Back to Journals » Infection and Drug Resistance » Volume 16
Prevalence of Quorum Sensing and Virulence Factor Genes Among Pseudomonas aeruginosa Isolated from Patients Suffering from Different Infections and Their Association with Antimicrobial Resistance
Authors Ghanem SM, Abd El-Baky RM , Abourehab MAS , Fadl GFM, Gamil NGFM
Received 18 January 2023
Accepted for publication 13 April 2023
Published 21 April 2023 Volume 2023:16 Pages 2371—2385
DOI https://doi.org/10.2147/IDR.S403441
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Shimaa M Ghanem,1 Rehab Mahmoud Abd El-Baky,1,2 Mohamed AS Abourehab,3 Gamal FM Fadl,1 Nancy GFM Gamil1
1Department of Microbiology and Immunology, Faculty of Pharmacy, Minia University, Minia, Egypt; 2Department of Microbiology and Immunology, Faculty of Pharmacy, Deraya University, Minia, Egypt; 3Department of Pharmaceutics, College of Pharmacy, Umm Al-Qura University, Makkah, 21955, Saudi Arabia
Correspondence: Mohamed AS Abourehab, Email [email protected]
Purpose: Antimicrobial resistance and virulence genes play important roles in increasing the severity of Pseudomonas aeruginosa infections, especially in hospitalized patients with high antibiotic pressure. Most genes that encode Pseudomonas aeruginosa virulence factors are controlled and regulated by the quorum sensing (QS) system. The aim of this study was to investigate the frequency of some virulence genes (rhlR, rhlI, lasR, lasI, lasB, toxA, aprA, algD, ExoS, and plcH genes) and their association with antibiotic resistance.
Methods: Antimicrobial susceptibility was determined by Kirby–Bauer agar disk diffusion method. A total of 125 clinical isolates of P. aeruginosa were tested for some virulence genes using polymerase chain reaction (PCR).
Results: The highest resistance was observed against cefepime (92.8%). Multi-drug resistant (MDR) P. aeruginosa represented 63.2% of total isolates with high distribution among wound isolates (21/79, 26.3% of MDR isolates). LasB was the most prevalent virulence gene among the tested isolates (89.6%) followed by aprA (85.6%), exoS (84%), algD (80%), toxA (76.8%), and plcH (75.2). Furthermore, a significant association (P < 0.05) among most of the tested virulence genes and MDR isolates was found. The presence of more than 5 virulence genes was highly observed among wound infections, otitis media, and respiratory tract infection isolates.
Conclusion: The complex association of virulence genes including QS system regulating genes with antibiotic resistance indicates the importance of the tested factors in the progression of infections, which is considered a great challenge for the health-care team with the need for specific studies for each area having different antibiotic resistance profiles and the development of effective treatment strategies such as anti-virulent and quorum sensing inhibiting drugs against P. aeruginosa infections.
Keywords: Pseudomonas aeruginosa, quorum sensing, virulence genes, antibiotic resistance
Introduction
P. aeruginosa is one of the most life-threatening and resistant bacteria according to the World Health Organization (WHO), for which new antibiotics are urgently needed.1 P. aeruginosa is also one of the most prevalent nosocomial pathogens worldwide.2 It affects people who are susceptible, such as those with postoperative immune suppression or immunosuppressed patients.3 Since this organism exhibits virtually all known mechanisms of antibiotic resistance, nosocomial infections caused by it are frequently challenging to be cured. These resistance mechanisms frequently occur in combination, conferring multi-resistant phenotypes.2 Infections caused by P. aeruginosa include urinary tract infections, bacteremia, cystic fibrosis, lung infections, wound infections, especially of thermal burns, surgical wound infections, and otitis media.4
It is well recognized that P. aeruginosa infections significantly increase the risk of morbidity and mortality because of the organism’s propensity to express a wide range of virulence factors, adapt readily to environmental changes, acquire antibiotic resistance5 and producing agents such as pyocyanin, hemolysin, gelatinase, and biofilms that cause tissue injury and shield P. aeruginosa from immune system detection and antibiotic action.6,7 Additional virulence factors that P. aeruginosa can produce include lipases, lecithinase, DNase, and proteases. Different mechanisms of P. aeruginosa resistance result in the emergence of isolates that are pan-drug-resistant (PDR) or multi-drug resistant (MDR).8 The pathogen exhibits a variety of these mechanisms such as multidrug efflux pump, β-lactamases, and aminoglycoside modifying enzymes9 that increase the chance of developing multidrug resistant strains representing a significant threat to health-care facilities and created increasing immense clinical global problem.10 Bacterial resistance syndrome is a property of P. aeruginosa, as this strain possesses almost all known antimicrobial resistance mechanisms.11 Additionally, infections brought on by isolates of the multi-drug-resistant (MDR) P. aeruginosa are linked to longer hospital stays, higher expenses, and higher rates of morbidity and mortality.12
In recent years, it has been discovered that P. aeruginosa can produce biofilm and many virulence factors under the regulation of quorum sensing (QS) mechanism that is a cell-to-cell communication process. It was found that QS systems are 2 systems named as Las and rhl system depending on N-acyl homoserine lactone (AHL) signal molecules (also named as autoinducers). Las system is comprised of the transcriptional regulatory protein LasR, its cognate autoinducer molecule N-(3-oxododecanoyl) homoserine lactone (3OC12-HSL) and the AHL synthase LasI, while Rhl system consists of RhlR, its cognate autoinducer molecule N-butyryl homoserine lactone (C4-HSL) and the AHL synthase RhlI. Autoinducers should reach a critical threshold concentration to bind its cognate transcriptional regulatory protein. Then, the autoinducer-transcriptional regulatory protein complex activates the expression of many target genes, along with the AHL synthase that results in forming a positive feedback loop. Las and rhl systems are two separate QS systems but related with each other by a hierarchical manner, with the dominance of las system over the rhl system.13
Numerous virulence factors present in P. aeruginosa may be crucial to the pathogenicity of this bacteria. It can produce many extracellular enzymes and toxins that can disrupt host cell membranes and can impair phagocytosis and host immune response. Toxin A (toxA) that can suppress protein synthesis and affect macrophage action, alkaline protease (aprA) can interfere with fibrin formation and inactivate host defense proteins, elastase that is able to split immunoglobulins and complement ingredients and impair neutrophil activity, and exoenzymes that are the primary virulence factors (S, U, T, exoS, exoU, and exoT) affecting protein synthesis. The breakdown of phospholipids in pulmonary surfactants may also be facilitated by two phospholipase C enzymes that are encoded by plcH (hemolytic phospholipase C) and plcN (non-hemolytic phospholipase C) which act together sequentially. plcH promote disruption of erythrocyte membranes exposing the inner leaflets followed by hydrolyzing phosphatidylserine in the inner leaflets by plcN.14,15
The present study was designed to study antimicrobial resistance patterns, identify Quorum sensing and virulence genes in the isolated strains of P. aeruginosa, and to study the association of QS genes and virulence genes with different types of infections and antibiotic resistance.
Materials and Methods
Bacterial Strains
In the current study, 125 P. aeruginosa strains were isolated from 350 different clinical specimens from patients admitted to the Minia University Hospital suffering from different infections (urinary tract infections 50 samples, respiratory tract infection, 87 sputum samples, otitis media, 83 ear discharge samples, wound infections; 85 samples and gastroenteritis; 45 samples). All clinical samples were obtained as part of the routine hospital laboratory procedures and labeled with the source and patient information. Ethical clearance for the study was granted by the ethics committee, faculty of Pharmacy, Minia University (No. HV16/2020). P. aeruginosa strains were confirmed using traditional microbiological methods and biochemical tests (culture on Cetrimide agar and Oxidase test). Biofilm formation was tested using the tissue culture plate method (TCP).16
The gold-standard technique for biofilm identification is the tissue culture plate (TCP) assay, which was first published by Christensen et al in 1995. In 10 mL of TSB with 1% glucose, isolates from freshly prepared agar plates were injected. Broths were incubated for 24 hours at 37°C. Then, fresh medium was added, and the cultures were diluted 1:100.
200 L of the diluted cultures were placed in each of the 96 wells of sterile flat-bottom tissue culture plates. For the purpose of testing media sterility and non-specific binding, only sterile broths were offered as a blank. Likewise, control organisms were diluted and incubated as well. The tissue culture plates contained the three controls as well as blanks.
The culture plates underwent a 24-hour incubation period at 37°C. The wells were cleaned four times with 0.2 mL of phosphate buffer saline (pH 7.2) before being let to dry naturally. The wells were next stained for 30 minutes at room temperature with 200 L of 0.1% crystal violet. To get rid of the excess color, the plates were rinsed with distilled water and then left to dry. The use of 200 L of 95% ethanol helped to dissolve the adherent stain. A micro ELISA auto reader operating at a wavelength of 630 nm was used to measure the optical densities (OD) of stained adherent biofilm. Three times of the experiment were carried out in duplicate. All test results were computed, and the average OD values of the sterile medium was subtracted. Non-biofilm producers were judged to have ODs below 0.120, moderate biofilm producers to have ODs between 0.120 and 0.240, and more than 0.240 as strong biofilm producers.
Antimicrobial Susceptibility Testing
Antimicrobial susceptibility of P. aeruginosa strains was performed determined by the Kirby–Bauer disc diffusion method using Muller-Hinton agar (HiMedia, India) according to Clinical & Laboratory Standards Institute guidelines (CLSI).17 The antibiotics evaluated were ciprofloxacin (CIP, 5 μg), amikacin (AK, 30 μg), cefepime (CPM, 30 μg), norfloxacin (NX, 10 μg), imipenem (IPM, 10 μg), gentamicin (GEN, 10 μg), tobramycin (TOB, 10 μg), aztreonam (ATM, 30 μg), piperacillin-tazobactam (PIT 100/10 μg), and colistin (CL, 10 μg). Zones of inhibition were recorded in mm. The susceptibility pattern was determined using the CLSI interpretation chart as susceptible (S), intermediate (I), or resistant (R).
Molecular Methods
A loop of bacterial cells was inoculated into Luria Bertani broth vials (Difco Laboratories, Detroit, Michigan, USA) containing 100 mg/mL ampicillin (Sigma, USA) and incubated at 37°C with shaking at 185 rpm for 16–18 h. DNA extraction was performed using an Extraction Kit (Qiagen kit, Germany) according to the manufacturer’s instructions. Forward and reverse primers for 10 virulence genes were used. PCR amplifications were conducted to detect the genes listed in Table 1. The amplifications were conducted in a volume of 25 μL containing 12.5 μL of PCR Master mix (DreamTaq Green PCR master mix, Thermo scientific, USA), 1 μL of each primer (10 pM/µL) (forward and reverse), 1 μL of template DNA, and nuclease-free water. The PCR conditions for amplification for all of the mentioned genomic regions were: initial denaturation for 5 min at 94° C, 35 cycles of denaturation for 60s at 94° C, annealing for 60s at 48°C, and extension for 90s at 72°C with a final extension at 72° C for 10 min.18,19
 |
Table 1 The Primers Used in This Study and Their Sequences |
Following amplification, aliquots were removed from each reaction mixture and 100-bp phage ladders (BIOMATIK, USA) were examined by electrophoresis (70 V, 45 min) in gels composed of 1.5% (w/v) agarose (Promega, USA) in 1X TBE buffer (40 mM Tris, 20 mM boric acid, 1 mM EDTA, pH 8.3), and stained with ethidium bromide (5 g/100 mL). The gel bands were visualized under UV illumination using a gel image analysis system.
Statistical Analysis
To compare the frequencies obtained for virulence genes and antibiotic resistance, chi-square (X2) and Fisher’s exact test were performed using SPSS version 17 statistical software (SPSS Inc., Chicago, IL). Correlations were determined using Spearman correlation (r) and Pearson’s correlation coefficient (r2) in bivariate linear correlations (P < 0.05). The P-value was significant if it was ≤0.05.
Results
P. aeruginosa Isolation and Identification
A total of 125 P. aeruginosa isolates were isolated from a total of 350 samples including: urine (15/50), sputum (27/87), ear discharges (32/83), wound exudates (35/85), and stool (16/45). TCP method was used to detect the ability of P. aeruginosa isolates to develop biofilm. Out of 125 P. aeruginosa isolates, 78 (62.4%) isolates were detected as strong biofilm producers, 32 (25.6%) as moderate biofilm producers and 15 (12%) as weak or non-biofilm producers.
Antimicrobial Susceptibility
In this study, antimicrobial susceptibility profiles for 10 antibiotics, representing seven different classes, demonstrated high rates of antibiotic resistance. The resistance to ciprofloxacin, amikacin, cefepime, norfloxacin, imipenem, gentamicin, tobramycin, aztreonam, piperacillin-tazobactam, and colistin ranged from 12.8 to 92.8% (Figure 1). High incidence of antibiotic resistance of P. aeruginosa isolates against ciprofloxacin, cefepime, and colistin antibiotics was shown by strains isolated from urine, wound infection and sputum.
Table 2 shows the distribution of MDR isolates among different types of infections. The table showed that 63.2% of total P. aeruginosa isolates were MDR (as they were resistant to 3 or more of the tested antibiotics). In addition, it was found that multi-drug resistant isolates were highly distributed among sputum samples as 70.4% of sputum isolates were MDR, followed by urine isolates (66.7% of urine isolates) while 9 stool isolates only showed MDR pattern (56.3%). In addition, our results showed that the highest incidence of MDR isolates were obtained from wound infections, otitis media and respiratory tract infections (Table 3).
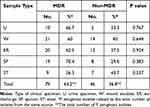 |
Table 2 Distribution of MDR Resistant P. aeruginosa Among Isolates Among Different Types of Infection |
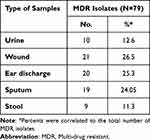 |
Table 3 Prevalence of MDR Isolates Among the Different Types of Infections |
Distribution of Virulence Genes Among Isolates of Different Type of Samples and MDR Isolates
The results indicated that 93.6% (117/125) of the isolates were positive for one or more of the tested virulence genes, while only 6.4% (8/125) were negative for all of these genes. The results were: 94.4% (118/125) of the isolates were positive for rhlR, 69.6% (87/125) were positive for rhlI, while 80% (100/125) were positive for LasR, and the remaining 81.6% (102/125) were positive for lasI. lasB was found to be the most common virulence gene detected in P. aeruginosa isolates (89.6%). Moreover, aprA (85.6%), exoS (84%), algD (80%), toxA (76.8%), and plcH (75.2%) genes that play important role in the pathogenesis of P. aeruginosa were identified in the evaluated isolates.
Regarding the distribution of virulence genes among isolates according to sample type, it was found that there is no significant correlation between virulence genes and the type of clinical sample, except for the lasI gene, which did exhibit a significant association, as shown in Table 4.
 |
Table 4 Distribution of Quorum Sensing and Virulence Factors Regulating Genes in P. aeruginosa Isolated from Different Clinical Specimens |
A significant correlation between the distribution of the tested virulence genes and MDR resistance in P. aeruginosa isolates, especially QS genes (rhlI, rhlR and LasR), LasB, aprA and exoS as shown in Table 5.
 |
Table 5 Distribution of Virulence Factor Genes Among MDR and Non-MDR P. aeruginosa Isolates |
Distribution of Biofilm Producers and Non-Producers Among MDR Isolates and Their Resistance Pattern Against Different Antimicrobial Agents
Most of MDR isolates were biofilm producers as biofilm acts as a barrier for the diffusion of antibiotics (Figure 2). Resistance pattern of biofilm and non-biofilm producing organisms showed that all producing P. aeruginosa were cefepime resistant, followed by ciprofloxacin (73.6%). Imipenem showed the highest activity against biofilm producing isolates. On the other hand, the highest activity against biofilm non-producing isolates was shown by tobramycin and imipenem (Table 6).
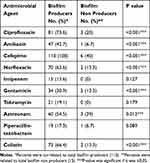 |
Table 6 Resistance Pattern of P. aeruginosa Among Biofilm Producers and Non-Producer |
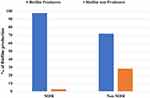 |
Figure 2 Correlation between biofilm formation and type of resistant P. aeruginosa isolates (P > 0.001). Abbreviation: MDR, Multi-drug resistant. |
Distribution of QS and Virulence Genes Among P. aeruginosa Isolated from Different Types of Infection and Resistance Profile of Highly Virulent Isolates
Distribution of the tested virulence genes was observed among strains isolated from wound, ear discharge and sputum samples. Also, high distribution of lasB gene encoding elastase enzymes and aprA genes encoding alkaline phosphatase were found among sputum isolates showing their important effect on P. aeruginosa respiratory tract infections. It was noticed that strains isolated from sputum samples were positive for at least 3 virulence genes and maximum for 10 virulence genes, which indicates that strains able to cause respiratory infections are more virulent and highly resistant to antimicrobials. Our results showed also that 20% of total isolates were positive for 6 virulence genes, followed by the presence of 8 virulence genes in 16.8% of isolates especially those were isolated from ear discharge and sputum samples (Table 7).
 |
Table 7 Distribution of Virulence Genes Among P. aeruginosa Clinical Isolates Collected from Different Sources |
Resistance Profile of Highly Virulent P. aeruginosa Isolates Contain More Than 6 Genes Including QS Sensing Genes
Table 8 shows that all isolates (biofilm producers) harbor both lasI/or R and rhlI/or R except 2 isolates were positive for rhl system only (Group 2). These 2 isolates were positive for all virulence genes except exoS gene and resistant for 3 antibiotics. Furthermore, there were 3 isolates positive for Las system only (Group 3), positive for all virulence genes and resistant for 5 antibiotics, which indicates that Las system plays an important role in regulating virulence factors. It was found that all isolates positive for all QS genes were positive for lasB and algD genes that indicates the important impact of QS genes in regulating both genes. In addition, these isolates were resistant to 6 to 8 antibiotics. Previous results in this study indicated the impact of QS genes on regulating different virulence factors, biofilm production, which lead to tissue destruction and impairment for the immune response and the emergence of non-responsive cells and the development of antibiotic resistance. Table 9 shows significant association (P < 0.001) between groups containing different numbers of virulence genes and number of antibiotics showing low activity against the tested strains due to their resistance. In addition, strong positive correlation (r = 0.815, P < 0.001) between number of virulence genes and resistance to antibiotics (Table 10).
 |
Table 8 Resistance Pattern of Highly Virulent Biofilm Producing P. aeruginosa Harboring ≥6 Virulence Genes |
 |
Table 9 Correlation Between Different Groups of Virulent Genes of Highly Virulent Biofilm Producing P. aeruginosa and Number of Resistant Antibiotics |
 |
Table 10 Correlation Between Number of Genes and Number of Antibiotics |
Discussion
It is well known that P. aeruginosa infections are associated with high morbidity and mortality due to its ability of adapting their environmental changes, to express many virulence factors and to acquire antibiotic resistance. In this study, 63.2% of total isolates were MDR. In addition, 92.8% of the isolates exhibited resistance to cefepime. A similar study from Iran reported approximately 56% resistance to cefotaxime in P. aeruginosa isolates from 600 isolates of P. aeruginosa that were cultured from patients at two hospitals in Tehran.20,21 Based on the results, 16% of the isolates exhibited resistance to piperacillin-tazobactam, which is higher than other results obtained from study by Khan in Makkah and Jeddah (4.9%).22 The highest activity against the tested isolates was shown by imipenem (12.8%). Similar results showing the highest activity of imipenem and piperacillin-tazobactam against MDR P. aeruginosa were reported by other studies (6% and 28%, respectively).23,24 In the present study, 73% of P. aeruginosa isolates exhibited sensitivity to aminoglycoside antibiotics, gentamicin, tobramycin, and amikacin. The results from this study regarding gentamicin are in agreement with those from Shahcheraghi et al23(69%), and Zahra et al25 (77%), but differed from those of Nakhael et al24 (45.31%). Moreover, Tambekar et al26 and Ehinmidu27 reported a high susceptibility to gentamicin. Low susceptibility to gentamicin was reported in two studies (32.2%, 33%, respectively) in 2014 by Fazeli et al15 and Nwanze et al.28 Also, our results showed that ciprofloxacin inhibited the growth of 32.8% of the isolates. Data from the present study showed a difference with those obtained previously by Saeidi et al,29 who reported that ciprofloxacin showed inhibitory activity against 85% of isolates. Akingbade et al30 reported that P. aeruginosa strains have moderate resistance to ciprofloxacin (43%), which disagrees with the results of this study. Ilham et al31 and Nwanze et al28 also reported moderate and high resistance to ciprofloxacin.15,28,31 The infection process is aided by a number of P. aeruginosa regulatory and virulence factors. Understanding the pathogenesis of this opportunistic disease will be enhanced by the knowledge gained from studying these virulence factors. Moreover, it will help to define new targets to improve therapeutic efficacy and regimens. In the present study, 10 virulence factors, including lasB, aprA, toxA, algD, plcH, and exoS, were detected using PCR. Most of the virulence factor genes were detected in most of isolates. In addition, quorum sensing system was mostly observed among most of isolates. The Las system regulates the production of some virulence factors elastase, exotoxin A, and alkaline protease. In addition, Las system positively regulates rhl system. The rhl system induces the regulation of some virulence factors such as alkaline protease, biofilm formation by enhancing the biosynthesis of pel polysaccharide, elastase, rhamnolipid, pyocyanin, and HCN.
It was found that biofilm non-producing isolates showed no resistance against either imipenem and tobramycin while biofilm producing isolates showed resistance to imipenem and tobramycin. These results agreed with that reported by Bagge et al,32 who reported that imipenem cause changes in biofilm structure in biofilm-producing isolates due to its ability to increase alginate production. Also, our results showed that non-biofilm producing isolates were completely susceptible to tobramycin. Susceptibility of pseudomonas aeruginosa to tobramycin was reported by Koeppen et al33 who reported that tobramycin reduced several outer membrane vesicles (OMVs) associated virulence determinants.
Our results showed that all MDR isolates were positive for rhlR. Regarding previous results obtained by previous studies, one can conclude that the resistance of P. aeruginosa strains to various antibiotics is relatively high. Resistance profile differs according to the time, the type of infection and if samples obtained from inpatient or outpatients. However, the results of these studies differ according to the time and location of isolation. In addition, the frequency of four QS genes (lasI, lasR, rhlI, and rhlR) in P. aeruginosa strains was analyzed in the present study. Different P. aeruginosa virulence factors are controlled by a gene system that is referred to as the QS system. In our study, results showed that the highest gene frequency was associated with rhlR in 118 strains (94.4%). The frequency of rhlI, lasR, and lasI genes was 69.6%, 80%, and 81.6%, respectively, in all tested isolates. By screening of 54 P. aeruginosa strains with simultaneous resistance to three antibiotics, 49 strains were found to be positive for the rhlR QS gene. A significant correlation between strains resistant to three antibiotics and rhlR gene distribution was observed. In comparison to other studies, Kadhim et al34 showed that the frequencies of lasR, lasI, rhlR, and laslI QS genes were 5%, 78.3%, 65%, and 43.3%, respectively. Aghamollaei et al35 reported the frequency of the LasI QS gene to be 48.5%. In a study performed by Senturk et al,36 four isolates were found to be positive for lasR, lasI, rhlR, and rhlI genes. One of the isolates lacked the lasR gene, and one isolate was negative for lasR, lasI, and rhlR.
Production of extracellular enzymes that break down QS coding genes may be the cause of QS gene deficiency.37 QS coding gene mutations may also contribute to variations in QS gene expression. The function of the LasR and RhlR genes is compromised, according to Bjarnsholt et al37 due to increased mutations in QS genes. The expression of QS genes may be compromised by the presence of many P. aeruginosa strains at the infection site.36
Additional studies are required to determine the reason for these variations in QS gene expression and how they relate to the drug resistance of different strains of P. aeruginosa. According to the results of the present study, the highest frequencies were shown by rhlR (94.4%) and lasI (81.6%) indicating that the QS system plays an important role in the pathogenicity of P. aeruginosa. Moreover, these genes were primarily observed in clinical isolates that are MDR. Therefore, further investigations for determining a relationship between these genes and drug resistance are required. Many studies reported the important role of QS system in regulating virulence genes. LasI produces 3-oxo-C12-HSL that is able to activate LasR (cytoplasmic receptor), which has the ability to regulate the expression of genes controlling exotoxin-A production, proteases, biofilm formation, hemolysins, elastases.38,39 On the other hand, RhlI produces the C4-HSL, which associates with its cognate receptor RhlR resulting in the expression of many virulence genes controlling the production of pyocyanin, hydrogen cyanide, siderophores, elastases, alkaline protease and regulating bacterial motility39,40 The prevalence of resistance to several antibiotics is the major problem linked to infections formed by biofilm producer bacteria.41 Extracellular matrix production is a sign of a formed biofilm and works as a barrier to antibiotics, decreasing sensitivity to them.42 Biofilm is known to prevent the distribution of antibiotics, makes it more challenging to treat patients with pseudomonal infections, which are confirmed by many researchers.41,43
Overall, lasB (89.6%) was the most detected virulence genes among P. aeruginosa isolates especially among sputum isolates due to its importance in lung infections as it leads to the degradation of connective tissues in lungs by inducing tissue injury and hemorrhage through destroying elastin, collagen types III and IV, laminin, fibronectin, and vitronectin of host cells. In chronic infections, elastase B destroy many components of the immune defense mechanism such as tumor necrosis factor-α, interferon-γ and interleukin-2 resulting in the damage of host cells.44 Regarding the previous destructive effects of elastase B, it was found that las B was the most common virulence genes among MDR which indicates its role in the emergence of non-responsive bacterial cells to antibiotics. aprA genes were found to be highly distributed in sputum isolates (88.9%). Moreover, aprA can destroy host proteins such as complement proteins and cytokines resulting in alveolar hemorrhage, necrosis of alveolar septal cells, and infiltration of mononuclear cells enhancing its survival in tissues.45 A study performed by Mittal et al,46 a high level of production of elastase, protease, phospholipase C and toxin A was reported among isolates obtained from urinary tract infections in comparison to burn, wound infections and acute pneumonia. Regarding previous results, levels of these virulence factors showed different patterns based on the infection site.
In the present study, a high level of algD was observed among the tested isolates (80%), algD gene, regulating alginate capsule production by P. aeruginosa, plays a significant role in chronic lung infections.47,48 Its expression may be associated with biofilm formation that also affects the therapeutic activity of antibiotics by acting as a barrier for their direct effect on the organism. It was documented that algD, algU, and rpoS genes are up-regulated in biofilm producing P. aeruginosa.49 algD, exoS genes were shown to be common among ear discharge samples, while the toxA gene was highly distributed among wound swabs’ samples, which in agreement with that reported by Morin et al.50
Regarding phospholipase C enzymes (PLCs), PlcH hemolytic and PlcN nonhemolytic, it was reported that injecting mice with large dosages may result in vascular permeability, organ damage, and death by the action of PlcH.51 In the present study, plcH gene was highly distributed among sputum isolates (81.5%) in comparison to other types of samples due to its important role in degrading pulmonary surfactants, destroying cytoplasmic membranes and inhibiting opsonin. Wargo et al52 reported that inhibition of hemolytic phospholipase C protects lung function during infections. Unlike previous studies from Iran, the results confirmed the low presence of these genes in 38.8% of P. aeruginosa isolates.15
In this study, more than 82.3% of MDR isolates expressed all tested genes that indicates their role in the development of resistance against antibiotics due to their pathophysiological destructive effect for host cell and different components of immune system leading to the selection of non-responsive bacterial cells and increasing the bacterial cell ability to adapt their environment. Galdino et al53 reported that targeting elastase B (metalloenzyme) with metal-based antibiotics can reduce the emergence of antibiotic resistance. On the other hand, co-existence of 3 to 10 virulence genes in association with high presentation of MDR activity against the tested antibiotics was shown by respiratory tract, otitis media and wound infections isolates, which is in agreement with results obtained from Moradali et al.54 Anju et al55 and Qin et al.56
A wide spread of antibiotic resistance especially among strains isolated from patients suffering from respiratory tract infections. Increasing resistance against ciprofloxacin, cefepime and colistin are of great concern. P. aeruginosa is a pathogen that can accommodate their environment, express several virulence factors and show multidrug resistance pattern making the treatment of infections caused by this bacterium difficult and a great challenge. P. aeruginosa isolates exhibit a great degree of variation in their pathogenicity. Association of virulence genes with the type of infection indicates the important role of these factors in the stabilization of infection and to resist host immune response, which was well expressed among strains isolated from respiratory tract infections. Our results showed that QS genes have an important role in increasing the virulence of pathogens in association with the increase of antibiotic resistance resulting in the spread of life-threatening infections that is considered as a great challenge facing the health-care team. Quorum sensing inhibitors development is an essential need for controlling P. aeruginosa infections. Many researchers reported the activity of some compounds as anti- quorum sensing agents. It was found that some phytochemicals sharing a heterocyclic ring structure similar to AHL molecules have the ability to interact with QS receptors and have the ability to degrade signal receptors such as ajoene, allicin, curcumin, cinnamaldehyde, eugenol. In addition, quercetin can inhibit biofilm production and twitching motility.57 Olaniyi et al.58 Benzene ethanamine, 4-methoxy- and Cyclopentadecanone, 2-hydroxy- from Psidium guajava leaves showed inhibitory properties against QS proteins of Salmonella Typhi.
Conclusion
P. aeruginosa causing respiratory tract, otitis media and wound infections are much more virulent and highly resistant strains in comparison to those isolated from other sources. A significant correlation between the abundance of the tested virulence genes and MDR resistance in P. aeruginosa isolates. Studying antimicrobial susceptibility patterns and distribution of virulence genes may be helpful for developing effective treatment strategies such as anti-virulent and Quorum sensing inhibiting drugs against P. aeruginosa infections.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, prior approval (No. HV16/2020) by the ethical committee of Faculty of Pharmacy, Minia University.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Acknowledgments
We are grateful to the medical staff of Minia University Hospital for collecting the specimens used in this study. The authors thank the Deanship of scientific research at Umm Al-Qura University for supporting this work by grant code (23UQU4290565DSR120).
Funding
The authors thank the Deanship of scientific research at Umm Al-Qura University for supporting this work by grant code (23UQU4290565DSR120).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Awan AB, Schiebel J, Böhm A, et al. Association of biofilm formation and cytotoxic potential with multidrug resistance in clinical isolates of Pseudomonas aeruginosa. Excli J. 2019;18:79.
2. Strateva T, Yordanov D. Pseudomonas aeruginosa – a phenomenon of bacterial resistance. J Med Microbiol. 2009;58(9):1133–1148. doi:10.1099/jmm.0.009142-0
3. Driscoll JA, Brody SL, Kollef MH. The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs. 2007;67(3):351–368. doi:10.2165/00003495-200767030-00003
4. Schaechter M. Encyclopedia of Microbiology. Academic Press; 2009.
5. Van Delden C, Iglewski BH. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg Infect Dis. 1998;4(4):551. doi:10.3201/eid0404.980405
6. Cevahir N, Demir M, Kaleli I, Gurbuz M, Tikvesli S. Evaluation of biofilm production, gelatinase activity, and mannose-resistant hemagglutination in Acinetobacter baumannii strains. J Microbiol Immunol Infect. 2008;41(6):513–518.
7. Ciofu O, Lee B, Johannesson M, Hermansen NO, Meyer P, Høiby N. Investigation of the algT operon sequence in mucoid and non-mucoid Pseudomonas aeruginosa isolates from 115 Scandinavian patients with cystic fibrosis and in 88 in vitro non-mucoid revertants. Microbiology. 2008;154(1):103–113. doi:10.1099/mic.0.2007/010421-0
8. Raafat MM, Ali-Tammam M, Ali AE. Phenotypic and genotypic characterization of Pseudomonas aeruginosa isolates from Egyptian hospitals. Afr J Microbiol Res. 2016;10(39):1645–1653. doi:10.5897/AJMR2016.8254
9. Mahmoud AB, Zahran WA, Hindawi GR, Labib AZ, Galal R. Prevalence of multidrug-resistant Pseudomonas aeruginosa in patients with nosocomial infections at a university hospital in Egypt, with special reference to typing methods. J Virol Microbiol. 2013;13:165.
10. Lim K-T, Yasin RM, Yeo -C-C, et al. Genetic fingerprinting and antimicrobial susceptibility profiles of Pseudomonas aeruginosa hospital isolates in Malaysia. J Microbiol Immunol Infect. 2009;42(3):197–209.
11. Talebi-Taher M, Gholami A, Rasouli-Kouhi S, Adabi M, Adabi M. Role of efflux pump inhibitor in decreasing antibiotic cross-resistance of Pseudomonas aeruginosa in a burn hospital in Iran. J Infect Dev Count. 2016;10(06):600–604. doi:10.3855/jidc.7619
12. Gales AC, Castanheira M, Jones RN, Sader HS. Antimicrobial resistance among gram-negative bacilli isolated from Latin America: results from SENTRY Antimicrobial surveillance program (Latin America, 2008–2010). Diagn Microbiol Infect Dis. 2012;73(4):354–360. doi:10.1016/j.diagmicrobio.2012.04.007
13. İnat G, Sırıken B, Başkan C, Erol İ, Yıldırım T, Çiftci A. Quorum sensing systems and related virulence factors in Pseudomonas aeruginosa isolated from chicken meat and ground beef. Sci Rep. 2021;11(1):15639. doi:10.1038/s41598-021-94906-x
14. Krall R, Schmidt G, Aktories K, Barbieri JT. Pseudomonas aeruginosa ExoT is a Rho GTPase-activating protein. Infect Immun. 2000;68(10):6066–6068. doi:10.1128/IAI.68.10.6066-6068.2000
15. Fazeli N, Momtaz H. Virulence gene profiles of multidrug-resistant Pseudomonas aeruginosa isolated from Iranian hospital infections. Iran Red Crescent Med J. 2014;16(10). doi:10.5812/ircmj.15722
16. Christensen GD, Simpson WA, Younger J, et al. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985;22(6):996–1006. doi:10.1128/jcm.22.6.996-1006.1985
17. Wayne P. Clinical and Laboratory Standards Institute: performance standards for antimicrobial susceptibility testing: 20th informational supplement. CLSI Doc M100-S20. 2010;85(4):355–359.
18. Agnello M, Wong-Beringer A. Differentiation in Quinolone Resistance by Virulence Genotype in Pseudomonas Aeruginosa. Public Library of Science San Francisco; 2012.
19. Badamchi A, Masoumi H, Javadinia S, Asgarian R, Tabatabaee A. Molecular detection of six virulence genes in Pseudomonas aeruginosa isolates detected in children with urinary tract infection. Microb Pathog. 2017;107:44–47. doi:10.1016/j.micpath.2017.03.009
20. Asghari-Moghadam N, Rasoolzadeh R, Hoseini-Moghadam S-M-M, Seifi M, Pour-Shafie MR, Talebi M. Isolation, antibiotic resistance determination and genotypic analysis of pseudomonas aeruginosa strains causing urinary tract infection in catheterized patients of shohada and Labafi Nejad Hospitals, Tehran, Iran. J Isfahan Med Sch. 2014;32(272):1–8.
21. Cowell BA, Twining SS, Hobden JA, Kwong MS, Fleiszig SM. Mutation of lasA and lasB reduces Pseudomonas aeruginosa invasion of epithelial cells. Microbiology. 2003;149(8):2291–2299. doi:10.1099/mic.0.26280-0
22. Khan MA, Faiz A. Antimicrobial resistance patterns of Pseudomonas aeruginosa in tertiary care hospitals of Makkah and Jeddah. Ann Saudi Med. 2016;36(1):23–28. doi:10.5144/0256-4947.2016.23
23. Shahcheraghi F, Nikbin V-S, Feizabadi MM. Prevalence of ESBLs genes among multidrug-resistant isolates of Pseudomonas aeruginosa isolated from patients in Tehran. Microb Drug Resist. 2009;15(1):37–39. doi:10.1089/mdr.2009.0880
24. Nakhaei MM, Hosseini HM, Mobaiyen H. Evaluation of antibiotic resistance and detection of CTX-M type extended spectrum beta-lactamase in clinical isolates of pseudomonas aeruginosa in mashhad. J Ilam Univ Med Sci. 2014;22(5):70–77.
25. Zahra T, Rezvan M, Ahmad K. Detection and characterization of multidrug resistance and extended-spectrum-beta-lactamase-producing (ESBLS) Pseudomonas aeruginosa isolates in teaching hospital. Afr J Microbiol Res. 2011;5(20):3223–3228. doi:10.5897/AJMR11.260
26. Tambekar D, Dhanorkar D, Gulhane S, Khandelwal V, Dudhane M. Antibacterial susceptibility of some urinary tract pathogens to commonly used antibiotics. Afr J Biotechnol. 2006;5:17.
27. Ehinmidu JO. Antibiotics susceptibility patterns of urine bacterial isolates in Zaria, Nigeria. Trop J Pharm Res. 2003;2(2):223–228.
28. Nwanze P, Nwaru L, Oranusi S, et al. Urinary tract infection in Okada village: prevalence and antimicrobial susceptibility pattern. Sci Res Essays. 2007;2(4):112–116.
29. Saeidi S, Alavi-Naini R, Shayan S. Antimicrobial susceptibility and distribution of tem and ctx-m genes among esbl-producing Klebsiella pneumoniae and Pseudomonas aeruginosa causing urinary tract infections. Zahedan J Res Med Sci. 2014;16(4):1–5.
30. Akingbade O, Balogun S, Ojo D, et al. Plasmid profile analysis of multidrug resistant Pseudomonas aeruginosa isolated from wound infections in South West, Nigeria. World Appl Sci J. 2012;20(6):766–775.
31. Ilham HH, Banyan A. Isolation of Pseudomonas aeruginosa from clinical cases and environmental samples, and analysis of its antibiotic resistant spectrum at Hilla Teaching Hospital; 2011.
32. Bagge N, Schuster M, Hentzer M, et al. Pseudomonas aeruginosa biofilms exposed to imipenem exhibit changes in global gene expression and beta-lactamase and alginate production. Antimicrob Agents Chemother. 2004;48(4):1175–1187. doi:10.1128/AAC.48.4.1175-1187.2004
33. Koeppen K, Barnaby R, Jackson AA, Gerber SA, Hogan DA, Stanton BA. Tobramycin reduces key virulence determinants in the proteome of Pseudomonas aeruginosa outer membrane vesicles. PLoS One. 2019;14(1):e0211290. doi:10.1371/journal.pone.0211290
34. Kadhim D, Ali Mi R. Prevalence study of quorum sensing groups among clinical isolates of Pseudomonas aeruginosa. Int J Curr Microbiol App Sci. 2014;3(11):204–215.
35. Aghamollaei H, Azizi BK, Moosazadeh MM. Rapid detection of Pseudomonas aeruginosa by PCR method using specific primers of quorum sensing LasI gene. Armaghane Danesh. 2014;18(9):723–735.
36. Senturk S, Ulusoy S, Bosgelmez-Tinaz G, Yagci A. Quorum sensing and virulence of Pseudomonas aeruginosa during urinary tract infections. J Infect Dev Count. 2012;6(06):501–507. doi:10.3855/jidc.2543
37. Bjarnsholt T, Jensen PØ, Fiandaca MJ, et al. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr Pulmonol. 2009;44(6):547–558. doi:10.1002/ppul.21011
38. Rutherford S, Bassler B. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med. 2012;2:a012427–a012427. doi:10.1101/cshperspect.a012427
39. Chadha J, Harjai K, Chhibber S. Revisiting the virulence hallmarks of Pseudomonas aeruginosa: a chronicle through the perspective of quorum sensing. Environ Microbiol. 2022;24(6):2630–2656. doi:10.1111/1462-2920.15784
40. Papenfort K, Bassler BL. Quorum sensing signal–response systems in Gram-negative bacteria. Nat Rev Microbiol. 2016;14(9):576–588. doi:10.1038/nrmicro.2016.89
41. Karthic A, Gopinath P. Detection of biofilm among clinical isolates of Pseudomonas aeruginosa by tissue culture plate (TCP) method. J Chem Pharm Sci. 2016;9(4):3236–3238.
42. Heydari S, Eftekhar F. Biofilm formation and β-lactamase production in burn isolates of Pseudomonas aeruginosa. Jundishapur J Microbiol. 2015;8(3). doi:10.5812/jjm.15514
43. Rewatkar A, Wadher B. Staphylococcus aureus and Pseudomonas aeruginosa-Biofilm formation Methods. J Pharm Biol Sci. 2013;8(5):36–40.
44. Azam MW, Khan AU. Updates on the pathogenicity status of Pseudomonas aeruginosa. Drug Discov Today. 2019;24(1):350–359. doi:10.1016/j.drudis.2018.07.003
45. Wretlind B, Olǵerts RP. Pseudomonas aeruginosa elastase and its role in pseudomonas infections. Rev Infect Dis. 1983;5:S998–S1004. doi:10.1093/clinids/5.Supplement_5.S998
46. Mittal R, Aggarwal S, Sharma S, Chhibber S, Harjai K. Urinary tract infections caused by Pseudomonas aeruginosa: a minireview. J Infect Public Health. 2009;2(3):101–111. doi:10.1016/j.jiph.2009.08.003
47. Berry A, DeVault JD, Chakrabarty A. High osmolarity is a signal for enhanced algD transcription in mucoid and nonmucoid Pseudomonas aeruginosa strains. J Bacteriol. 1989;171(5):2312–2317. doi:10.1128/jb.171.5.2312-2317.1989
48. Kamath S, Kapatral V, Chakrabarty A. Cellular function of elastase in Pseudomonas aeruginosa: role in the cleavage of nucleoside diphosphate kinase and in alginate synthesis. Mol Microbiol. 1998;30(5):933–941. doi:10.1046/j.1365-2958.1998.01121.x
49. Donlan RM. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002;8(9):881. doi:10.3201/eid0809.020063
50. Morin CD, Déziel E, Gauthier J, Levesque RC, Lau GW. An organ system-based synopsis of pseudomonas aeruginosa virulence. Virulence. 2021;12(1):1469–1507. doi:10.1080/21505594.2021.1926408
51. Terada LS, Johansen KA, Nowbar S, Vasil AI, Vasil ML. Pseudomonas aeruginosa hemolytic phospholipase C suppresses neutrophil respiratory burst activity. Infect Immun. 1999;67(5):2371–2376. doi:10.1128/IAI.67.5.2371-2376.1999
52. Wargo MJ, Gross MJ, Rajamani S, et al. Hemolytic phospholipase C inhibition protects lung function during Pseudomonas aeruginosa infection. Am J Respir Crit Care Med. 2011;184(3):345–354. doi:10.1164/rccm.201103-0374OC
53. Galdino ACM, Viganor L, de Castro AA, et al. Disarming Pseudomonas aeruginosa Virulence by the Inhibitory Action of 1, 10-Phenanthroline-5,6-Dione-Based Compounds: elastase B (LasB) as a chemotherapeutic target. Front Microbiol. 2019;10:1701. doi:10.3389/fmicb.2019.01701
54. Moradali MF, Ghods S, Rehm BH. Pseudomonas aeruginosa Lifestyle: a Paradigm for Adaptation, Survival, and Persistence. Front Cell Infect Microbiol. 2017;7:39. doi:10.3389/fcimb.2017.00039
55. Anju VT, Busi S, Imchen M, et al. Polymicrobial infections and biofilms: clinical significance and eradication strategies. Antibiotics. 2022;11(12):1731. doi:10.3390/antibiotics11121731
56. Qin S, Xiao W, Zhou C, et al. Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct Target Ther. 2022;7(1):199. doi:10.1038/s41392-022-01056-1
57. Chadha J, Harjai K, Chhibber S. Repurposing phytochemicals as anti-virulent agents to attenuate quorum sensing-regulated virulence factors and biofilm formation in Pseudomonas aeruginosa. Microb Biotechnol. 2022;15(6):1695–1718. doi:10.1111/1751-7915.13981
58. Olaniyi TD, Adetutu A. In silico anti-quorum sensing activities of phytocompounds of Psidium guajava in Salmonella enterica serovar Typhi. J Umm Al-Qura Univ Appl Sci. 2023. doi:10.1007/s43994-023-00029-6
59. Martins VV, Pitondo‐Silva A, de Melo Manço L, et al. Pathogenic potential and genetic diversity of environmental and clinical isolates of P seudomonas aeruginosa. Apmis. 2014;122(2):92–100. doi:10.1111/apm.12112
60. Woods DE, Cryz SJ, Friedman R, Iglewski B. Contribution of toxin A and elastase to virulence of Pseudomonas aeruginosa in chronic lung infections of rats. Infect Immun. 1982;36(3):1223–1228. doi:10.1128/iai.36.3.1223-1228.1982
61. Lima JL, Alves LR, Jacomé PR, Bezerra Neto JP, Maciel MAV, Morais MM. Biofilm production by clinical isolates of Pseudomonas aeruginosa and structural changes in LasR protein of isolates non biofilm-producing. Braz J Infect Dis. 2018;22:129–136. doi:10.1016/j.bjid.2018.03.003
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

