Back to Journals » Veterinary Medicine: Research and Reports » Volume 12
Prevalence of Staphylococcus aureus, Methicillin-Resistant Staphylococcus aureus and Potential Risk Factors in Selected Dairy Farms at the Interface of Animal and Human in Bishoftu, Ethiopia
Authors Tibebu L, Belete Y, Tigabu E, Tsegaye W
Received 30 July 2021
Accepted for publication 7 September 2021
Published 23 September 2021 Volume 2021:12 Pages 241—251
DOI https://doi.org/10.2147/VMRR.S331968
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Lakech Tibebu,1 Yerega Belete,2 Eyasu Tigabu,3 Wondewossen Tsegaye2
1Disease Surveillance Expert Epidemiology Directorate, Ministry of Agriculture, Addis Ababa, Ethiopia; 2Department of Microbiology, Immunology and Parasitology, St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia; 3Research Directorate, Ethiopian Public Health Institute, Addis Ababa, Ethiopia
Correspondence: Yerega Belete
Department of Microbiology, Immunology and Parasitology, St. Paul’s Hospital Millennium Medical College, P.O. Box 1271, Addis Ababa, Ethiopia
Tel +251 920735677
Email [email protected]
Background: Staphylococcus aureus (S. aureus) has been reported as the most commonly isolated highly contagious pathogen from human, animals and animal products. Methicillin-resistant Staphylococcus aureus (MRSA) has emerged as a significant pathogen with zoonotic potential that could have devastating consequence for the health and well-being of animals and human.
Methods: A cross-sectional study was conducted from July 2020 to January 2021. A total of 233 samples from cow milk, udder swabs and milkers’ hand swabs were collected for culture and identification based on the standard protocol. Antimicrobial susceptibility tests were performed for all isolates by using Kirby Bauer’s disk diffusion test. MRSA was detected by cefoxitin disk diffusion test.
Results: S. aureus was isolated from 50 (21.46%) of 233 samples and the prevalence of MRSA was 4%. The highest prevalence was found in cow milk 36 (25.53%) followed by hand swabs 10 (19.23%) and udder swabs 4 (10%). S. aureus prevalence was 58.33%, 30.0%, 21.43%, 17.92%, 15.79% in farm D, C, E, A, B respectively. A large percentage (58.33% and 30%) were from farm D and C. S. aureus isolation rate showed statistically significant association with farm types (p = 0.011) and with previous mastitis exposure (p = 0.001). High level of resistance was observed to penicillin (94%) and ampicillin (92%), but low level resistance to gentamicin (0%), amikacin (0%), ceftriaxone (0%), chloramphenicol (4%), ciprofloxacin and cefoxitin (4%). The overall prevalence of multidrug resistance (MDR) was 10.42%.
Conclusion: Prevalence of S. aureus in milk showed statistically significant association with respect to previous mastitis exposure and farm types (p = 0.011). High level of resistant to penicillin and ampicillin was observed. Therefore, effective mastitis control programs, best veterinary practice among all farms and use of antibiotics in the farm should be strictly controlled.
Keywords: S. aureus, MRSA, MDR, animal, human, interface, multidrug resistance, methicillin-resistance staphylococcus aureus, Staphylococcus aureus
Introduction
Staphylococcus aureus (S. aureus) colonizes most human and animal bodies and causes variety of infections like bacteremia, necrotizing pneumonia and toxic shock syndrome in human, and mammary gland infection (mastitis) in animals.1,2 It was released to the milk supply and causes food poisoning in human.3 The source of S. aureus contamination of raw milk in dairy farm could be originated from animal itself, contaminated feed, bedding, housing and water processing environment and the burden of S. aureus increased when there were less personnel and less utensils hygiene.4,5
S. aureus acquires antimicrobial resistance very quickly and methicillin resistance is of particular relevance one because this cause resistance to all beta-lactam antibiotics.3 In the world three forms of methicillin-resistant S. aureus (MRSA) have been occurred. These are health care-acquired MRSA (HA-MRSA) occurred in immune compromised persons, the community-associated MRSA (CA-MRSA) occurred in the healthy persons and the recent one occurred in livestock animal which is called Livestock-associated MRSA (LA-MRSA) and there is a risk of zoonotic transmission for a person who have contact with LA-MRSA infected animals.6,7 The emergence of MRSA in animals causes multidrug-resistance (MDR).8
Staphylococcus aureus, especially MRSA has been regarded as zoonotic agents and there is high concern on this pathogen. A cross-sectional study conducted in the US, on 2005 at the annual American College of Veterinary Internal Medicine forum with the aim of determining methicillin-resistant S. aureus colonization in Veterinary personnel found that 7% of veterinarians and 12% of technician attendees were colonized with MRSA ST398. These studies show that transmission of MRSA can occur from human to animal and vice versa and direct exposure to MRSA-positive animals may lead to transmission to humans. Furthermore, it is due to the consuming of MRSA infected animal products.9,10
Food borne diseases are among the most widespread public health problems at the globe. Food normally becomes a potential source of human infection due to contamination during production, collection, transportation and processing. It is also an important vehicle for the transfer of antimicrobial resistant (AMR) bacteria. Furthermore, transfer of AMR bacteria to humans via the food chain and from livestock has been well documented. Public health concern arises when either milk is consumed raw or when pasteurization is not standard.11
MRSA causes many infections which in turn result in higher costs, longer treatment times, and higher rates of hospitalization. MRSA infected cattle acts as a reservoir and later transmit the infections to other animals and humans,12 and it has a clear zoonotic relevance, especially in the case of occupational exposure. The detection of MRSA in bovine milk and dairy cattle herds is increasingly reported worldwide. The resistance mechanism is due to the acquisition of mecA or mecC gene in mobile genetic element called staphylococcal cassette chromosome (SCCmec). In this mec genes code for alternative penicillin-binding proteins, PBP2a, that has reduced affinity to most β-lactam antibiotics.2,13
MRSA occurred after the use of methicillin to clinical practice. The biggest challenge occurred by MRSA globally is decreased susceptibility to other antibiotics including the beta-lactam drugs. At this time MRSA rapidly spread to entire human community and in livestock. However, the colonization rates are high in some group of people. Like, children, hospitalized elderly patients, and young women overcrowding, sharing personal items coupled with poor hygiene, over populated areas and in intravenous drug. Furthermore, this lead in facilitating the spread of the infection.6
The prevalence of MRSA was higher in America than Europe. Different studies reported that prevalence rates of greater than 70% in South Korea and Vietnam, and less than 50% in Portugal, Greece, and Italy.6 In Egypt, a study shows prevalence of S. aureus was 17.2%. In another study, 70–73% of S. aureus strains isolated from various foods were resistant to β-lactam such as Penicillin and Ampicillin.9 In Ethiopia, overall prevalence of S. aureus with different study showed that 15.3% originating from raw cows’ milk, 25% from swabs of milkers’ hand, 20% from swabs of milking bucket, and 10% from swabs of drying towel.14
In study done in South Africa the prevalence of MRSA was 5.7–7% in commercial farms. In other African country study the prevalence of MRSA were higher in Ethiopia (60.3%) in Nigeria 28.57%, in Morocco 15% and low prevalence in Kenya 7.8% were recorded.15 However, information on prevalence of S. aureus and MRSA in milkers personnel and in lactating dairy cows has not been extensively studied in Ethiopia and specifically in the study area. Therefore, this research aimed to determine the prevalence of S. aureus, MRSA and potential risk factors from selected dairy farm at the interface of human and animal in Bishoftu.
Methods and Materials
Study Area
The study was conducted in Bishoftu (Debre-Zeit). Bishoftu is found in the central high lands of Ethiopia at 47 km Southeast of Addis Ababa, the capital city of Ethiopia. It is located to Ada district of east Shewa zone of Oromia region. The total human population of the town was 200,00016 and cattle population of the area is 146,312.17 It is located at 8°45′ N longitude and 38°59′ E latitude at an altitude of 1880 meter above sea level. It has an average annual rainfall of 1150 mm of which 84% falls during the long rainy season that extends from June to September, and the remaining during the short rainy season that extends from March to May. The mean annual minimum and maximum temperatures are 8.5 and 30.7 °C respectively and the mean relative humidity is 61.3%.18
In the town there are 40 (small and large scale dairy farm)18 and three dairy processing companies which are namely called Ada’a Dairy Cooperative, Lema Dairy and Holland Dairy farm.19 The study area had five farms which were arbitrary named as A, B, C, D and E. Farm-A has a herd size of 400 cows and 150 lactating cows, farm-B has a herd size of 113 and 60 lactating cows, farm-C has a herd size of 50 and 34 lactating cows, farm-D has a herd size of 29 and 18 lactating cows, and farm-E has a herd size of 67 and 30 lactating cows.
Study Design and Period
A cross-sectional study was conducted to determine the prevalence of S. aureus, methicillin resistant S. aureus and potential risk factors from selected dairy farm at the interface of human and animal in Bishoftu town from July to January, 2021.
Source of Population
The source population were dairy cattle, and people who were working on dairy farms.
Study Population
The study population were apparently healthy lactating cows and milking personnel on dairy farms.
Inclusion and Exclusion Criteria
Inclusion Criteria
Dairy farms that are willing to participate in the study and all lactating cows in the selected farms were included. Milking personnel who were willing to give consent.
Exclusion Criteria
Sick animals who are already being treated with antibiotics and people who are critically ill.
Sample Size Calculation
Sample size was calculated by applying the single population proportion formula, n=(Zα/2)2 × p (1−p)/d2),where, n = sample size, z = statistic for a level of confidence, d = margin of error, and p = expected prevalence or proportional, 95% level of confidence with a margin of error of 5% and 13.6% prevalence from a previous study done at Ambo and Guder town.20 Therefore, 181 lactating cows were included. Since a total of 52 milkers were available in the dairy farms during the sampling time, we took these samples making the total sample size 233.
Sampling Method and Sampling Techniques
The dairy farms were selected based on the availability of one or more lactating cows and willingness of the dairy farm owners and farm workers to be part of the study. Then lactating cows from the selected farms were included by using lottery method with simple random sampling techniques after assigning of identification tags for each lactating cows. A total of 233 samples were collected. From this, 181 samples were collected from lactating cows and 52 samples were collected from milkers’ hand. From 181 lactating cows’ samples, 141 samples were cow milk and 40 of the samples were udder swabs.
Data Collection
To determine the potential risk factors for S. aureus and MRSA in milkers’ both self-administer and interview administered questionnaires were used after training of the data collectors. The purpose of the study as well as any related harm and benefit were explained to the study participants accordingly.
Specimen Collection
Composite milk samples of 250 milliliter (mL) were taken from each cow after cleaning the udder with water and soap and dried using clean towels and disinfected the teat with 70% alcohol. After discarding the first four strip of milk, the next strips of milk were transferred into sterile milk collection bottle. Udder and milkers’ hand swabs were taken with sterile swab before milking. Prior to sampling hand and udder swabs, swab tips were moistened with sterile sodium chloride (NaCl) solution. The hand and udder swabs were placed inside a screw-capped tube containing sterile NaCl solution and transported using a cold chain to Microbiology laboratory of the Ethiopian Public Health Institute (EPHI), Addis Ababa, Ethiopia, and stored at +4 °C for a maximum of 24 h until it is being processed and cultured.9
Laboratory Testing Procedures
Culturing and Identification Procedure
Bacteriological culture was performed following the standard microbiological technique. A common selective medium used for the isolation of pathogenic staphylococci was Mannitol salt agar. 25 mL of milk sample was taken from the sterile milk collection tube and transferred into 225 mL of a normal saline solution and homogenized. Appropriate dilution of 1mL of the suspension was inoculated into labeled sterilized petri dish and 20 mL of melted MSA (45–50°C) pour on each petri dish and mixed by rotating. For hand and udder swabs, the tip of the swabs were squeezed in the tube that contain normal saline solution then 1mL of the suspension was inoculated into labeled sterilized petri dish and 20 mL of melted MSA pour on each petri dish and mixed by rotating. Then all the plates were incubated at 37°C for 24–48 hrs. The colonies of S. aureus were counted manually by using a pen clicked on the colony which is on backside of the petri dish. A loopful of the colonies were streaked onto Tryptic soya agar (TSA) and incubates at 37°C for 24 hrs then pure colonies were preserved and maintained on tryptic soya broth (TSB) for further characterization of the isolates. Eventually, identification of the isolates were done based on biochemical tests such as catalase, coagulase and growth on mannitol salt agar. Samples were considered as positive for S. aureus when the isolates were catalase and coagulase positive and showed fermentation of mannitol (strong golden yellow color colonies).11
Antibiotic Susceptibility Testing and Identification of MRSA
Antimicrobial susceptibility was done by Kirby-Bauer disc diffusion method based on the standard procedure. Three to five well-isolated colonies of similar appearance were picked and homogenized in 3–4 mL of sterile saline solution and make a suspension until the turbidity match to 0.5 McFarland standard (Mary-l’Etoil, France). A sterile cotton swab was dipped into the suspension, and excess suspension was removed by gentle rotation of the swab against the surface of the tube. The swab was then used to distribute the bacteria evenly over the entire surface of Mueller Hinton agar (Oxoid). The inoculated plates were left at room temperature to dry for 3–5 minutes. With the aid of disc dispenser, the following concentration of antibiotic discs were put on the surface of Mueller-Hinton agar (Oxoid), cefoxitin (30µg), penicillin G (10 μg), ampicillin (10 μg), ciprofloxacin (5 μg), chloramphenicol (30 μg), tetracycline (30 μg), gentamicin (10 μg), amikacin (30 μg) and ceftriaxone (30 μg). The plates were incubated at 37°C for 18–24 hours. Then, the diameter of the “zone of inhibition” around the antibiotic discs was measured and reported as sensitive, intermediate and resistance. Zone of inhibitions values obtained was compared with the Clinical and Laboratory Standard Institute (CLSI) and interpret the results obtained.21,22 MRSA is identified by assessing zone of inhibitions with cefoxitin ≤ 21 mm. Cefoxitin disc diffusion test is considered superior to oxacillin disc diffusion test due to its ease of reading and higher sensitivity.12
Enumeration of Staphylococcus aureus
Serial dilutions of milk samples were prepared up to 101 with a normal saline solution and from the dilution one-milliliter sample suspension of milk and swab were aseptically transferred to petri dish and pour on Mannitol salt agar. As the plate containing colonies with typical growth on MSA appearance of circular, smooth, convex, moist, golden- yellow color and medium in size was taken as S. aureus and count them manually by clicking with pen on the colony.
Quality Assurance
Data collection was conducted after the data collectors were given a training, and participants were informed about the purpose of the study and after given consent. The sample were collected, transported using a cold chain and stored at +4 °C for a maximum of 24 hrs until it being processed and cultured. All participant information collected during the study period was checked for its clarity and completeness in a regular basis. Each lot of the medium was checked for expiration dates prior to use as part of quality control.
Standard operating procedures (SOPs) of the laboratory were ensured the reliability and validity of test result. Bach of the media was incubated at 37°C for 24 hrs and media were checked for pH, sterility, ability and support growth before use. In addition to these, visually check media for depth, smoothness, hemolysis, excessive bubbles, contamination, check for cracked or damaged plates, and frozen or melted agar prior to use. The media performances were checked with a known positive controls standard American Type Culture Collection (ATCC) reference S. aureus ATCC 25923 as positive control and E. coli ATCC 25922 as negative control. MRSA is identified by assessing zone of inhibitions with cefoxitin disc ≤ 21 mm and a report of susceptible, intermediate or resistant can be obtained by referring to the standardized tables compiled by CLSI.
Data Analysis and Interpretation
Data were presented into descriptive statistics with frequency, proportion, percentages, measures of central tendency and standard deviation. Data were also entered and analyzed using the statistical software SPSS version 25. The chi-square test was calculated to determine the association between variables, P-value of ≤ 0.05 was considered to test statistically significant association.
Operational Definition
CA-MRSA
MRSA isolates were considered to be community acquired if they were recovered within 72 h of admission to the hospital.
Interface
A point where two systems or subjects meet and interact.
MDR
Resistant to three or more antimicrobial class.
MRSA
S. aureus strain that is resistant to penicillin, β-lactams such as methicillin, oxacillin or cloxacillin.
Results
In this study a total of 233 samples from five intensive and semi-intensive dairy farms were collected and analyzed microbiologically. The overall prevalence of S. aureus was 50/233 (21.46%) in five dairy farms. The prevalence of S. aureus in cow milk, hand swabs and udder swabs were 25.53%, 19.23% and 10% respectively. However, there was no statistically significant association in the isolation rate of S. aureus among the different sample types (P >0.05) (Table 1).
 |
Table 1 Isolation Rate of S. aureus Between Different Sample Types Conducted in Bishoftu Dairy Farm, Oromia Region, Ethiopia, from July 2020 to January 2021 |
The isolation rates of S. aureus at the five dairy farm was 17.92, 15.79, 30.0, 58.33 and 21.43 in farm A, B, C, D and E respectively. A large percentage of S. aureus isolates (58.33% and 30%) were from farms D and C. Surprisingly, Statistically significant association was observed between farm types and S. aureus isolation rate (Table 2).
 |
Table 2 Isolation Rate of S. aureus at Different Farm Level in Bishoftu Dairy Farms, Oromia Region, Ethiopia, from July 2020 to January 2021 |
Associated Factors with Prevalence of S. aureus
In this study, the prevalence of S. aureus was higher in adult milkers’ hands with the age of 30–40 years, no awareness about S. aureus and MRSA and in milkers who did not use antiseptic before/after milking. In addition, higher prevalence of S. aureus was observed in farms which had poor barn drainage system, semi-intensive management system, and in cows with previous mastitis exposure. None of the associated factors showed statistically significant association with the prevalence of S. aureus except previous mastitis exposure (p< 0.05) (Table 3).
 |
Table 3 Associated Risk Factors for Isolation Rate of S. aureus and MRSA from Cow Milk, Udder Swabs and Hand Swabs in Bishoftu Dairy Farms, Oromia Region, Ethiopia, from July 2020 to January 2021 |
Staphylococcus aureus Load in Raw Milk Samples from Cow Udder
In this study, from the total of 36 milk samples which were positive for S. aureus, 14 (37.84%) of them were above the recommended level. The highest S. aureus colony count was observed in dairy farm A, which was 9.8×102 followed 5.9×102 (Table 4).
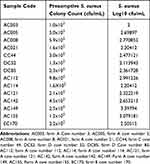 |
Table 4 Staphylococcus aureus Load from Raw Milk Collected Directly from Cows’ Udder in Bishoftu Dairy Farms, Oromia Region, Ethiopia, from July 2020 to January 2021 |
Antimicrobial Susceptibility Test
Antimicrobial susceptibility test was performed for all 50 S. aureus isolates. Nine different antibiotic disks were used. In this study, bacterial isolates showed varying degree of susceptibility to the antimicrobial agents used. From overall isolates the highest resistance was 47 (94%) to penicillin followed by ampicillin 46 (92%) and tetracycline 37 (74%). MRSA was detected as 2 (4%) in one dairy farm from cow milk samples. Intermediate resistance was observed for amikacin 7 (14%) and tetracycline 1 (2%). High level of sensitivity was observed for gentamicin (100%), ceftriaxone (100%), chloramphenicol (96%), cefoxitin (96%) and ciprofloxacin (96%) (Table 5).
 |
Table 5 Antimicrobial Susceptibility Test Result of S. aureus in Bishoftu Dairy Farms, Oromia Region, Ethiopia, from July 2020 to January 2021 |
From nine antibiotic disks, mono-drug resistance was observed in 1(2.08%) isolate and other isolates showed for two, three, four, and five antimicrobials resistance which is 11 (22.92%), 32 (66.67%), 3 (6.25%), and 1 (2.08%) respectively. The overall MDR was 10.42% (Table 6).
 |
Table 6 MDR Pattern of S. aureus Isolates in Bishoftu Dairy Farms, Oromia Region, Ethiopia, from July 2020 to January 2021 |
Discussion
This study showed that the overall prevalence of S. aureus was 21.46%. From this, 25.53% was from cow milk, 10% was from udder swabs and 19.23% was from hand swabs. The prevalence of S. aureus among different samples in this study showed no statistically significant association (P>0.05). The prevalence of S. aureus in the present study is higher than other studies conducted at Mukaturi and Sululta 16.6%,14 Sebeta 19.6%,11 Iran 6.61%23 and Egypt 19%,24 North west India 19.84%.25 These variation might be due to difference in sample size, isolation techniques, awareness and skills of the farm workers, geographic regions and variation in study subjects for example for Iran the sample was collected from buffalo and camel. It is comparable with a study reported at Alage Veterinary College Dairy Farm 21.2%,17 Uganda 20.3%8 and China 22.3%.26 However, our result is lower than the prevalence conducted in Addis Ababa 50%,9 Hawassa 51.2%,27 Bishoftu 28.65%,28 Hawassa 78%,29 Mekele 32.81%,30 China 46.2%,31 Italy 47.2%13 and India 54.3%.32 These variations might be the different in management system used by the farm, types of sample for example in Italy bulk tank milk and in Hawassa29 only milk sample was analyzed, diagnostic test like PCR test was used for a studies in Mekele and India.
The prevalence of S. aureus from cow milk in this study 25.53% was higher than the study conducted in Mukaturi and Sululta 15.3%,14 but lower than Tigray 38.09%.30 This is due to variation in hygienic procedure, sample size and variation in clinical cases for example in a study conducted in Tigray all study subjects had mastitis. On the other hand, the isolation rate of S. aureus in milkers’ hand swabs and udder swabs was also (19.2%) and (10%) respectively. The rate of isolation of S. aureus in milkers’ hand swabs is lower than the study conducted at Mukaturi and Sululta 25%14 and India 41.2%.32 The variation might be difference in diagnostic techniques in India PCR was conducted.
The current study revealed that the prevalence of S. aureus in farm D, C, E, A and B was 58.33%, 30%, 21.43%, 17.92% and 15.79% respectively. Statistically significant association was observed between farm types and S. aureus isolation rate.
Staphylococcus aureus infection is spread during milking when S. aureus contaminated milk from an infected gland comes in contact with an uninfected gland, and the bacteria penetrate the teat canal and infections persist and even not well respond to antibiotic therapy which act as source of infection.33 The present study showed a high prevalence of S. aureus (66.67%) in dairy cows that had a history of previous mastitis exposure. Statistically significant association was observed between prevalence of S. aureus and dairy cows that have previous mastitis exposure (p<0.001).Our study is in agreement with a study conducted in Bishoftu18 and Hawassa.27
In microbial study conducted in London described as the total S. aureus count (102–104 cfu/g/mL) was described as unsatisfactory level of bacterial quality in the foods.34 In the current study the total S. aureus count in each raw milk sample was within the range of 102–103. Higher range of S. aureus count was reported in a study conducted at Mukturi and Sululta in which 42.9%14 Staphylococcus aureus positive raw milk samples had 104−105 cfu/mL S. aureus count. Which are unsatisfactory level and the milk that was consumed is a serious risk to the health of the population.
In the present study, a high level of sensitivity was observed for gentamicin 100%, ceftriaxone 100%, chloramphenicol 96%, Cefoxitin 96% and Ciprofloxacin 96%. However, high level of resistance was observed to penicillin (94%). The highest resistance pattern to penicillin was in agreement with the study conducted in Bishoftu 91.1%,35 Mukaturi and Sululta 97.6%,14 Tigray >90%,2 Addis Ababa 95.3%,9 Hawassa 100%,29 Iran 100%36 and China26. This high resistance is due to production of β-lactamase by S. aureus that inactivates penicillin and related antibiotics. The beta-lactams are the drugs of choice for intramammary infections. However, the inappropriate and regular use of these medications has contributed to the emergence of resistant bacteria.
Furthermore, high S. aureus resistance was observed to ampicillin 92% and intermediate resistance to tetracycline 74%. This finding is in agreement with other studies for ampicillin resistance in Tigray 100%,30 Ethiopia 98.1%,37 Hawassa 70.9%,29 Uganda 73.2%,8 India 74.42%.38 Similarly, the tetracycline resistance value was comparable with study Mekele 32–35%2 and China 68.53%.26 The reason for the high resistance might be ampicillin and tetracycline are commonly used for the treatment in humans and animals.
Infected animals and associated products have been supposed to be a potential source of community acquired MRSA. Recently, the isolation of MRSA in animal and human have become a worldwide concern.39 The current study revealed that resistance to cefoxitin was 4% which is indicator of MRSA occurrence. This finding was similar with a study conducted in Mekelle 4.5%,2 Tanzania 4.4%40 and Italy 3.8%, but much lower than in Sebeta 100%.11 The reason of MRSA occurrence is due to occurrence of sub-clinical mastitis in dairy farms and this transmits to raw milk and by this the opportunity of MRSA transmission from the milking halls and from infected milkiers’ hands into the raw milk, other human and animal. In addition, the presence of substandard hygienic procedure practiced by milkers are the most important probable reasons for occurrence of MRSA.23 Identification of MRSA in dairy farms within this study emphasizes the need for increased milkers’ awareness regarding safe milk collection and applies good hygiene procedure which helps to prevents cross-contamination and administer antimicrobial regularly and prescribed antibiotics with authorized prescription.
The prevalence of MDR in this study was 10.42%. Our study is much lower than in studies conducted in Egypt 100%.24 The reason for the occurrence of this MDR is due to most antimicrobials used for this study are frequently used antibiotics that produce β-lactamase, that inactivates penicillin and closely related antibiotics and as a result of repeated therapeutic and or indiscriminate use of them in the dairy farms and human for the treatment of infection. Furthermore, the presence of antimicrobial residues in milk which render AMR pathogens which was released in the milk and finally used by different person.41
Conclusion
The occurrence of S. aureus and MRSA in cow milk, udder of the cow and milkers’ hands was the source of the pathogen transmission to other human and animals. This study revealed that high prevalence of S. aureus was reported in dairy cows that have a previous mastitis exposure and statistically significant association was observed between previous mastitis exposure and S. aureus isolation rate (p<0.005). High level of resistance was observed to gentamicin, ceftriaxone, penicillin and moderate resistance to ampicillin. Isolates also showed resistance to cefoxitin. Moreover, the isolates showed MDR and which is an alarming situation for designing prevention, control measures and monitoring rational use of drugs.
Abbreviations
AMR, Antimicrobial resistance; BTM, Bulk Tank Milk; CA-MRSA, Community Associated Methicillin-Resistant Staphylococcus aureus; CCs, Clonal Complexes; CFU, Colony Forming Unit; CLSI, Clinical and Laboratory Standard Institute; HA-MRSA, Health care-Associated Methicillin-Resistant Staphylococcus aureus; LA-MRSA, Livestock-Associated Methicillin-Resistant Staphylococcus aureus; MCC, Milk Collection Center; MDR, Multidrug Resistance; MRSA, Methicillin-Resistant Staphylococcus aureus; NaCl, Sodium Chloride; PBP2a, Penicillin Binding Protein 2a; SCCmec, Staphylococcal chromosome cassette mec; SFP, Staphylococcal Food Poisoning; SPHMMC, Saint Paul’s Hospital Millennium Medical College.
Data Sharing Statement
Data is available upon request from the corresponding author.
Ethical Approval and Consent to Participate
Ethical clearance was obtained from Institution Review Board (IRB) of St Paul’s Hospital Millennium Medical College and Department of Microbiology, Parasitology and Immunology. Official permission was also obtained from Bishoftu dairy farm. In addition, written consent was obtained from the milkers’ personnel before the initiation of data collection, and that it was conducted in accordance with the Declaration of Helsinki, and dairy cattle owners were informed and aware about the purpose of the study so that the cattle were treated and examined with best practice veterinary care. The individual results of any investigation remained confidential. All identified cases of S. aureus in cow milk and udder swabs were referred to attending veterinary physicians.
Consent for Publication
Not applicable. This study does not contain any individual or personal data.
Acknowledgments
The authors would like to thank Saint Paul’s Hospital Millennium Medical College, Department of Microbiology, Immunology and Parasitology for sponsoring the research. Our special thanks and appreciation go to the staff members of Bishoftu dairy farm workers for their cooperation and organizing the preconditions of sample and data collection. We also acknowledged the Ethiopian public health Microbiology laboratory, especially the food microbiology laboratory staffs for their helpful assistance and material supporting during the laboratory work. We also extend our profound gratitude to the study participants for their willingness without whom this research work would not have been possible.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was sponsored by St. Paul’s hospital millennium medical college, Addis Ababa, Ethiopia.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Dittmann KK, Chaul LT, Lee SHI, et al. Staphylococcus aureus in some Brazilian dairy industries: changes of contamination and diversity. Front Microbiol. 2017;8:2049.
2. Kalayu AA, Woldetsadik DA, Woldeamanuel Y, Wang S-H, Gebreyes WA, Teferi T. Burden and antimicrobial resistance of S. aureus in dairy farms in Mekelle, Northern Ethiopia. BMC Vet Res. 2020;16(1):20. doi:10.1186/s12917-020-2235-8
3. Mcmillan K, Moore SC, Mcauley CM, Fegan N, Fox EM. Characterization of Staphylococcus aureus isolates from raw milk sources in Victoria, Australia. BMC Microbiol. 2016;16(1):1–12. doi:10.1186/s12866-016-0789-1
4. Mcauley CM, Mcmillan K, Moore SC, Fegan N, Fox EM. Prevalence and characterization of foodborne pathogens from Australian dairy farm environments. J Dairy Sci. 2014;97(12):7402–7412. doi:10.3168/jds.2014-8735
5. Tarekgne EKT. Staphylococcus aureus from milk and milk products in Ethiopia: prevalence, enterotoxigenic potential, antibiotic resistance and spa types. Norwegian Univ Life Sci. 2016;48:1–188.
6. Chukwunonso E, Veronica B, Toyo P, et al. Methicillin-resistant Staphylococcus aureus. Int J Med Res Health Sci. 2018;7(1):122–127.
7. Carfora V, Giacinti G, Sagrafoli D, et al. S. aureus in dairy sheep and in-contact humans: an Intra-Farm Study. Am Dairy Sci Assoc. 2016;99(6):4251–4258. doi:10.3168/jds.2016-10912
8. Asiimwe BB, Baldan R, Trovato A, Cirillo DM. Prevalence and molecular characteristics of Staphylococcus aureus, including methicillin resistant strains, isolated from bulk can milk and raw milk products in pastoral communities of South-West Uganda. BMC Infect Dis. 2017;17(1):422. doi:10.1186/s12879-017-2524-4
9. Beyene T, Hayishe H, Gizaw F, et al. Prevalence and antimicrobial resistance profile of Staphylococcus in dairy farms, abattoir and humans in Addis Ababa, Ethiopia. BMC Res Notes. 2017;10(1):171. doi:10.1186/s13104-017-2487-y
10. Hanselman BA, Kruth SA, Rousseau J, et al. Methicillin-resistant Staphylococcus aureus colonization in veterinary personnel. Emerg Infect Dis. 2006;12(12):1933–1938. doi:10.3201/eid1212.060231
11. Ayele Y, Gutema FD, Edao BM, et al. Assessment of Staphylococcus aureus along milk value chain and its public health importance in Sebeta, Central Oromia, Ethiopia. BMC Microbiol. 2017;17(1):141. doi:10.1186/s12866-017-1048-9
12. Joshi L, Devkota SP. Methicillin-resistant Staphylococcus aureus (MRSA) in cattle: epidemiology and zoonotic implications. Int J Appl Sci Biotech. 2014;2(1):29–33. doi:10.3126/ijasbt.v2i1.9861
13. Cortimiglia C, Luini M, Bianchini V, et al. Prevalence of Staphylococcus aureus and of methicillin-resistant S. aureus clonal complexes in bulk tank milk from dairy cattle herds in Lombardy Region (Northern Italy). Epidemiol Infect. 2016;144(14):3046–3051. doi:10.1017/S0950268816001576
14. Regasa S, Mengistu S, Abraha A. Milk safety assessment, isolation, and antimicrobial susceptibility profile of staphylococcus aureus in selected dairy farms of Mukaturi and Sululta Town, Oromia Region, Ethiopia. Vet Med Int. 2019;2019:e3063185. doi:10.1155/2019/3063185
15. Lozano C, Gharsa H, Ben SK, Zarazaga M, Torres C. Staphylococcus aureus in animals and food: methicillin resistance, prevalence and population structure. A review in the African continent. Microorganisms. 2016;4(12):1–19.
16. Bersisa A, Tulu D, Negera C. Investigation of bacteriological quality of meat from abattoir and butcher shops in Bishoftu, Central Ethiopia. Int J Microbiol. 2019;1:1–3. doi:10.1155/2019/6416803
17. Bekele M, Mamo G, Mulat S, Ameni G, Beyene G, Tekeba E. Epidemiology of bovine tuberculosis and its public health significance in Debre-Zeit intensive dairy farms, Ethiopia. J Biomed Nurs. 2016;2(2):8–18.
18. Birhanu M, Leta S, Mamo G, Tesfaye S. Prevalence of bovine subclinical mastitis and isolation of its major causes in Bishoftu Town, Ethiopia. BMC Res Notes. 2017;10(1):767. doi:10.1186/s13104-017-3100-0
19. Yilma Z, Guernebleich E, Sebsibe A. A Review of the Ethiopian Dairy Sector. FAO Report. 2011:83.
20. Megersa L. Identification and antimicrobial susceptibility profiles of Staphylococcus species isolated from raw milk, swabs of udders, milking utensils and milkers hands in small holder and dairy farms in Ambo and Guder Town [Internet]. Addis Ababa university; 2015 [
21. Mekonnen YT. Occurrence, Antimicrobial Susceptibility and Public Health Implication of MRSA in Ready to Eat Dairy Foods in Harar Town and Its Surrounding Areas. Addis Ababa: Addis Ababa University; 2017:130.
22. Limbago B. M100-S11, performance standards for antimicrobial susceptibility testing. Clin Microbiol Newsl. 2001;23(6):49.
23. Rahi A, Kazemeini H, Jafariaskari S, Seif A, Hosseini S, Safarpoor Dehkordi F. Genotypic and phenotypic-based assessment of antibiotic resistance and profile of staphylococcal cassette chromosome mec in the methicillin-resistant Staphylococcus aureus recovered from raw milk. Infect Drug Resist. 2020;30(13):273–283. doi:10.2147/IDR.S229499
24. Seedy FRE, Samy AA, Salam HSH, Khairy EA, Koraney AA. Polymerase chain reaction detection of genes responsible for multiple antibiotic resistance Staphylococcus aureus isolated from food of animal origin in Egypt. Vet World. 2017;10(10):1205–1211. doi:10.14202/vetworld.2017.1205-1211
25. Sharma V, Sharma S, Dahiya DK, Khan A, Mathur M, Sharma A. Coagulase gene polymorphism, enterotoxigenecity, biofilm production, and antibiotic resistance in Staphylococcus aureus isolated from bovine raw milk in North West India. Ann Clin Microbiol Antimicrob. 2017;16(1):65. doi:10.1186/s12941-017-0242-9
26. Liu B, Sun H, Pan Y, et al. Prevalence, resistance pattern, and molecular characterization of Staphylococcus aureus isolates from healthy animals and sick populations in Henan Province, China. Gut Pathog. 2018;10(1):31. doi:10.1186/s13099-018-0254-9
27. Abebe R, Hatiya H, Abera M, Megersa B, Asmare K. Bovine mastitis: prevalence, risk factors and isolation of Staphylococcus aureus in dairy herds at Hawassa milk shed, South Ethiopia. BMC Vet Res. 2016;12(1):270. doi:10.1186/s12917-016-0905-3
28. Hika WA, Biruk TM, Ashenafi SB, Mekonnen SB. Isolation and identification of methicillin-resistant Staphylococcus aureus from mastitis dairy cows in Bishoftu town, Ethiopia. Afr J Microbiol Res. 2017;11(44):1606–1613. doi:10.5897/AJMR2017.7088
29. Daka D, G/silassie S, Yihdego D. Antibiotic-resistance Staphylococcus aureus isolated from cow’s milk in the Hawassa area, South Ethiopia. Ann Clin Microbiol Antimicrob. 2012;11(1):26. doi:10.1186/1476-0711-11-26
30. Girmay W, Gugsa G, Taddele H, et al. Isolation and identification of methicillin-resistant Staphylococcus aureus (MRSA) from milk in shire dairy farms, Tigray, Ethiopia. Vet Med Int. 2020;2020:e8833973. doi:10.1155/2020/8833973
31. Wang W, Lin X, Jiang T, et al. Prevalence and characterization of Staphylococcus aureus cultured from raw milk taken from dairy cows with mastitis in Beijing, China. Front Microbiol. 2018:9:1123.
32. Bhati T, Gaurav K, Khichar V, Kataria AK. Prevalence of Staphylococcus aureus isolated from mastitic milk, udder Surfaces and milkers’ hands from different farms in Bikaner, Rajasthan. Indian J Anim Res. 2018;8(5):867. doi:10.30954/2277-940X.10.2018.19
33. Petersson-Wolfe CS, Mullarky IK, Jones GM. Staphylococcus aureus Mastitis: Cause, Detection, and Control. Virginia Cooperative Extension; 2010:7.
34. Gilbert RJ, Louvois J, Donovan T, Little C, Nye K, Ribeiro CD. Guidelines for the microbiological quality of some ready-to-eat foods sampled at the point of sale. Commun Dis Public Health. 2000;3(3):165.
35. Kassa MK. The prevalence, distribution of staphylococcus and its antimicrobial susceptibility status of the isolates from meat and dairy milks of Bishoftu, Ethiopia. Int J Adv Res Biol Sci. 2020;7(3):152–167.
36. Massawe HF, Mdegela RH, Kurwijila LR. Antibiotic resistance of Staphylococcus aureus isolates from milk produced by smallholder dairy farmers in Mbeya Region, Tanzania. Int J Health. 2019;5(5):31–37.
37. Eshetie S, Tarekegn F, Moges F, Amsalu A, Birhan W, Huruy K. Methicillin resistant Staphylococcus aureus in Ethiopia: a meta-analysis. BMC Infect Dis. 2016;16(1):1–8. doi:10.1186/s12879-016-2014-0
38. Sudhanthiramani S, Swetha CS, Bharathy S, Veterinary SV. Prevalence of antibiotic resistant Staphylococcus aureus from raw milk samples collected from the local vendors in the region of Tirupathi, India. Vet World. 2015;8(4):478–481. doi:10.14202/vetworld.2015.478-481
39. Tessema F. Review on the prevalence and drug resistance patterns of staphylococcus aureus in food producing animals, their products and humans. Int J Biol Sci Med. 2017;1:5.
40. Mohammed J, Ziwa MH, Mahuton Y, Hounmanou G, Kisanga A, Tuntufye HN. Molecular typing and antimicrobial susceptibility of methicillin-resistant staphylococcus aureus isolated from bovine milk in Tanzania. Int J Microbiol. 2018;1:4.
41. Msalya G. Contamination levels and identification of bacteria in milk sampled from three regions of Tanzania: evidence from literature and laboratory analyses. Hindawi. 2017;1:1–7.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
