA and panD from this study do not support correlation between these two genes and PZA resistance.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was in line with the Declaration of Helsinki and ethically approved (LS2022060701) by the Ethics Committee of Hunan Chest Hospital in 2022. All methods were performed in accordance with the relevant guidelines and regulations. Each patient signed an informed consent form during hospitalization and treatment.
Funding
This study was supported by the Hunan Provincial Natural Science Foundation of China (2019JJ50299) and Hunan Provincial Health Commission (20200935). The funders had no role in the study design, data collection and analysis, preparation of the manuscript, or decision to publish.
Disclosure
The authors declare there are no conflicts of interest.
References
1. World Health Organization. Global Tuberculosis Report. Geneva: World Health 0rganization; 2022.
2. Zhao Y, Xu S, Wang L, et al. National survey of drug-resistant tuberculosis in China. N Engl J Med. 2012;366(23):2161–2170. doi:10.1056/NEJMoa1108789
3. Singh R, Dwivedi SP, Gaharwar US, et al. Recent updates on drug resistance in mycobacterium tuberculosis. J Appl Microbiol. 2020;128(6):1547–1567. doi:10.1111/jam.14478
4. Kempker RR, Heinrichs MT, Nikolaishvili K, et al. Lung tissue concentrations of pyrazinamide among patients with drug-resistant pulmonary tuberculosis. Antimicrob Agents Chemother. 2017;61(6): doi:10.1128/AAC.00226-17
5. Zhang Y, Chiu Chang K, Leung CC, et al. ‘Z(S)-MDR-TB’ versus ‘Z(R)-MDR-TB’: improving treatment of MDR-TB by identifying pyrazinamide susceptibility. Emerg Microbes Infect. 2012;1(7): e5. doi:10.1038/emi.2012.18
6. Whitfiels MG, Soeters HM, Warren RM, et al. A global perspective on pyrazinamide resistance: systematic review and meta-analysis. PLoS One. 2015;10(7): e0133869. doi:10.1371/journal.pone.0133869
7. Morlock GP, Tyrrell FC, Baynham D, et al. Using reduced inoculum densities of mycobacterium tuberculosis in MGIT pyrazinamide susceptibility testing to prevent false-resistant results and improve accuracy: a multicenter evaluation. Tuberc Res Treat. 2017;2017:3748163. doi:10.1155/2017/3748163
8. Mustazzolu A, Piersimoni C, Iacobino A, et al. Revisiting problems and solutions to decrease mycobacterium tuberculosis pyrazinamide false resistance when using the bactec MGIT 960 system. Ann Ist Super Sanita. 2019;55(1):51–54. doi:10.4415/ANN_19_01_09
9. Konno K, Feldmann F, McDermott W. Pyrazinamide susceptibility and amidase activity of tubercle bacilli. Am Rev Respir Dis. 1967;95(3):461–469. doi:10.1164/arrd.1967.95.3.461
10. Wayne LG. Simple pyrazinamidase and urease tests for routine identification of mycobacteria. Am Rev Respir. 1974; 109: 147–51.
11. Aono A, Chikamatsu K, Yamada H, et al. A simplified pyrazinamidase test for pyrazinamide drug susceptibility in mycobacterium tuberculosis. J Microbiol Methods. 2018;154:52–54. doi:10.1016/j.mimet.2018.09.018
12. Singh P, Wesley C, Jadaun GP, et al. Comparative evaluation of Lowenstein-Jensen proportion method, bact/ALERT 3D system, and enzymatic pyrazinamidase assay for pyrazinamide susceptibility testing of mycobacterium tuberculosis. J Clin Microbiol. 2007;45(1):76–80. doi:10.1128/JCM.00951-06
13. World Health Organization. WHO operational handbook on tuberculosis: module 3: diagnosis-rapid diagnostics for tuberculosis detection: World Health Organization; 2021.
14. Li K, Yang Z, Gu J, et al. Characterization of pncA mutations and prediction of PZA resistance in mycobacterium tuberculosis clinical isolates from Chongqing, China. Front Microbiol. 2020;11:594171. doi:10.3389/fmicb.2020.594171
15. Yadon AN, Maharaj K, Adamson JH, et al. A comprehensive characterization of PncA polymorphisms that confer resistance to pyrazinamide. Nat Commun. 2017;8(1):588. doi:10.1038/s41467-017-00721-2
16. Miotto P, Cabibbe AM, Feuerriegel S, et al. Mycobacterium tuberculosis pyrazinamide resistance determinants: a multicenter study. mBio. 2014;5(5): e01819–14. doi:10.1128/mBio.01819-14
17. Ei PW, Mon AS, Htwe MM, et al. Pyrazinamide resistance and pncA mutations in drug resistant mycobacterium tuberculosis clinical isolates from Myanmar. Tuberculosis. 2020;125:102013. doi:10.1016/j.tube.2020.102013
18. Naluyange R, Mboowa G, Komakech K, et al. High prevalence of phenotypic pyrazinamide resistance and its association with pncA gene mutations in mycobacterium tuberculosis isolates from Uganda. PLoS One. 2020;15(5): e0232543. doi:10.1371/journal.pone.0232543
19. Shi W, Zhang X, Jiang X, et al. Pyrazinamide inhibits trans-translation in mycobacterium tuberculosis. Science. 2011;333(6049):1630–1632. doi:10.1126/science.1208813
20. Dillon NA, Peterson ND, Feaga HA, et al. Anti-tubercular activity of pyrazinamide is independent of trans-translation and RpsA. Sci Rep. 2017;7(1):6135. doi:10.1038/s41598-017-06415-5
21. Liu W, Chen J, Shen Y, et al. Phenotypic and genotypic characterization of pyrazinamide resistance among multidrug-resistant mycobacterium tuberculosis clinical isolates in Hangzhou, China. Clin Microbiol Infect. 2018;24(9): e1–1016 e5. doi:10.1016/j.cmi.2017.12.012
22. Khan MT, Rehaman AU, Junaid M, et al. Insight into novel clinical mutants of RpsA-S324F, E325K, and G341R of Mycobacterium tuberculosis associated with pyrazinamide resistance. Comput Struct Biotechnol J. 2018;16:379–387. doi:10.1016/j.csbj.2018.09.004
23. Khan MT, Khan A, Rehman AU, et al. Structural and free energy landscape of novel mutations in ribosomal protein S1 (rpsA) associated with pyrazinamide resistance. Sci Rep. 2019;9(1):7482. doi:10.1038/s41598-019-44013-9
24. Tan Y, Hu Z, Zhang T, et al. Role of pncA and rpsA gene sequencing in detection of pyrazinamide resistance in Mycobacterium tuberculosis isolates from southern China. J Clin Microbiol. 2014;52(1):291–297. doi:10.1128/JCM.01903-13
25. Xia Q, Zhao LL, Li F, et al. Phenotypic and genotypic characterization of pyrazinamide resistance among multidrug-resistant Mycobacterium tuberculosis isolates in Zhejiang, China. Antimicrob Agents Chemother. 2015;59(3):1690–1695. doi:10.1128/AAC.04541-14
26. Zhang S, Chen J, Shi W, et al. Mutations in panD encoding aspartate decarboxylase are associated with pyrazinamide resistance in Mycobacterium tuberculosis. Emerg Microbes Infect. 2013;2(6):e34. doi:10.1038/emi.2013.38
27. Gopal P, Sarathy JP, Yee M, et al. Pyrazinamide triggers degradation of its target aspartate decarboxylase. Nat Commun. 2020;11(1):1661. doi:10.1038/s41467-020-15516-1
28. Shi J, Su R, Zheng D, et al. Pyrazinamide resistance and mutation patterns among multidrug-resistant mycobacterium tuberculosis from Henan Province. Infect Drug Resist. 2020;13:2929–2941. doi:10.2147/IDR.S260161
29. Burgos MV, Pym AS. Molecular epidemiology of tuberculosis. Eur Respir J Suppl. 2002;36(Supplement 36):54s–65s. doi:10.1183/09031936.02.00400702
30. Eva Nathanson MS, Paul Nunn FRCP, Mukund Uplekar MD, et al. MDR Tuberculosis — critical Steps for Prevention and Control. New Engl J Med. 2010;363 (11) : 1050 -8. doi:10.1056/NEJMra0908076
31. Che Y, Bo D, Lin X, et al. Phenotypic and molecular characterization of pyrazinamide resistance among multidrug-resistant mycobacterium tuberculosis isolates in Ningbo, China. BMC Infect Dis. 2021;21(1):605. doi:10.1186/s12879-021-06306-1
32. Alene KA, Xu Z, Bai L, et al. Spatial clustering of drug-resistant tuberculosis in Hunan province, China: an ecological study. BMJ Open. 2021;11(4): e043685. doi:10.1136/bmjopen-2020-043685
33. World health Organization. Policy guidance on drug-susceptibility testing (DST) of second-line antituberculosis drugs. World health Organization;2008.
34. World health Organization. Technical report on critical concentrations for drug susceptibility testing of medicines used in the treatment of drug-resistant tuberculosis. World health Organization;.2018.
35. Wayne LG. Simple pyrazinamidase and urease tests for routine identification of mycobacteria. Am Rev Respir Dis. 1974;109(1):147–151. doi:10.1164/arrd.1974.109.1.147
36. World Health Organization. Catalogue of Mutations in Mycobacterium Tuberculosis Complex and Their Association with Drug Resistance. Geneva: World Health Organization; 2021.
37. Supply P, Allix C, Lesjean S, et al. Proposal for standardization of optimized mycobacterial interspersed repetitive unit-variable-number tandem repeat typing of mycobacterium tuberculosis. J Clin Microbiol. 2006;44(12):4498–4510. doi:10.1128/JCM.01392-06
38. Pang Y, Zhu D, Zheng H, et al. Prevalence and molecular characterization of pyrazinamide resistance among multidrug-resistant mycobacterium tuberculosis isolates from Southern China. BMC Infect Dis. 2017;17(1):711. doi:10.1186/s12879-017-2761-6
39. Gu Y, Yu X, Jiang G, et al. Pyrazinamide resistance among multidrug-resistant tuberculosis clinical isolates in a national referral center of China and its correlations with pncA, rpsA, and panD gene mutations. Diagn Microbiol Infect Dis. 2016;84(3):207–211. doi:10.1016/j.diagmicrobio.2015.10.017
40. Pwasmmsmktllnn E. Pyrazinamide resistance and pncA mutations in drug resistant mycobacterium tuberculosis clinical isolates from Myanmar. Tuberculosis. 2020;125: doi:10.1016/j.tube.2020.102013
41. Calderon RI, Velasquez GE, Becerra MC, et al. Prevalence of pyrazinamide resistance and Wayne assay performance analysis in a tuberculosis cohort in Lima, Peru. Int J Tuberc Lung Dis. 2017;21(8):894–901. doi:10.5588/ijtld.16.0850
42. Park S, Jo KW, Shim TS. Treatment outcomes in multidrug-resistant tuberculosis according to pyrazinamide susceptibility. Int J Tuberc Lung Dis. 2020;24(2):233–239. doi:10.5588/ijtld.19.0314
43. Alame-Emane AKXP, Pierre-Audigier C, Cadet-Daniel V, et al. Pyrazinamide resistance in mycobacterium tuberculosis arises after rifampicin and fluoroquinolone resistance. Int J Tuberc Lung Dis. 2015;19(6):679–684. doi:10.5588/ijtld.14.0768
44. Daum LT, Konstantynovska OS, Solodiankin OS, et al. Characterization of novel Mycobacterium tuberculosis pncA gene mutations in clinical isolates from the Ukraine. Diagn Microbiol Infect Dis Apr. 2019;93(4):334–338. doi:10.1016/j.diagmicrobio.2018.10.018
45. Sengstake S, Bergval IL, Schuitema AR, et al. Pyrazinamide resistance-conferring mutations in pncA and the transmission of multidrug resistant TB in Georgia. BMC Infect Dis. 2017;17(1):491. doi:10.1186/s12879-017-2594-3
46. Rajendran V, Sethumadhavan R. Drug resistance mechanism of PncA in Mycobacterium tuberculosis. J Biomol Struct Dyn. 2014;32(2):209–221. doi:10.1080/07391102.2012.759885
47. Jonmalung J, Prammananan T, Leechawengwongs M, et al. Surveillance of pyrazinamide susceptibility among multidrug-resistant mycobacterium tuberculosis isolates from Siriraj Hospital, Thailand. BMC Microbiol. 2010;10(1):223. doi:10.1186/1471-2180-10-223
48. Rueda D, Bernard C, Gandy L. susceptibility in Mycobacterium tuberculosis. Int J Mycobacteriol. 2018;7(1):16–25. doi:10.4103/ijmy.ijmy_187_17
49. Ramirez-Busby SM, Rodwell TC, Fink L, et al. A multinational analysis of mutations and heterogeneity in PZase, RpsA, and PanD associated with pyrazinamide resistance in M/XDR mycobacterium tuberculosis. Sci Rep. 2017;7(1):3790. doi:10.1038/s41598-017-03452-y
50. Karmakar M, Rodrigues CHM, Horan K, et al. Structure guided prediction of pyrazinamide resistance mutations in pncA. Sci Rep. 2020;10(1):1875. doi:10.1038/s41598-020-58635-x
51. Suzuki S, Horinouchi T, Furusawa C. Prediction of antibiotic resistance by gene expression profiles. Nat Commun. 2014;5(1):5792. doi:10.1038/ncomms6792
52. Vallejos-Sanchez K, Lopez JM, Antiparra R, et al. Mycobacterium tuberculosis ribosomal protein S1 (RpsA) and variants with truncated C-terminal end show absence of interaction with pyrazinoic acid. Sci Rep. 2020;10(1):8356. doi:10.1038/s41598-020-65173-z
53. Gopal P, Nartey W, Ragunathan P, et al. Pyrazinoic acid inhibits mycobacterial coenzyme a biosynthesis by binding to aspartate decarboxylase PanD. ACS Infect Dis. 2017;3(11):807–819. doi:10.1021/acsinfecdis.7b00079
54. World health Organization. High-Priority Target Product Profiles for New Tuberculosis Diagnostics: Report of a Consensus Meeting. Geneva: World Health Organization; 2014.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
