Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Prevalence of Microalbuminuria Among Diabetes Patients in Africa: A Systematic Review and Meta-Analysis
Authors Mohammed O, Alemayehu E , Bisetegn H , Debash H, Gedefie A , Ebrahim H , Tilahun M , Fiseha T
Received 25 February 2023
Accepted for publication 3 July 2023
Published 11 July 2023 Volume 2023:16 Pages 2089—2103
DOI https://doi.org/10.2147/DMSO.S409483
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Ousman Mohammed, Ermiyas Alemayehu, Habtye Bisetegn, Habtu Debash, Alemu Gedefie, Hussen Ebrahim, Mihret Tilahun, Temesgen Fiseha
Department of Medical Laboratory Sciences, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
Correspondence: Ousman Mohammed, Tel +251911641754, Email [email protected]
Background: Microalbuminuria (MAU) is considered the earliest sign of diabetic nephropathy among diabetes patients. In order to effectively manage diabetic nephropathy and its consequences early, detection of microalbuminuria as soon as possible, especially for diabetes patients, is critical. Therefore, the present study aimed to determine the pooled prevalence of microalbuminuria among diabetes patients in Africa.
Methods: Electronic databases such as Google Scholar, PubMed, African Journals Online, Web of Science, Cochrane Library, EMBASE, and ResearchGate were searched for articles and grey literature. The STATA version 14 software was used to conduct the meta-analysis. I2 and Cochran’s Q test were employed to assess the presence of heterogeneity between studies. Due to the presence of heterogeneity, a random effect model was used. The publication bias was assessed using the symmetry of the funnel plot and Egger’s test statistics. Moreover, subgroup analysis, trim and fill analysis, and sensitivity analysis were also done.
Results: The overall pooled prevalence of microalbuminuria among diabetes patients in Africa was 37.11% (95% CI 31.27– 42.95). Substantial heterogeneity was observed between studies, with I2 values of 94.7%. Moreover, this meta-analysis showed that the pooled estimate of microalbuminuria among type 1 and type 2 diabetes patients was 35.34% (95% CI: 23.89– 46.80, I2=94.2), and 40.24% (95% CI: 32.0– 48.47, I2=94.9) respectively. MAU, on the other hand, was more common in people with diabetes for more than 5 years 38.73% (95% CI: 29.34– 48.13) than in people with diabetes for less than 5 years 31.48% (95% CI: 18.73– 44.23).
Conclusion: This systematic review and meta-analysis found a high prevalence of microalbuminuria among diabetes patients. As a result, early detection of microalbuminuria is critical for preventing and treating microvascular complications such as diabetic nephropathy and the onset of end-stage renal disease.
Keywords: microalbuminuria, diabetes, Africa, meta-analysis
Introduction
Globally, the prevalence of diabetes is increasing at an alarming rate and has become a public health concern.1–3 According to the 10th edition of the International Diabetes Federation (IDF) Diabetes Atlas, there will be 537 million people living with diabetes worldwide in 2021. The global prevalence of diabetes is now estimated to be over 10%.4 In Africa, the number of people with diabetes is expected to increase by 162.5% by the year 2045.5 Diabetes mellitus (DM) is associated with the derangement of the normal metabolism of carbohydrates, lipids, and proteins. The primary cause of morbidity and mortality in DM is macrovascular and microvascular complications.6 Long-term complications of diabetes can lead to visual impairment (retinopathy), blindness, kidney disease (nephropathy), nerve damage, amputation, heart disease, and stroke.7 Diabetic nephropathy (DN) is a common complication in diabetic patients characterised by persistent albuminuria, a progressive decline in glomerular filtration rate (GFR), and raised arterial blood pressure.8 Diabetic nephropathy is the leading cause of end-stage renal disease and premature mortality in diabetic patients due to its insidious onset.9–13 Approximately one-third to half of patients with diabetes develop renal manifestations.14,15
According to studies, 20 to 40% of type 2 DM patients eventually develop nephropathy.16,17 The development of DN consists of several stages, the earliest being microalbuminuria, which can progress to overt proteinuria and ultimately end-stage renal disease (ESRD).18,19 Microalbuminuria (MAU) remains the best-documented predictor of the high risk of the development of diabetic nephropathy in DM patients.20 It can be defined as a urinary albumin excretion rate (UAE) of between 30–300 mg/24 hours or as an albumin/creatinine ratio (ACR) of 30–300 mg albumin/g of creatinine.21 According to the theory that MAU does reflect both phases of glomerular failure and generalised endothelial dysfunction, MAU and extra renal vascular damage in diabetic patients are related.22–24
MAU in diabetic patients is more likely to progress to overt proteinuria and, eventually, renal failure.25–28 Typically, diabetic nephropathy advances irreversibly from the onset of clinical proteinuria to ESRD. However, it has been demonstrated that early detection, medical care, and appropriate lifestyle changes can stop or reverse the progression from micro- to macroalbuminuria.29 According to some data, after 10 to 15 years of untreated type 1 diabetes with persistent MAU, over 80% of patients will have overt nephropathy, and 50% will eventually progress to end-stage renal disease (ESRD).30 20–40% of type 2 diabetes individuals with MAU advance to overt nephropathy after 20 years from the time of onset, and about 20% develop ESRD, according to research.31
Renal replacement therapy is not widely available in African settings. Therefore, it is essential to identify microalbuminuria as soon as possible, especially in this high-risk group of people.32 Therefore, MAU is a highly valuable indicator of kidney health and therapy outcomes in diabetic patients. There have been a lot of studies published that evaluate the prevalence of MAU in African DM patients. However, the majority of these studies used a limited sample size and only one healthcare facility, and they revealed a widely varied frequency of MAU. As a result, the current study was conducted with the goal of merging studies from the existing literature to evaluate the combined prevalence of microalbuminuria in African diabetic patients.
Methods
Search Strategy and Selection Criteria
This systematic review and meta-analysis were registered at PROSPERO with registration ID 2022: CRD42022344430. In accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines, the current review was conducted.33 Systematic electronic searches using databases such as Google Scholar, PubMed, African Journals Online, Web of Science, Cochrane Library, EMBASE, and ResearchGate were done from May to July 4, 2022, to retrieve all relevant primary articles reporting the prevalence of MAU among diabetes patients in Africa. In addition, we extended our search by retrieving reference lists of eligible studies. The search protocol was formulated using the following keywords combined by Boolean logic (AND/OR): microalbuminuria, diabetes, diabetes mellitus, type 1 diabetes, non-insulin-dependent diabetes mellitus, type 2 diabetes, insulin-dependent diabetes mellitus, and each African country: Searched articles were entered into Endnote Software to avoid duplicates, and the list was consolidated into one. The two reviewers (OM and EA) blindly screened the titles, abstracts, and full-text search results to identify potentially eligible studies. And the full text of selected articles was assessed in detail against the inclusion criteria.
Eligibility Criteria
Inclusion Criteria
Full-length articles that report the prevalence of microalbuminuria and/or are able to calculate the prevalence of MAU among DM patients were included. Studies that reported increased urinary albumin excretion using a urinary albumin excretion rate (UAE) of between 30–299 mg/24 hours or an albumin/creatinine ratio (ACR) of 30–299 mg albumin/g of creatinine were included. Furthermore, grey literature written in the English language was also included.
Exclusion Criteria
Articles whose full length were not available and lacked the necessary data to be extracted, as well as full-length articles published in a language other than English, were excluded. Case reports, clinical trials, case series studies, letters to the editor, and studies that either failed to describe the prevalence of MAU or lacked pertinent data were also excluded.
Data Extraction
The information on the name of the primary author, publication year, country where the study was conducted, study design, sample size, types of diabetes, the overall prevalence of MAU, the prevalence of MAU between genders, and the prevalence of MAU between type 1 and type 2 were all summarised by Microsoft Excel as part of the data extraction format. Consensus-based talks and an independent evaluation by a third author were used to settle any differences or discrepancies. Consensus-based talks and an independent evaluation by a third researcher were used to settle any differences or disagreements. When more information was required, we tried to get in touch with the primary authors. For studies appearing in more than one publication, we considered the most recent and comprehensive studies and those with the largest sample size. In addition, studies conducted in both type 1 and type 2 DM subjects and reporting the prevalence of MAU independently were extracted as separate studies.
Quality Assessment
A Newcastle-Ottawa scale adapted for cross-sectional studies and a quality assessment tool were used to assess the quality of the included studies.34 All eligible studies were evaluated, and studies of good quality or above were included in the final analysis. Two authors (OM and EA) evaluated each featured paper’s quality independently. Before determining the final evaluation score, the reviewers compared their quality appraisal scores and eliminated any discrepancies.
Operational Definitions
Patients with a significant rise in the excretion of urine albumin-creatinine ratio (ACR) within the particular range of 30–299 mg of albumin per g of creatinine or a urinary albumin excretion rate (UAE) of between 30–299 mg per 24 hours were regarded as having microalbuminuria. DM was defined as HbA1c (≥6.5%), a fasting blood sugar level (≥126 mg/dl), or a random blood sugar level (≥200 mg/dl).
Statistical Analysis
STATATM version 14 software was used for all data analysis. Using a random-effects model, a meta-analysis of the pooled prevalence of MAU was conducted, producing a pooled prevalence with 95% confidence intervals. Cochran’s Q and the I2 statistics were used to assess the degree of heterogeneity between studies. With I2 values of 25%, 50%, and 75%, respectively, the degree of heterogeneity is classified as low, moderate, or high.35 Subgroup analyses were conducted in accordance with the predefined criteria, including sub-region, publication year, sex, and forms of DM, in order to investigate the origins of heterogeneity. The symmetry of the funnel plot was visually examined, and Egger’s test statistics were considered to evaluate the publication bias among the studies.36,37 The random effect analysis was utilised to do a nonparametric trim and fill analysis because both techniques revealed publication bias. A leave-one-out sensitivity analysis was additionally conducted to assess the impact of single research on the combined estimate of the other studies.38 For all computations, statistical significance was set at p <0.05 for all calculations.
Result
Flow Chart
Figure 1 shows the flow chart and selection process for determining the pooled prevalence of microalbuminuria (MAU) in diabetes patients. A total of 747 articles were discovered using electronic searches and other sources, and 521 non-duplicate articles were screened. About 483 of them were removed after looking over their titles and abstracts. The full texts of the remaining 38 articles were reviewed. Only 29 studies met our inclusion criteria, and the remaining 9 articles were rejected because the intended outcomes were not attained. Moreover, the four studies that reported the prevalence of MAU in type 1 DM and type 2 DM were extracted separately and treated as independent investigations. Subsequently, 29 articles were included in the final analysis (Figure 1).
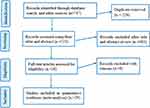 |
Figure 1 Flow chart of studies’ search and retrieval process. |
Overview of Included Studies
In this study, 29 original articles published from 1992 to 2022, consisting of 3885 study participants, were included.39–67 Among the study participants, 824 (21.2%) had type 1 diabetes, 2343 (60.3%) had type 2 diabetes, and for the remaining 718 (18.5%), the specific types of DM were not reported. Eleven (33.3%) studies were conducted on type 1 DM patients, seventeen studies (51.5%) on type 2 DM patients, and five studies (15.2%) on type 1 and 2 DM patients. All the included studies were conducted at the health institution level, and no community-based study was found. Except for one case-control study,44,61,66 and one cohort study,58 all the studies used a cross-sectional study design with sample sizes ranging from 2067 to 289 patients.56 The mean age of the study participants varied from 8.4 to 61.3 years. Moreover, Omar et al61 reported the highest (77.5%) prevalence of MAU in type 1 DM patients, while Amolo60 reported the lowest (6.2%) prevalence. For analysis purposes, studies done by Rahlenbeck et al, Martin et al, Lutale et al, and Fetni et al were extracted twice because they reported the prevalence of MAU separately for type 1 and type 2 DM. The studies were conducted in 14 different countries, including Ethiopia (2 studies),40,41 Nigeria (6 studies),42–45,66,67 Cameroon (3 studies),46,47,64 Sudan (2 studies),48,65 Tanzania (3 studies),49–51 Uganda (2 studies),52–54 South Africa (1 study),55 Botswana (2 studies),56,57 Algeria (1 study),58 Ghana (1 study),59 Kenya (1 study),60 Egypt (1 study),61 Senegal (1 study),62 and Zambia (2 studies)39,63 (Table 1).
 |
Table 1 Overview of Included Studies Conducted in Africa (N=3885) |
The Pooled Prevalence of Microalbuminuria Among Diabetes Patients in Africa
The pooled prevalence of microalbuminuria in the studies conducted in Africa between 1992 and July 4, 2022, was found to be 37.11% (95% CI 31.27–42.95; p < 0.001). The Cochran’s Q values for the heterogeneity test are 569.22 (degree of freedom, d.f = 32), and I2 with 94.4% suggests that there is a significant amount of heterogeneity between studies (Figure 2).
 |
Figure 2 Forest plot showing the pooled prevalence of microalbuminuria among diabetes patients in Africa. |
Subgroup Analysis
Subgroup analysis was done based on sub-region, types of DM, mean duration of DM, study design, sex, and publication year in order to investigate the cause of heterogeneity among the included studies in this meta-analysis. Eastern Africa accounted for more than one-third (12 (36.4%) of the studies in our review, followed by Western Africa (8 (24.2%), Central Africa (3 (9.1%), Northern Africa (5 (15.2%), and Southern Africa (5 (15.2%). In five African sub-regions, the combined prevalence of MAU among DM patients ranged from 30.57% (95% CI: 20.3–40.83) in Eastern Africa to 46.5% (95% CI: 32.29–60.71) in Northern Africa. The prevalence estimates between studies per sub-region showed significant heterogeneity (total heterogeneity, p<0.0001) (Table 2). 33.95 (26.83, 41.08).
 |
Table 2 The results of Subgroup Analysis by Different Categories of the Studies in Africa |
Furthermore, this meta-analysis revealed that the overall pooled estimates of MAU among type 1 and type 2 DM patients in Africa were 35.34% (95% CI: 23.89–46.80, I2=94.2), and 40.24% (95% CI: 32.0–48.47, I2=94.9), respectively. Based on the subgroup analysis of MAU, the pooled point estimates for diabetic male patients were 36.6% (95% CI: 29.50–43.69, I2=87.4), and diabetic female patients were 33.95% (95% CI: 26.83–41.08, I2=91.4). High levels of heterogeneity were seen in both sexes (p<0.0001) (Table 2). In order to see the prevalence of MAU over time, we divided the included studies into three groups (1992–2002, 2003–2012, and 2013–2022), or every ten years intervals, based on the publication year. Among studies conducted between 1992 and 2002, a pooled prevalence of 43.3% (95% CI: 32.6–54.1, I2=76.7) of MAU was found. The lowest prevalence of MAU, however, was observed in studies conducted from 2003 to 2012, when it was 27.11% (95% CI: 16.37–37.85, I2=93.7). MAU, on the other hand, was more common in people with diabetes for more than 5 years 38.73% (95% CI: 29.34–48.13) than in people with diabetes for less than 5 years 31.48% (95% CI: 18.73–44.23). Moreover, the subgroup analysis revealed that among case-control studies, the highest pooled prevalence of MAU was found to be 48.46% (95% CI: 24.14–72.78) (Table 2).
Publication Bias
A funnel plot test was used to assess the presence of publication bias. And this showed the presence of publication bias, which is supported by the asymmetry displayed in the funnel plot. Moreover, Egger’s test statistics also indicated the presence of publication bias with a p-value of <0.001. Therefore, this is an indication of the presence of unpublished data that can modify the prevalence of MAU among DM patients in Africa (Figure 3).
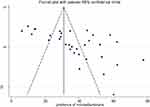 |
Figure 3 Bias assessment plot of reported prevalence of microalbuminuria among diabetes patients across studies published in Africa. |
Trim and Fill Analysis of Pooled Prevalence of Microalbuminuria Among Diabetes Patients
The pooled prevalence of microalbuminuria among DM patients in Africa was 23.94% (95% CI: 17.56–30.33) based on trim and fill analysis following the addition of fourteen studies, with a p-value <0.001 (Table 3).
 |
Table 3 Trim and Fill Analysis of Overall Pooled Prevalence of Microalbuminuria Among Diabetes Patients in Africa |
Sensitivity Analysis
Sensitivity analysis was employed to determine the impact of a single study on the combined effect size. A single study’s exclusion had no discernible impact on the pooled burden estimates when studies were eliminated one at a time since the resulting pooled effect size was within the 95% confidence interval of the combined pooled effect size. Because of this, random-effects sensitivity analysis revealed that no particular study had an effect on the overall prevalence of MAU among diabetic patients (Table 4).
 |
Table 4 Sensitivity Analysis for Single Study Influence of Pooled Estimate |
Discussion
End-stage renal disease has become more common as the prevalence of diabetes mellitus has increased.68 Microalbuminuria is a risk factor for cardiovascular disease complications and an early indicator of diabetic nephropathy.20 The presence of microalbumin in diabetic patients’ urine is an early warning sign of systemic vasculopathy and other microvascular complications.69 According to the current study, the overall pooled prevalence of MAU among diabetes patients in Africa was 37.11% (95% CI: 31.27–42.95). This finding indicated that a significant proportion of diabetes patients had microalbuminuria. Furthermore, the current study discovered that 35.34% (95% CI: 23.89–46.80) of type 1 diabetes patients and 40.24% (95% CI: 32.0–48.47) of type 2 diabetes patients have microalbuminuria. This high prevalence of MAU in African diabetics could be attributed to the presence of comorbidities such as hypertension, which exacerbates systemic vasculopathy and other microvascular complications.
This pooled prevalence of MAU among type 2 diabetes patients is comparable to the findings (39%) of a global cross-sectional study on type 2 diabetes patients conducted in 33 countries.70 A similar result of 35.1% (12.3–74.5%) was reported in large, multiple international cohort studies including >3 million participants.71 Likewise, the current pooled prevalence of microalbuminuria among diabetic subjects was in agreement with the Pakistan multi-centre study (34%),72 Albania (40.8%),73 Asians (39.8%),70 and Saudi Arabians (41.3%).74 Furthermore, Dinneen et al discovered that the prevalence of microalbuminuria in type 2 diabetes mellitus patients ranged from 20% to 36% across 8 cohorts.75 However, our finding was higher than the European prevalence of microalbuminuria (26% to 29%),76 Australia (26.1%),77 North India (25.5%),78 and Iran (14.2%).79 Moreover, Klein et al found a prevalence of microalbuminuria of 22.0% in those type 2 DM subjects.80 On the other hand, the MAU in our study was lower than a study conducted by Parving et al (58%)81 and the United Arab Emirates (61%).82
The current overall estimated prevalence of MAU in type 1 diabetes was 35.34%, which was comparable to a study done by Klein et al (29.2%).80 In contrast, the current result was lower than that reported by Warram et al (60%).83 Changes in sample size, study design, data processing method, assay method, ethnicity, cut-off levels for albumin excretion, duration of DM, renal status, and other clinical features could explain this disparity. Furthermore, the prevalence of microalbuminuria was heavily influenced by the variability and concentration of urine on a daily basis. According to this review’s evidence, more than one-third of diabetes patients have microalbuminuria, which is the first step in the development of microvascular complications such as renal nephropathy.20 Renal function deteriorates as a result of diabetic nephropathy, resulting in renal insufficiency. Treatment is required at this stage to slow the rate of progression. If left untreated, the kidney can malfunction, leading to kidney failure and the need for dialysis or kidney transplants,84,85 but such kidney disease treatments and management are difficult to come by in resource-limited countries.32,86
The present review also attempted to conduct a subgroup analysis based on sub-region, gender, publication year, and diabetes type. MAU had the highest pooled prevalence of 46.50% (95% CI: 32.29–60.71) in studies conducted in Northern Africa,48,58,61,65 followed by 40.81 (95 CI: 30.85–50.76) in studies conducted in Western Africa.42–45,59,62,66,67 This disparity could be attributed to differences in study subjects, urine collection methods, the presence of comorbidity, and assay methods used in those African countries. In terms of gender, the subgroup analysis revealed that 36.60% (95% CI: 29.50–43.69) of male DM patients had MAU compared to 33.95% (95% CI: 26.83–41.08) of female DM patients. In previous studies,87,88 male participants had a higher proportion of MAU than their female counterparts. This slightly higher prevalence of MAU in male subjects could be explained by males being more vulnerable to other risk factors that could aggravate diabetes and affect renal glomerulus function.
In the current review, 40.24% of type 2 diabetic patients had MAU, which was higher than that of type 1 diabetes patients by 5%. In addition, this review also indicated that the prevalence of MAU among studies conducted on type 1 and type 2 DM was even lower (30.02%). Albuminuria may increase in type 2 diabetes patients due to other comorbidities, and renal function, particularly glomerular filtration rate, may decrease. Studies published twenty years ago40,45,64 revealed a higher prevalence (43.3%) of MAU than recent ones. Even after a subgroup analysis, this review found significant heterogeneity in studies on the prevalence of microalbuminuria in DM patients. There are several potential causes of heterogeneity in this study. One possible explanation is sample size differences; for example, Yarhere et al67 included only 20 participants in their study, whereas Molefe Baikai et al56 included 289 participants. Another possible explanation for this heterogeneity is the variety of urine sample collection methods, assay methods, and differences in diabetes duration among participants. This review also revealed a publication bias, which is supported by the symmetry of the funnel plot and the results of Egger’s test. After adding 14 studies, we performed trim and fill analysis, and the pooled prevalence of microalbuminuria among DM patients was found to be 23.94%. The sensitivity analysis, however, revealed that no single study had an effect on the total pooled effect size.
One of the current review’s limitations is that it was unable to provide information on the risk factors associated with MAU. However, this is the first review to combine the findings of several African studies, providing stronger evidence on the prevalence of microalbuminuria among diabetes patients. Furthermore, the review attempted to conduct a subgroup analysis based on diabetes type and demonstrated the pooled prevalence of MAU among type 1 and 2 DM patients.
Conclusion
This review discovered a high prevalence of microalbuminuria among diabetes patients in Africa. Microalbuminuria was more common in Northern Africans, people with type 2 diabetes, and people who had diabetes for more than five years. As a result, early detection of microalbuminuria is critical for preventing and treating microvascular complications such as diabetic nephropathy and the onset of end-stage renal disease. This is especially important in developing countries, where recommended renal therapies such as dialysis and transplantation are not easily accessible due to financial constraints.
Data Sharing Statement
All the datasets used and/or analyzed during the current study are available in the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no potential competing interests in this work.
References
1. Shah N, Amanullah SJ, Marwat MA. Association of hyperuricemia with diabetic nephropathy in type 2 diabetes mellitus. KJMS. 2014;7(2):267.
2. Li Y, Zhao L, Yu D, Ding G. The prevalence and risk factors of dyslipidemia in different diabetic progression stages among middle-aged and elderly populations in China. PLoS One. 2018;13(10):e0205709. doi:10.1371/journal.pone.0205709
3. Khan MA, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of type 2 diabetes–global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10(1):107. doi:10.2991/jegh.k.191028.001
4. Sun H, Saeedi P, Karuranga S, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi:10.1016/j.diabres.2021.109119
5. Atlas D. International diabetes federation. In: IDF Diabetes Atlas.
6. Naicker S, Ashuntantang G. End stage renal disease in sub-Saharan Africa. Chronic Kidney Dis Disadv Popul. 2017;2017:125–137.
7. Story M, Stang J. Nutrition needs of adolescents. Guidelines Adolesc Nutr Services. 2005;3(1):21–34.
8. Obineche EN, Adem A. Update in diabetic nephropathy. Int J Diabetes Metabol. 2005;13(1):1. doi:10.1159/000497567
9. John S. Complication in diabetic nephropathy. Diabetes Metabol Synd. 2016;10(4):247–249. doi:10.1016/j.dsx.2016.06.005
10. Bikbov B, Purcell CA, Levey AS, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–733. doi:10.1016/S0140-6736(20)30045-3
11. Cockwell P, Fisher LA. The global burden of chronic kidney disease. Lancet. 2020;395(10225):662–664. doi:10.1016/S0140-6736(19)32977-0
12. Wolf G. New insights into the pathophysiology of diabetic nephropathy: from haemodynamics to molecular pathology. Eur J Clin Invest. 2004;34(12):785–796. doi:10.1111/j.1365-2362.2004.01429.x
13. Singh VP, Bali A, Singh N, Jaggi AS. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol. 2014;18(1):1. doi:10.4196/kjpp.2014.18.1.1
14. Yadav B, Shashidhar KN, Raveesha A, Muninarayana C. Assessment of cystatin C and microalbumin as biomarkers for nephropathy in patients with type 2 diabetes mellitus. J Evol Med Dent Sci. 2022;10(25):1866–1871. doi:10.14260/jemds/2021/386
15. Pecoits-Filho R, Abensur H, Betônico CC, et al. Interactions between kidney disease and diabetes: dangerous liaisons. Diabetol Metab Syndr. 2016;8(1):1–21. doi:10.1186/s13098-016-0159-z
16. American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care. 2010;33(Supplement_1):S11–61. doi:10.2337/dc10-S011
17. Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab. 2008;4(8):444–452. doi:10.1038/ncpendmet0894
18. Warram JH, Scott LJ, Hanna LS, et al. Progression of microalbuminuria to proteinuria in type 1 diabetes: nonlinear relationship with hyperglycemia. Diabetes. 2000;49(1):94–100. doi:10.2337/diabetes.49.1.94
19. De Boer IH, Rue TC, Cleary PA, et al.; Diabetes Control and Complications Trial, Epidemiology of Diabetes Interventions and Complications Study Research Group. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med. 2011;171(5):412–420. doi:10.1001/archinternmed.2011.16
20. Hovind P, Tarnow L, Rossing P, et al. Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. BMJ. 2004;328(7448):1105. doi:10.1136/bmj.38070.450891.FE
21. Ammirati AL. Chronic kidney disease. Revista da Associação Médica Brasileira. 2020;66:s03–9. doi:10.1590/1806-9282.66.S1.3
22. Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. Diabetologia. 1989;32(4):219–226. doi:10.1007/BF00285287
23. Ho YW, Chau KF, Leung CB, et al. Hong Kong registry report 2004. Hong Kong J Nephrol. 2005;7(1):38–46. doi:10.1016/S1561-5413(09)60179-4
24. Jerums G, Maclsaac RJ. Treatment of microalbuminuria in patients with type 2 diabetes mellitus. Treat Endocrinol. 2002;1(3):163–173. doi:10.2165/00024677-200201030-00004
25. Dadhania BP, Aravat AH, Dhruva GA. Study of microalbuminuria in diabetes type 2 patients as a marker of morbidity (a study of 100 cases in Rajkot city). Int J Biomed Adv Res. 2012;3(10):763–766. doi:10.7439/ijbar.v3i10.769
26. Chen C, Wang C, Hu C, et al. Normoalbuminuric diabetic kidney disease. Front Med. 2017;11(3):310–318. doi:10.1007/s11684-017-0542-7
27. Goud MB, Devi SO, Nayal B, Begum S. Bio-chemical aspects, pathophysiology of microalbuminuria and glycated hemoglobin in type 2 diabetes mellitus. In: Pathophysiology and Complications of Diabetes Mellitus. IntechOpen; 2012.
28. Roscioni SS, Heerspink HJ, De Zeeuw D. Microalbuminuria: target for renoprotective therapy PRO. Kidney Int. 2014;86(1):40–49. doi:10.1038/ki.2013.490
29. Bakris GL, Molitch M. Microalbuminuria as a risk predictor in diabetes: the continuing saga. Diabetes Care. 2014;37(3):867–875. doi:10.2337/dc13-1870
30. Orchard TJ, Dorman JS, Maser RE, et al. Prevalence of complications in IDDM by sex and duration: Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes. 1990;39(9):1116–1124. doi:10.2337/diab.39.9.1116
31. Zelmanovitz T, Gerchman F, Balthazar AP, Thomazelli FC, Matos JD, Canani LH. Diabetic nephropathy. Diabetol Metab Syndr. 2009;1:10. doi:10.1186/1758-5996-1-10
32. Ogundele SB. Chronic kidney disease in sub-Saharan Africa. Saudi J Kidney Dis Transpl. 2018;29(5):1188. doi:10.4103/1319-2442.243945
33. Moher D, Liberati A, Tetzlaff J, Altman DG; Prisma Group. Reprint—preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther. 2009;89(9):873–880. doi:10.1093/ptj/89.9.873
34. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi:10.1007/s10654-010-9491-z
35. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi:10.1136/bmj.327.7414.557
36. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed‐effect and random‐effects models for meta‐analysis. Res Synthesis Methods. 2010;1(2):97–111. doi:10.1002/jrsm.12
37. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi:10.2307/2533446
38. Higgins JP. Commentary: heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. 2008;37(5):1158–1160. doi:10.1093/ije/dyn204
39. Rasmussen JB, Thomsen JA, Rossing P, Parkinson S, Christensen DL, Bygbjerg IC. Diabetes mellitus, hypertension and albuminuria in rural Z ambia: a hospital‐based survey. Trop Med Int Health. 2013;18(9):1080–1084. doi:10.1111/tmi.12139
40. Rahlenbeck SI, Gebre-Yohannes A. Prevalence and epidemiology of micro-and macroalbuminuria in Ethiopian diabetic patients. J Diabetes Complications. 1997;11(6):343–349. doi:10.1016/S1056-8727(96)00122-5
41. Muse AI, Osman MO, Wedajo GT, et al. Prevalence of microalbuminuria and associated factors among type 2 diabetes mellitus patients in Jigjiga Town Public Hospitals, Somali Regional State, Ethiopia. Res Square. 2020. doi:10.21203/rs.3.rs-936394/v1
42. Ogiator M, Ojobi J, Ijachi O, Asor P. Association between microalbuminuria and hypertension among type 2 diabetes mellitus patients attending Benue State University Teaching Hospital, Makurdi, North Central, Nigeria. Int J Med Rev Case Rep. 2020;4(5):24.
43. Ufuoma C, Ngozi JC, Kester AD, Godwin YD. Prevalence and risk factors of microalbuminuria among type 2 diabetes mellitus: a hospital-based study from, Warri, Nigeria. Sahel Med J. 2016;19(1):16. doi:10.4103/1118-8561.181889
44. Halliru HA, Musa B, Dahiru SD, Koki YA, Adamu S, Adamu SM. Microalbuminuria as an index of diabetic nephropathy among chronic diabetic patients in Gumel, North Western Nigeria. J Diabetol. 2016;7(3):155.
45. Erasmus RT, Oyeyinka G, Arije A. Microalbuminuria in non-insulin-dependent (type 2) Nigerian diabetics: relation to glycaemic control, blood pressure and retinopathy. Postgrad Med J. 1992;68(802):638–642. doi:10.1136/pgmj.68.802.638
46. Bissong ME, Teke GN, Sanje M, Goneh H, Foka FE, Kamga HL. Microalbuminuria in diabetic patients in the Bamenda health district. Sci J Clin Med. 2017;6(4):63–67. doi:10.11648/j.sjcm.20170604.14
47. Efundem NT, Assob JC, Feteh VF, Choukem SP. Prevalence and associations of microalbuminuria in proteinuria-negative patients with type 2 diabetes in two regional hospitals in Cameroon: a cross-sectional study. BMC Res Notes. 2017;10(1):1–5. doi:10.1186/s13104-017-2804-5
48. Rahamtalla FA, Elagib AA, Mahdi A, Ahmed SM. Prevalence of microalbuminuria among sudanese type 2 diabetic patients at elmusbah center at ombadda—omdurman. IOSR J Pharm. 2012;2(5):51–55.
49. Lutale JJ, Thordarson H, Abbas ZG, Vetvik K. Microalbuminuria among type 1 and type 2 diabetic patients of African origin in Dar es Salaam, Tanzania. BMC Nephrol. 2007;8(1):1–8. doi:10.1186/1471-2369-8-2
50. Ghosh S, Lyaruu I, Yeates K. Prevalence and factors associated with microalbuminuria in type 2 diabetic patients at a diabetes clinic in northern Tanzania. Afri J Diabetes Med. 2012;20(2):247.
51. Kantarama E, Uwizeye D, Mselle T. Prevalence and correlates of microalbuminuria among type 2 diabetes patients at Muhimbili National Hospital, Dar es Salaam, Tanzania. Rwanda J Med Health Sci. 2021;4(1):84–97. doi:10.4314/rjmhs.v4i1.7
52. Martin M, Edrisa M, SSinabulya I, Samuel K, Frank M, Kiiza MC. Microalbuminuria among newly diagnosed diabetic patients at mulago national referral hospital in Uganda: a cross sectional study. J Obes Weight Loss Med. 2018;4(1). doi:10.23937/2572-4010.1510021
53. Kiconco R, Rugera SP, Kiwanuka GN. Microalbuminuria and traditional serum biomarkers of nephropathy among diabetic patients at Mbarara regional referral hospital in south western Uganda. J Diabetes Res. 2019;2019:1–7. doi:10.1155/2019/3534260
54. Lubwama SK. Prevalence and factors associated with microalbuminuria in children and adolescents with Type 1 Diabetes in Mulago and Nsambya Hospitals in Uganda (Doctoral dissertation); Makerere University; 2022. Available from: http://hdl.handle.net/10570/10104.
55. Kalk WJ, Raal FJ, Joffe BI. The prevalence and incidence of and risk factors for, micro-albuminuria among urban Africans with type 1 diabetes in South Africa: an inter-ethnic study. Int J Diabetes Mellit. 2010;2(3):148–153. doi:10.1016/j.ijdm.2010.10.003
56. Molefe-Baikai OJ, Molefi M, Cainelli F, Rwegerera GM. The prevalence of microalbuminuria and associated factors among patients with type 2 diabetes mellitus in Botswana. Niger J Clin Pract. 2018;21(11):1430–1437. doi:10.4103/njcp.njcp_224_18
57. Ramaphane T, Gezmu AM, Tefera E, et al. Prevalence and factors associated with microalbuminuria in pediatric patients with type 1 diabetes mellitus at a large tertiary-level hospital in Botswana. Diabetes Metabol Syndr Obes. 2021;14:4415. doi:10.2147/DMSO.S322847
58. Fetni S, Goudjil T, Zekri S, Lemdaoui MC. the diagnosis of diabetic kidney disease; frequencies of urinary microalbumin and creatinine ratios, and the establishment of causal relationships between lipid and glycemic levels and obesity in an Algerian population. Acta Medica. 2021;37:2433. doi:10.19193/0393-6384_2021_5_377
59. Eghan BA, Frempong MT, Adjei-Poku M. Prevalence and predictors of microalbuminuria in patients with diabetes mellitus: a cross-sectional observational study in Kumasi, Ghana. Ethn Dis. 2007;17(4):726.
60. Amolo PA. The prevalence of microalbuminuria in children and adolescents with type 1 Diabetes mellitus at the outpatient clinic in Kenyatta National Hospital (Doctoral dissertation), University of Nairobi; 2021. Available from: http://erepository.uonbi.ac.ke:8080/xmlui/handle/11295/25090.
61. Omar MA, Rezk MM, El-Kafoury AA, Kandil MS. Microalbuminuria and glycated hemoglobin in children with type 1 diabetes mellitus. Alexandria J Med. 2015;51(1):83–88. doi:10.1016/j.ajme.2014.04.005
62. Djiby S, Demba D, Assane NM, et al. Prevalence of microalbuminury and associated risk factors in a population of diabetics followed at the Marc Sankale Center of Dakar. Open J Internal Med. 2018;8(1):24–32. doi:10.4236/ojim.2018.81004
63. Chaamba SG. Microalbuminuria and serum creatinine in type 2 diabetes mellitus patients attending renal clinic at university teaching hospital in Lusaka, Zambia (Doctoral dissertation). The University of Zambia; 2019. Available from: http://dspace.unza.zm/handle/123456789/6184.
64. Sobngwi E, Mbanya JC, Moukouri EN, Ngu KB. Microalbuminuria and retinopathy in a diabetic population of Cameroon. Diabetes Res Clin Pract. 1999;44(3):191–196. doi:10.1016/S0168-8227(99)00052-2
65. Mohamed AA. Prevalence of Microalbuminuria & Retinopathy among adolescents with Insulin Dependent Diabetes Mellitus (IDDM) (Doctoral dissertation). University of Khartoum; 2005.
66. Bunza FU, Mainasara AS, Dallatu MK, Bunza JM, Wasagu IZ. Prevalence of microalbuminuria among diabetic patients in Usmanu Danfodiyo University Teaching Hospital, Sokoto. Bayero J Pure Appl Sci. 2014;7(1):1–5. doi:10.4314/bajopas.v7i1.1
67. Yarhere IE, Jaja T, Anolue M. Microalbuminuria in type 1 diabetes mellitus children in University of Port Harcourt Teaching Hospital, Nigeria. Pan Afri Med J. 2020;36(1). doi:10.11604/pamj.2020.36.161.23782
68. Raji L. Recommendations for the management of special populations, renal disease in diabetes. Am J Hypertens. 2003;16:46–49. doi:10.1016/j.amjhyper.2003.07.006
69. Weir MR. Microalbuminuria in type 2 diabetes: an important overlooked cardiovascular risk factor. J Clin Hypertens. 2004;6:134–143. doi:10.1111/j.1524-6175.2004.02524.x
70. Parving HH, Lewis JB, Ravid M, Remuzzi G, Hunsicker LG. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int. 2006;69(11):2057–2063. doi:10.1038/sj.ki.5000377
71. Shin JI, Chang AR, Grams ME, et al. Albuminuria testing in hypertension and diabetes: an individual-participant data meta-analysis in a global consortium. Hypertension. 2021;78(4):1042–1052. doi:10.1161/HYPERTENSIONAHA.121.17323
72. Ahmedani MY, Hydrie MZ, Iqbal A, Gul A, Mirza WB, Basit A. Prevalence of microalbuminuria in type 2 diabetic patients in Karachi: Pakistan a multi-center study. Hypertension. 2005;2194:99–107.
73. Pasko N, Toti F, Strakosha A, et al. Prevalence of microalbuminuria and risk factor analysis in type 2 diabetes patients in Albania: the need for accurate and early diagnosis of diabetic nephropathy. Hippokratia. 2013;17(4):337.
74. Alzaid AA, Sobki S, De Silva V. Prevalence of microalbuminuria in Saudi Arabians with non-insulin-dependent diabetes mellitus: a clinic-based study. Diabetes Res Clin Pract. 1994;26(2):115–120. doi:10.1016/0168-8227(94)90148-1
75. Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus: a systematic overview 17 of the literature. Arch Intern Med. 1997;157(13):1413–1418. doi:10.1001/archinte.1997.00440340025002
76. Gall MA, Rossing P, Skøtt P, et al. Prevalence of micro-and macroalbuminuria, arterial hypertension, retinopathy and large vessel disease in European type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1991;34(9):655–661. doi:10.1007/BF00400995
77. Atkins RC, Polkinghorne KR, Briganti EM, Shaw JE, Zimmet PZ, Chadban SJ. Prevalence of albuminuria in Australia: the AusDiab kidney study. Kidney Int. 2004;66:S22–4. doi:10.1111/j.1523-1755.2004.09206.x
78. Kanakamani J, Ammini AC, Gupta N, Dwivedi SN. Prevalence of microalbuminuria among patients with type 2 diabetes mellitus—a hospital-based study from north India. Diabetes Technol Ther. 2010;12(2):161–166. doi:10.1089/dia.2009.0133
79. Afkhami-Ardekani M, Modarresi M, Amirchaghmaghi E. Prevalence of microalbuminuria and its risk factors in type 2 diabetic patients. Indian J Nephrol. 2008;18(3):112. doi:10.4103/0971-4065.43690
80. Klein R, Klein BE, Moss SE. Prevalence of microalbuminuria in older-onset diabetes. Diabetes Care. 1993;16(10):1325–1330. doi:10.2337/diacare.16.10.1325
81. Parving HH, Persson F, Rossing P. Microalbuminuria: a parameter that has changed diabetes care. Diabetes Res Clin Pract. 2015;107(1):1–8. doi:10.1016/j.diabres.2014.10.014
82. Al-Maskari F, El-Sadig M, Obineche E. Prevalence and determinants of microalbuminuria among diabetic patients in the United Arab Emirates. BMC Nephrol. 2008;9(1):1–8. doi:10.1186/1471-2369-9-1
83. Warram JH, Gearin G, Laffel L, Krolewski AS. Effect of duration of type 1 diabetes on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. J Am Soc Nephrol. 1996;7:930–937. doi:10.1681/ASN.V76930
84. Gross JL, De Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28(1):164–176. doi:10.2337/diacare.28.1.164
85. Dabla PK. Renal function in diabetic nephropathy. World J Diabetes. 2010;1(2):48–56. doi:10.4239/wjd.v1.i2.48
86. Toyama T, Furuichi K, Ninomiya T, et al. The impacts of albuminuria and low eGFR on the risk of cardiovascular death, all-cause mortality, and renal events in diabetic patients: meta-analysis. PLoS One. 2013;8(8):e71810. doi:10.1371/journal.pone.0071810
87. Adebisi SA, Okesina AB, Abu EO. Microalbuminuria in type 2 diabetic patients in ilorin, Nigeria. 2001.
88. Chowta NK, Pant P, Chowta MN. Microalbuminuria in diabetes mellitus: association with age, sex, weight, and creatinine clearance. Indian J Nephrol. 2009;19(2):53. doi:10.4103/0971-4065.53322
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
