Back to Journals » Nature and Science of Sleep » Volume 15
Prevalence of Obstructive Sleep Apnea Using Home Sleep Test in Taiwan During the Coronavirus Disease Pandemic
Authors Chou TTC , Hsu HC, Twu CW, Huang WK, Huang HM, Weng SH, Chen MC
Received 7 August 2023
Accepted for publication 12 December 2023
Published 22 December 2023 Volume 2023:15 Pages 1107—1116
DOI https://doi.org/10.2147/NSS.S434278
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sarah L Appleton
Tyron Tai-Chun Chou,1,* Hsin-Chien Hsu,1– 3,* Chih-Wen Twu,4,5 Wen-Kuan Huang,6 Hung-Meng Huang,1,7 Shih-Han Weng,8 Ming-Chih Chen2
1Department of Otolaryngology, Taipei City Hospital, Taipei City, Taiwan; 2Graduate Institute of Business Administration, College of Management, Fu Jen Catholic University, New Taipei City, Taiwan; 3General Education Center, University of Taipei, Taipei City, Taiwan; 4Department of Otorhinolaryngology, Head and Neck Surgery, Changhua Christian Hospital, Changhua County, Taiwan; 5Department of Post-Baccalaureate Medicine, National Chung Hsing University, Taichung City, Taiwan; 6Division of Hematology/Oncology, Department of Internal Medicine, Chang Gung Memorial Hospital at Linkou, Chang Gung University College of Medicine, Taoyuan City, Taiwan; 7Department of Otolaryngology, School of Medicine, College of Medicine, Taipei Medical University, Taipei City, Taiwan; 8Department of Education and Research, Taipei City Hospital, Taipei City, Taiwan
*These authors contributed equally to this work
Correspondence: Ming-Chih Chen, Institute of Business Administration, College of Management, Fu Jen Catholic University, No. 510, Zhongzheng Road, Xinzhuang District, New Taipei City, 242062, Taiwan, Email [email protected]
Background: Obstructive sleep apnea syndrome (OSAS) is a common disorder associated with serious sequelae. The current gold standard diagnostic method, polysomnography, is costly and time consuming and requires patients to stay overnight at a facility.
Aim: This study aimed to reveal the prevalence of OSAS in general adult population using a home sleep test (HST) during the coronavirus disease 2019 (COVID-19) pandemic.
Methods: This prospective cohort study was conducted by the Department of Otolaryngology, Taipei City Hospital, Taipei, Taiwan, between January 2020 and December 2021. A total of 1372 patients aged 30– 70 years completed an HST using a Type 3 portable sleep monitor (PM). The apnea-hypopnea index (AHI) was analyzed to assess the association of OSAS with age, body mass index (BMI), sex, Epworth Sleepiness Scale (ESS) and the Sleep Apnea Risk Assessment questionnaire (STOP-Bang questionnaire) rating.
Results: The mean age of the patients (782 men, 57%; 590 women, 43%) was 49.24 ± 11.04 years. OSAS was detected in 954 (69.5%) patients with 399 (29.1%) mild OSAS; 246 (17.9%) moderate OSAS; and 309 (22.5%) severe OSAS. Among these, the prevalence of moderate-to-severe OSAS was 143 (10.4%) in women and 412 (30.0%) in men. The mean age was the highest (51.29 ± 11.29) in the mild OSAS group and lowest (47.08 ± 10.87) in the healthy group. OSAS severity was greater with increasing BMI, 23.39 ± 3.44 in the healthy group and 29.29 ± 5.01 in the severe OSAS group. A positive correlation was also noted between the ESS/STOP-Bang questionnaire rating and OSAS severity.
Conclusion: The prevalence of OSAS in Taiwan was 69.5% in our study. It showed strong evidence that OSAS has important public health consequences and PMs are simple, fast, feasible, and cost-effective tools for OSAS screening in the home environment, especially during the COVID-19 pandemic.
Keywords: COVID-19, obstructive sleep apnea, portable sleep monitor, home sleep test, apnea-hypopnea index
Introduction
Sleep-disordered breathing (SDB) is a major public health concern worldwide. Obstructive sleep apnea syndrome (OSAS) is the most predominant form of SDB, accounting for more than 80% of cases.1,2 The general prevalence of OSAS in the United States is approximately 15%.3 Obstruction or collapse of the upper respiratory tract occurs during sleep, resulting in apnea or hypopnea, which in turn causes hypoxia.4 In the long term, this condition is associated with serious sequelae, such as hypertension, cerebrovascular disease, heart failure, atrial fibrillation, and coronary heart disease.5 In addition, it increases the risk of chronic diseases, such as diabetes mellitus,6 male impotence,7 anxiety, and depression.8 The most serious risk is an increase in the probability of sudden death,9 which is 2.5 times higher in patients affected with OSAS than in an average person.10
The gold standard diagnostic test for OSAS is polysomnography (PSG), which is expensive to perform, requires the patient to stay overnight in a sleep laboratory, and to be monitored by a sleep technician. PSG is cumbersome because multiple sensors are used, and patients may experience anxiety and discomfort associated with sleeping in unfamiliar environment. Furthermore, depending on the geographic location, PSG might be unavailable, or there might be a long wait time before a patient can undergo this test.11,12
Treatments for OSAS involve lifestyle modification, medical intervention, and surgery. These include weight management, continuous positive airway pressure (CPAP), or medical drugs such as melatonin that may help to improve sleep quality.13 Although CPAP is the first-line and gold standard for OSAS management, various surgical techniques such as uvulopalatopharyngoplasty, lateral pharyngoplasty, or barbed reposition pharyngoplasty may be an alternate for those unwilling or intolerant to CPAP.14,15
The purpose of our study was to share our experience in using the home sleep test (HST) in the Taiwanese adult population during the coronavirus disease (COVID-19) pandemic. Owing to the rapid spread of COVID-19 during the pandemic, people confined themselves to limited activities and refrained from staying overnight in medical facilities. We considered that, in such situations, the use of a portable HST device could play a key role in screening for OSAS. Compared with PSG, a portable sleep monitor (PM) is an easier and more cost-effective tool for sleep monitoring at home; moreover, these devices can be used for screening several individuals with OSAS, particularly when the need to avoid the complexity of in-hospital stay arises.
Methods
Study Design and Participants
This prospective cohort study was conducted by the Department of Otolaryngology, Taipei City Hospital, Taipei, Taiwan, between January 2020 and December 2021. Our research was aimed at identifying the prevalence of OSAS using HST in Taiwan during the COVID-19 pandemic. The patients were referred from twelve district health centers in Taipei who were considered as potential candidates for the study if they had symptoms such as excessive sleepiness during the daytime, snoring, breathlessness, and episodes of hypopnea or apnea, which are associated with OSAS, as reported by bed partners and assessed by the senior otolaryngologist. Thereafter, a total of 1372 patients completed the HST using a Type 3 PM. The apnea-hypopnea index (AHI) was analyzed for age, body mass index (BMI), sex, Epworth Sleepiness Scale (ESS) and Sleep Apnea Risk Assessment questionnaire (STOP-Bang questionnaire) ratings.
Details of the PM
According to the classification of the American Academy of Sleep Medicine (AASM), PMs can be classified into four categories. Type 1 PM requires technicians to operate and monitor the device using at least seven monitoring items and body position sensors. The PSG devices used at hospitals or sleep centers belong to this category. A Type 2 PM does not require technicians to operate and monitor the device; however, the monitoring items are similar to those in Type 1 PMs, and at least seven monitoring items and body posture sensors are required. The next two types of PMs are classified as portable PSG devices. A Type 3 PM is a portable home sleep detection instrument with at least four monitoring items (including blood oxygen concentration, nasal flow, breathing action, heart rhythm, and electrocardiogram). A Type 4 PM is a portable home sleep detection instrument with at least two monitoring items (such as, blood oxygen concentration and nasal flow).16–18
In our study, we used a Type 3 PM (ApneaLink AirTM, Resmed, San Diego, CA, USA), which is an easy-to-use, lightweight, self-operated instrument with high sensitivity and specificity. This PM can monitor five items: blood oxygen concentration, nasal flow, breathing movement, heart rhythm, and snoring. It consists of a chest elastic band, pulse oximeter, and nasal cannula. After every test, the PM must be disinfected using ozone (O3) and the nasal cannula must be disposed of. In addition to recording the AHI, it can also automatically analyze the hypopnea index, degree of airflow limitation, number of snores, and blood oxygen saturation index. Further, it can distinguish between obstructive and central SDB. The variables had configurable thresholds, and the definition of a hypopnea event was set to 3% oxygen desaturation, which met the requirements of the AASM.
The AHI can be used as an indicator of severity in adults, as follows: AHI < 5, normal; AHI ≥ 5 and < 15, mild SDB; AHI ≥ 15 and < 30, moderate SBD; and AHI ≥ 30, severe SDB.
Questionnaire
Study participants were recruited and interviewed by a sleep technician and research assistant. Consenting individuals completed the ESS and STOP-Bang questionnaire. The ESS is a self-administered questionnaire with 8 questions on a 4-point scale (0–3) (0= No chance of dozing, 1= Slight chance of dozing, 2= Moderate chance of dozing, 3= High chance of dozing). Total scores range from 0 to 24 and an ESS score of 0–10 was considered mild daytime sleepiness, 11–15 moderate daytime sleepiness, and 16–24 severe daytime sleepiness. The STOP-Bang questionnaire consists of eight dichotomous (yes/no) items related to the clinical features of sleep apnea. The total score ranges from 0 to 8. The risk is assessed as follows: low risk, “yes” responses to 0–2 questions; intermediate risk, “yes” responses to 3–4 questions; and high risk, “yes” responses to 5–8 questions, “yes” responses to 2 or more of the 4 STOP questions + male sex, “yes” responses to 2 or more of the 4 STOP questions + BMI > 35 kg/m2, or “yes” responses to 2 or more of the 4 STOP questions + neck circumference > 40 cm.
Statistical Analysis
Data were analyzed on a personal computer using SAS version 9.4 software (SAS Institute Inc., Cary, NC, USA). Differences between the means of independent variables with a normal distribution between men and women were assessed using a two-sample t-test (Table 1). Differences in the mean values between the healthy and OSAS groups were assessed using the Welch and Brown–Forsythe test (Table 2). A box plot was used to present the comparison between the ESS/STOP-Bang ratings and AHIs. In addition, the ROC curve (receiver operating characteristic curve) and intraclass correlation coefficient (ICC) were used to evaluate the accuracy of PM. Statistical significance was set at P < 0.05.
 |
Table 1 Distribution of AHIa, Age, BMIb, ESSc and STOP-Bang by Patient Sex |
 |
Table 2 Distribution of AHIa, Age, and BMIb in the Healthy and OSASc Groups |
Ethical Considerations
All patients consented to study participation by signing informed consent forms before enrolment. This study was approved by the Institutional Review Board of Taipei City Hospital, Taipei, Taiwan (TCHIRB-10908003-E). Type 3 PM (ApneaLink Air) did not pose any hazard to the patients.
Results
Figure 1 shows the flow of patients through this study. There were 2793 patients who registered for our study, of whom 1481 were 30–70 years old and were included in this study. We focused mainly on economically productive population and excluded those older than 70 years old with potential systemic diseases. Among them, 109 patients recorded for less than 4 h, resulting in a failure rate of 7.4% (109/1481). The reasons for failure were as follows: nasal cannula detachment (12/109), pulse oximeter detachment (20/109), and interruption of the examination due to poor adaptation to the HST device (77/109). A total of 1372 patients, 782 men (57%) and 590 women (43%), with an average age of 47.53 ± 10.83 years and 51.52 ± 10.91 years, respectively, were finally included in the analysis. The BMI was 27.30 ± 4.60 in men and 24.40 ± 4.34 in women. The mean AHI was greater in men (23.72 ± 21.3) than in women (11.77 ± 15.53). Differences in AHI, age, BMI, and ESS/STOP-Bang between men and women are shown in Table 1 (p < 0.001).
 |
Figure 1 Patient flow diagram. Abbreviation: AHI, apnea-hypopnea index. |
Table 2 shows the mean and standard deviation of the selected variables for various categories. OSAS was detected in 954 patients (69.5%) in the study population. Among these, 399 (29.1%) had mild OSAS; 246 (17.9%), moderate OSAS; and 309 (22.5%) severe OSAS. The prevalence of moderate-to-severe OSAS was 412 (30.0%) in men and 143 (10.4%) in women. The age difference between the healthy and OSAS groups was significant, and the difference was greater in the patients in the mild and moderate OSAS group than in the healthy controls and patients in the severe OSAS group (p < 0.0001). Regarding BMI, the severity of OSAS was significantly greater in patients with increased BMI, and there was a positive correlation between BMI and OSAS severity (p < 0.0001).
Box plots of the comparisons between the ESS/STOP-BANG questionnaire ratings and AHIs are shown in Figure 2. A positive correlation was also observed between the ESS/STOP-BANG questionnaire rating and AHI severity (p < 0.0001). Table 3 revealed Apnea-hypopnea index (AHI) based on type 3 portable sleep monitor (PM) for questionnaire data for 1372 patients.
 |
Table 3 AHIa Based on Type 3 PMb for Questionnaire Data for 1372 Patients |
Ninety-seven people also received a type 1 polysomnography (PSG) test in sleep laboratory of Taipei city hospital within one month after HST. Based on the receiver operating characteristic curve (ROC) for the AHI threshold, when the AHI values were 5, 10, 15, and 30, the area under the curve (AUC) was 0.778, 0.832, 0.924, and 0.989, respectively (Figure 3). The intraclass correlation coefficient (ICC) between PSG and PM was 0.929 in Figure 4, indicating that the larger the AHI value cut-off point, the higher the accuracy, correlation, and reliability between PM and PSG. Table 4 showed the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) in PM (ApneaLink Air) for 97 patients.
 |
Table 4 The Sensitivity, Specificity, PPVa, and NPVb in PMc for 97 Patients |
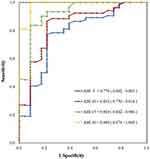 |
Figure 3 Receiver operating characteristic curve for 97 patients with polysomnography. Abbreviation: AHI, apnea-hypopnea index. |
 |
Figure 4 Intraclass correlation coefficient (ICC) for the PM-AHI and PSG-AHI for 97 patients. Abbreviations: AHI, apnea-hypopnea index; PM, portable sleep monitor; PSG, polysomnography. |
Discussion
The use of the HST to diagnose OSAS remains controversial.19 The arguments in using HST are regarding whether a PM can be used as the final diagnostic tool and whether the sensitivity of a patient self-operated PM is sufficient. These arguments were especially prevalent in the past when the sensitivity observed in most studies using PMs to detect OSAS was insufficient.19,20 Conversely, other studies have shown that using a PM is feasible for diagnosing OSAS and that the results are accurate.21 Using a PM has high clinical value, as the sensitivity is as high as 90%.19,22 Type 3 PM is the most widely accepted among the PM types.17 The sensitivity and specificity of Type 3 PM (ApneaLink Air) in identifying OSAS (AHI ≥ 5) were 85% and 50%, respectively. However, the sensitivity and specificity in the case of moderate-to-severe OSAS (AHI ≥ 15) were 91% and 95%, respectively. In our study, the sensitivity and specificity when the AHI ≥ 15 were 88.24% and 96.83%, respectively (Table 4). Therefore, this highlights the use of Type 3 PM as a valuable tool for diagnosing high-risk moderate-to-severe OSAS; however, OSAS severity may be underestimated in patients with a normal or low AHI.23 Furthermore, another benefit of using a PM in diagnosing OSAS is the cost of the device. The average cost of an examination completed by a PSG is approximately USD 700, whereas the per test cost by a PM is only USD 250, thus making the PM a much more cost effective tool.24
To our knowledge, this is the first comprehensive study that has used the HST to reveal the prevalence of OSAS in general adult population during the COVID-19 pandemic in Taiwan. It was believed that it would be difficult to teach patients how to use a PM and that they would find it difficult to use the device on their own, especially in case of older patients or those with insufficient education and low socioeconomic status. However, research has shown that even in the aforementioned groups, only 15% of patients could not perform the assessment for more than 4 h when using the device for the first time. After repeated testing, at least 4 h of recording could be completed. In our study, 109 patients recorded for less than 4 h, with a failure rate of 7.4%. There was also no theft or loss of PMs. During the COVID-19 pandemic, refraining from staying overnight in a medical facility was important in reducing the risk of transmission. In addition, to avoid COVID-19 infection during the HST, all portable PMs were disinfected with O3, and a disposable nasal cannula was used.25 This study screened for potential patients with OSAS using a large amount of HST screening data and achieved the advantages of early diagnosis. Thus, a PM is a simple, fast, economical, and efficient tool for screening patients for OSAS, especially during the COVID-19 pandemic.
Furthermore, sex, age, and BMI are the three most important factors that affect the OSAS prevalence rate.26 Recent studies have suggested that there are approximately one billion patients with OSAS globally.27 Middle-aged patients (30–70 years old) with moderate to severe OSAS (AHI ≥ 15) account for 6–13% of cases (women, 6%; men, 13%). Among men, the prevalence rate is approximately 10% and 17% in the 30–49-year-old group and 50–70-year-old group, respectively. Among women, the prevalence rate is approximately 3% and 10% in the 30–49-year-old group and 50–70-year-old group, respectively.28 However, the prevalence of OSAS in the general population in another research recorded a much higher prevalence of OSAS and was higher in men. An AHI ≥ 5 was recorded 83.8% in men and 60.8% in women, whereas an AHI ≥ 15 was noted 49.7% in men and 23.4% in women.28 The prevalence of OSAS can vary and ranged from 3.7% to 97.3% in Asian adults depending on the population studied and the criteria used for diagnosis.29 In our study, the prevalence of OSAS was also detected higher in 954 patients (69.5%) in general adult population. The prevalence of moderate-to-severe OSAS was 412 (30.0%) in men and 143 (10.4%) in women. There is strong evidence that OSAS has important public health consequences and might be revised the definition of the disorder.
Obesity plays a large role in OSAS. Although, whilst previously believed that patients with normal BMI were less likely to have OSAS, 20–40% of patients with OSAS are not obese,30 and most patients with moderate OSAS have BMIs within the normal range.19 Nevertheless, our study found a relationship among obesity, age, and sex. In terms of obesity, the mean BMI was 29.29 ± 5.01, 27.07 ± 4.25, 25.71 ± 5.67, and 23.39 ± 3.44 in the severe OSAS, moderate OSAS, mild OSAS, and healthy groups, respectively. This indicates a positive correlation between obesity severity and OSAS. As for age, mild and moderate OSAS is more common in older age groups. Finally, the male sex was also associated with a higher risk of OSAS than was the female sex.
The questionnaire used in our study also contributed to the screening of OSAS patients. A higher rating on the questionnaire indicated an increased risk of OSAS severity. When considering the questionnaire rating together with obesity, age, and sex, we could precisely screen for potential high-risk OSAS patients without the need for them to visit medical facilities. In our study, even if the rating in the questionnaire was mild in ESS, the mean AHI was higher at 15.12 ± 19.37. Previous study also found that no association was noted between severity of sleep-disordered breathing and presence of daytime sleepiness, measured with the ESS.31 Using questionnaires to screen general population alone may mis-diagnosed many potential OSAS patients.
A few limitations of this study must be acknowledged. The AHI value detected with the HST may have been underestimated, which implies that severity may also have been underestimated. This might have been due to patients were forced to sleep in supine position during PSG examinations conducted at a facility.32 Also, the absence of EEG in type 3 PM to detect arousals that form part of the hypopnea definitions may underestimate the AHI value. In addition, the total sleep period was estimated from the total recording period, which commenced from switching the light off to switching it on. This may have resulted in an overestimation of the actual sleep period and AHI value may have been underestimated.33,34 In the future, the use of a PM will be more widely accepted in clinical management. The sensitivity and specificity of PMs and PSG may become important indicators.
Conclusion
The prevalence of OSAS in general adult population aged 30–70 years in Taiwan was higher in our results (69.5%) than previous studies. There is strong evidence that OSAS has important public health consequences and PMs are simple, fast, feasible, and cost-effective tools for OSAS screening in the home environment, especially during the COVID-19 pandemic.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
This study complies with the Declaration of Helsinki.
Ethics Approval
All patients consented to study participation by signing written informed consent forms before enrolment. This study was approved by the Institutional Review Board of Taipei City Hospital, Taipei, Taiwan (TCHIRB-10908003-E). The Type 3 PM (ApneaLink Air) did not pose any hazard to the patients.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent to Publish
Patients signed written informed consent regarding publishing their data.
Acknowledgments
Acknowledgments to the Taipei City Health Bureau for their support.
Funding
Funding was provided by Taipei City Health Bureau, Taipei, Taiwan.
Disclosure
The authors declare that they have no conflict of interest.
References
1. Javaheri S. Central sleep apnea in congestive heart failure: prevalence, mechanisms, impact, and therapeutic options. Semin Respir Crit Care Med. 2005;26(1):44–55. doi:10.1055/s-2005-864206
2. Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69(7):841–858. doi:10.1016/j.jacc.2016.11.069
3. Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ. 2009;108(5):246–249.
4. Osman AM, Carter SG, Carberry JC, Eckert DJ. Obstructive sleep apnea: current perspectives. Nat Sci Sleep. 2018;10:21–34. doi:10.2147/NSS.S124657
5. Drager LF, McEvoy RD, Barbe F, Lorenzi-Filho G, Redline S; Initiative I. Sleep apnea and cardiovascular disease: lessons from recent trials and need for team science. Circulation. 2017;136(19):1840–1850. doi:10.1161/CIRCULATIONAHA.117.029400
6. Reutrakul S, Mokhlesi B. Obstructive sleep apnea and diabetes: a state of the art review. Chest. 2017;152(5):1070–1086. doi:10.1016/j.chest.2017.05.009
7. Kellesarian SV, Malignaggi VR, Feng C, Javed F. Association between obstructive sleep apnea and erectile dysfunction: a systematic review and meta-analysis. Int J Impot Res. 2018;30(3):129–140. doi:10.1038/s41443-018-0017-7
8. Garbarino S, Bardwell WA, Guglielmi O, Chiorri C, Bonanni E, Magnavita N. Association of anxiety and depression in obstructive sleep apnea patients: a systematic review and meta-analysis. Behav Sleep Med. 2020;18(1):35–57. doi:10.1080/15402002.2018.1545649
9. Blackwell JN, Walker M, Stafford P, Estrada S, Adabag S, Kwon Y. Sleep apnea and sudden cardiac death. Circ Rep. 2019;1(12):568–574. doi:10.1253/circrep.cr-19-0085
10. Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352(12):1206–1214. doi:10.1056/NEJMoa041832
11. Flemons WW, Douglas NJ, Kuna ST, Rodenstein DO, Wheatley J. Access to diagnosis and treatment of patients with suspected sleep apnea. Am J Respir Crit Care Med. 2004;169(6):668–672. doi:10.1164/rccm.200308-1124PP
12. Masa Jimenez JF, Barbe Illa F, Capote Gil F, et al. [Resources and delays in the diagnosis of sleep apnea-hypopnea syndrome]. Recursos y demoras en el diagnostico del sindrome de apneas-hipopneas durante el sueno (SAHS). Arch Bronconeumol. 2007;43(4):188–198. Spanish. doi:10.1016/s1579-2129(07)60050-0
13. Aiello G, Cuocina M, La Via L, et al. Melatonin or ramelteon for delirium prevention in the intensive care unit: a systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2023;12:2.
14. Iannella G, Lechien JR, Perrone T, et al. Barbed reposition pharyngoplasty (BRP) in obstructive sleep apnea treatment: state of the art. Am J Otolaryngol. 2022;43(1):103197. doi:10.1016/j.amjoto.2021.103197
15. Maniaci A, Di Luca M, Lechien JR, et al. Lateral pharyngoplasty vs. traditional uvulopalatopharyngoplasty for patients with OSA: systematic review and meta-analysis. Sleep Breath. 2022;26(4):1539–1550. doi:10.1007/s11325-021-02520-y
16. Standards of Practice Committee of the American Sleep Disorders Association. Practice parameters for the use of portable recording in the assessment of obstructive sleep apnea. Sleep. 1994;17(4):372–377.
17. Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable monitoring task force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3(7):737–747.
18. Lux L, Boehlecke B, Lohr KN. Effectiveness of portable monitoring devices for diagnosing obstructive sleep apnea: update of a systematic review. AHRQ Technology Assessments; 2004.
19. Dzieciolowska-Baran E, Gawlikowska-Sroka A, Szczurowski J. Diagnosis of sleep-disordered breathing in the home environment. Adv Exp Med Biol. 2020;1271:107–112. doi:10.1007/5584_2020_497
20. Mendonca F, Mostafa SS, Ravelo-Garcia AG, Morgado-Dias F, Penzel T. Devices for home detection of obstructive sleep apnea: a review. Sleep Med Rev. 2018;41:149–160. doi:10.1016/j.smrv.2018.02.004
21. Morales CR, Hurley S, Wick LC, et al. In-home, self-assembled sleep studies are useful in diagnosing sleep apnea in the elderly. Sleep. 2012;35(11):1491–1501. doi:10.5665/sleep.2196
22. Flemons WW, Littner MR, Rowley JA, et al. Home diagnosis of sleep apnea: a systematic review of the literature. An evidence review cosponsored by the American Academy of Sleep Medicine, the American College of Chest Physicians, and the American Thoracic Society. Chest. 2003;124(4):1543–1579. doi:10.1378/chest.124.4.1543
23. Erman MK, Stewart D, Einhorn D, Gordon N, Casal E. Validation of the ApneaLink for the screening of sleep apnea: a novel and simple single-channel recording device. J Clin Sleep Med. 2007;3(4):387–392. doi:10.5664/jcsm.26861
24. Kapoor M, Greenough G. Home Sleep Tests for Obstructive Sleep Apnea (OSA). J Am Board Fam Med. 2015;28(4):504–509. doi:10.3122/jabfm.2015.04.140266
25. Cristiano L. Could ozone be an effective disinfection measure against the novel coronavirus (SARS-CoV-2)? J Prev Med Hyg. 2020;61(3):E301–E303. doi:10.15167/2421-4248/jpmh2020.61.3.1596
26. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. doi:10.1164/rccm.2109080
27. Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–698. doi:10.1016/S2213-2600(19)30198-5
28. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi:10.1093/aje/kws342
29. Mirrakhimov AE, Sooronbaev T, Mirrakhimov EM. Prevalence of obstructive sleep apnea in Asian adults: a systematic review of the literature. BMC Pulm Med. 2013;13:10. doi:10.1186/1471-2466-13-10
30. Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383(9918):736–47. doi:10.1016/S0140-6736(13)60734-5
31. Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. doi:10.1016/S2213-2600(15)00043-0
32. Liu WT, Lin SY, Tsai CY, et al. Comparison of hospital-based and home-based obstructive sleep apnoea severity measurements with a single-lead electrocardiogram patch. Sensors. 2021;21:23.
33. Ng SS, Chan TO, To KW, et al. Validation of a portable recording device (ApneaLink) for identifying patients with suspected obstructive sleep apnoea syndrome. Intern Med J. 2009;39(11):757–762. doi:10.1111/j.1445-5994.2008.01827.x
34. Gantner D, Ge JY, Li LH, et al. Diagnostic accuracy of a questionnaire and simple home monitoring device in detecting obstructive sleep apnoea in a Chinese population at high cardiovascular risk. Respirology. 2010;15(6):952–960. doi:10.1111/j.1440-1843.2010.01797.x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

