Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 13
Prevalence of Intestinal Parasitic Infection and Associated Factors Among HAART Initiated Children Attending at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia
Authors Bayleyegn B , Woldu B, Yalew A, Kasew D , Asrie F
Received 19 October 2020
Accepted for publication 12 December 2020
Published 25 January 2021 Volume 2021:13 Pages 81—90
DOI https://doi.org/10.2147/HIV.S287659
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bassel Sawaya
Biruk Bayleyegn,1 Berhanu Woldu,1 Aregawi Yalew,1 Desie Kasew,2 Fikir Asrie1
1Department of Hematology and Immunohematology, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia; 2Department of Medical Microbiology, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia
Correspondence: Biruk Bayleyegn Email [email protected]
Background: Human immunodeficiency virus (HIV) and intestinal parasites co-infections are the most common causes of clinical illness and death, especially for children living in resource constrained setting. Therefore, the aim of this study was to determine the prevalence and associated factors of intestinal parasites among highly active anti-retroviral therapy (HAART) initiated children.
Methods: Cross-sectional study was conducted among 255 HAART initiated HIV-infected children at the University of Gondar Comprehensive Specialized Hospital from January to April 2020. Socio-demographic characteristics were collected using a structured questionnaire via a face-to-face interview. Clinical data of the children were collected by reviewing the medical records. Venous blood was collected for complete blood counts, viral load determination, and blood film examination. Flotation concentration technique was done in addition to direct wet mount for parasitological examination. Bi-variable and multi-variable logistic regression analysis were used to check the presence of significant association, and P-value< 0.05 was considered as statistically significant.
Results: The overall prevalence of intestinal parasite infection (IPI) among the study participants was 22.4% (95% CI=17– 28%). The presence of opportunistic infection (AOR=2.09 95% CI=1.81– 5.43), no eating under-cooked animal products (AOR=0.38 95% CI=0.16– 0.94), male sex (AOR=0.45 95% CI=0.22– 0.90), viral load rate > 1,000 copies/mL (AOR=1.80 95% CI=1.67– 4.19), and cytopenia (AOR=2.71 95% CI=1.59– 12.25) showed significant association with the prevalence of IPI.
Conclusion: Entamoeba histolytica and Ascaris lumbricoides were the most prevalent intestinal parasites among HAART initiated children. Among HAART initiated children, IPI were associated with gender, cytopenia, viral load, undercooked animal products, and the presence of opportunistic infections. Therefore, health education, prompt treatment, and regular deworming should be implemented to alleviate the burden of intestinal parasites in HIV-infected children.
Keywords: intestinal parasite, HIV, children, Ethiopia
Introduction
Intestinal parasitic infection is one of the major childhood health problems worldwide, particularly in the poorest and most deprived communities.1 Human immunodeficiency virus is an immunosuppressive infection that degrades the body’s defense of the host and predisposes the patient to other infections including intestinal parasitic attacks.2 Globally, there were 39.9 million people living with HIV by 2018. Among these, 1.7 million were under 15 years old. About 160,000 new HIV infections and 100,000 acquired immunodeficiency syndrome (AIDS) related death of these children have been reported. Regarding Ethiopia, there were 36,000 children under 15 years living with HIV, with 6,200 new infections and 1,800 AIDS related deaths.3 Despite the sustained lives due to antiretroviral therapy, about 80% of HIV/AIDS patients depart from their lives due to infections such as IPI related to AIDS.4
Intestinal parasites and HIV co-infections are the most common causes of clinical illness and death worldwide, particularly in resource limited settings. In SSA the parasitic infection rate is remarkably high, with some areas reporting 95% incidence,5,6 which underscores the IPI are poverty associated.7 The most important intestinal helminths are soil transmitted helminths (STHs), such as Ascaris lumbricoides, Trichuris Trichiura, Strongyloides stercoralis, and hookworms. Another mode of transmission for Ascaris and Trichuris trichiura were by faeco–oral route and hookworm which is transmitted by percutaneous or oral routes.8 Intracellular intestinal opportunistic infestation (Cryptosporidium paravum, Isospora belli, and Microsporidium spp) and non-opportunistic extracellular intestinal protozoan infestation (Entamoeba histolytica and Giardia lamblia) can result in serious problems in HIV-positive patients. This is because HIV causes a progressive decline of the mucosal immunological defense mechanism and alteration of the production of IgA antibodies.9
Even if people of all age groups are affected, children are more vulnerable to intestinal parasitic infections. Especially in these immunosuppressed children, intestinal parasites can have a devastating consequence, such as iron deficiency (anemia), malabsorption of fat and vitamin A&B12 deficiency, diarrhea, delayed growth, physical, and mental health problems.10–13
A variety of risk factors including poverty, climatic conditions, poor personal and environmental hygiene, lack of clean water, lack of toilet, inadequate healthcare, and lack of awareness about transmission are key factors contributing to the increased prevalence of parasitic infection.14,15
Control of IPI is an important tool to combat the HIV pandemic;6 hence Ethiopia has been practicing a health extension program since 2004. But Ethiopia contributes to the highest burden of IPI, which accounts for 8% of the global STH infections16 and the epidemiology of IPI is too worrisome, ranging from 54.5–84%14 and that reaches 95% in children.17
Although there are prevalence studies on IPI in different localities among different segments of the population, the burden of intestinal parasitic infections and associated factors in children living with HIV/AIDS and taking HAART seems to be overlooked in the study area.18 Hence, this study was aimed to determine the prevalence and associated risk factors of IPI among HAART experienced HIV-infected children at the University of Gondar Comprehensive Specialized Hospital (UOGCSH), Northwest Ethiopia.
Materials and Methods
Study Area, Design, and Period
A hospital-based cross-sectional study was conducted at the University of Gondar Comprehensive Specialized Hospital from January to April 2020 among HIV-infected children. The hospital is one of the pioneering healthcare institutions in Ethiopia, which provides both teaching and referral services to more than 7 million populations in the region and from the neighboring regions. The hospital provides multiple healthcare services such as psychiatric clinic, dialysis, leishmaniasis, medical, surgical, gynecological, antenatal care clinic, as well as HIV/AIDS prevention, treatment, and prognostic activities.
Study Population
The study population recruited in this study was all HIV-infected children under 15 years old, who were on HAART and had regular follow-up visits at the UoGCSH ART clinic. HIV-positive children who had taken antiparasitic treatment in the last 15 days or took deworming medication and those children without a legal guardian or unaccompanied children during the study period were excluded from the study.
In order to have a representative sample of our entire pediatrics ART follow-up clinic, the sample size was determined using a single population proportion formula. The sample size was calculated using the formula: 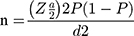 by considering a 16.9% reference prevalence19 with a 5% margin of error (d=±0.05) and 95% confidence level (Za/2=1.96). Then, computing with this formula and after applied 15% non-response rate the final sample size was 249, but for more accuracy 255 available samples were considered for analysis. The convenient sampling technique was used to recruit study participants.
by considering a 16.9% reference prevalence19 with a 5% margin of error (d=±0.05) and 95% confidence level (Za/2=1.96). Then, computing with this formula and after applied 15% non-response rate the final sample size was 249, but for more accuracy 255 available samples were considered for analysis. The convenient sampling technique was used to recruit study participants.
Data Collection
Socio-Demographic/Clinical Data Collection and Anthropometric Measurement
Socio-demographic characteristics of children and caregivers (such as age, gender, residence, educational status, family income, family size, family occupation, and life status) and history of diarrhea were collected using a structured questionnaire via a face-to-face interview. Diarrhea was defined as a minimum of three or more watery or loose stools within 24 hours.20 Clinical data of the children, such as HIV disease stage, OI, type of HAART, and HAART experience and duration of HAART were collected by reviewing the medical records.
Anthropometric measurements, including weight and height, were measured by trained clinical nurses using a digital scale with an attached wall-mounted stadiometer to the nearest 0.1 kg and 0.1 cm, respectively. Then Z-scores of nutritional indices, such as weight-for-age (WAZ), height-for-age (HAZ), and weight-for-height (WHZ) were calculated using World Health Organization (WHO) Anthro (for children aged ≤5 years) and Anthro-plus (for children aged >5 years) software. Since WAZ is an inadequate indicator for monitoring child growth >5 years due to its inability to distinguish between relative height and body mass, BMI-for-age is recommended by the WHO and USCDC to assess thinness/wasting in children and adolescents. Finally, children were classified as underweight when WAZ<−2 SD for under-5 children and BAZ<−2 SD for older children, and stunted when the HAZ scores were less than −2 SD.21
Laboratory Procedures
Hematological Parameters
About 5 mL of venous blood was collected in an EDTA anticoagulant coated test tube with a sterile disposable syringe and/or butterfly from each study participant. The blood samples were used for the complete blood count (CBC), viral load determination, and blood film examination. Only 2 mL of blood was analyzed for determining hematological parameters using a Sysmex KX21 hematology analyzer. Moreover, both thin and thick blood films using 10% Giemsa were examined for the detection and identification of malaria parasites and any quantitative as well as qualitative cell abnormality were reported by an experienced hematologist.
Viral Load Quantification
TaqMan® AMPLICOR HIV-1 MONITOR (Roche Molecular Systems), an advanced molecular technique, was used for the quantification of HIV-1 RNA in plasma collected by EDTA tubes containing plasma separator gel from each study participant. Then, the amount of circulating HIV was measured and reported as HIV RNA copies/mL of plasma by well-trained laboratory technologists.
Stool Collection and Examination
Approximately 3–5 grams fresh stool was collected by clean and dry plastic stool cup and transported in screw-capped cups in 5% formalin from a sample reception area to the laboratory. Test tub salt flotation concentration procedure was done for the detection of protozoan cysts, helminthic ova, and larvae, in addition to using the direct wet mount. About 2 grams of feces were emulsified with 10 mL of saturated salt solution in the test tube and stirred thoroughly. It was stirred well and more salt solution was added till a slight positive meniscus was observed. The tube was placed on a leveled surface of test tube rack with a glass coverslip been placed over the top of the tube. Any coarse matter which floated up was in contact with the coverslip. Then, after it was allowed to stand for 15 minutes, the coverslip was removed from the tube and placed on a labeled slide. The slide was examined for the detection and identification of eggs/cysts by a well experienced parasitologist.
Quality Assurance
To assure the quality of data, training was given for data collectors prior to data collection and daily close supervision was made during the data collection period. The questionnaire was pre-tested on 5% of the total sample size, and necessary modification was done. The blood sample was checked whether it was in the acceptance criteria. The performance of Sysmex KX-21 hematology analyzer and COBAS AmpliPrep/COBAS TaqMan HIV-1 test was checked according to the manufacturer’s instruction. Moreover, known malaria-positive and negative blood were used to check the quality and performance of the Giemsa staining reagent as well as the stool examination was performed after checking the specific gravity of the salt solution was above 1.25 using a Hydrometer once a week. Two readings were obtained for each measurement, and the mean of two anthropometric measurements was calculated and used for the analysis.
Data Analysis
The data were entered into EPI-info version 4.4 and were exported to the statistical package for social sciences (SPSS) version 20 software for analysis. Socio-demographic characteristics of the participants were presented as frequencies and percentages for the categorical variables. Multivariate logistic regression analysis was performed to estimate the odds ratio (OR) and 95% confidence interval (95% CI) to assess the associations between potential risk factors and prevalence of IPI. A P-value of <0.05 was considered to be statistically significant.
Ethical Consideration
Ethical approval was obtained from Ethical review committee of School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar. Furthermore, support and a permission letter were secured from UoGCSH. In accordance with the Declaration of Helsinki, written informed consent and/or assent were sought from each study participant and their family/guardians after an explanation of the purpose, the benefits and the possible risks of the study. It was made clear that participation in the study was purely on a voluntarily basis and refusal was possible. To ensure confidentiality of data, a unique code was assigned to each study participant, and those children positive with intestinal parasites were linked to the physicians working at ART Clinic for treatment.
Results
Socio-Demographic and Clinical Characteristics
A total of 255 HIV-positive children who were HAART initiated were recruited in this study, half of them were female (128, 50.2%). The median age of the participants was 13 years (IQR=10–14). Based on WHO clinical stage criteria of HIV, most of the study participants (245, 96.1%) were in WHO stage I. About 221 (86.7%) of study participants were under ART treatment for the duration of ≥1 year and 106 (41.6%) were taking a TDF containing HAART regimen. Nearly half (44%) of the study participants were stunted based on WHO nutritional assessment guidelines. In the current study, 29 (11.4%) of the study participants had a current history of OI, the commonest being TB infection, seen in 16 (6.3%), and pneumonia was seen in 12 (4.7%), followed by fungal infection in one(0.4). Consequently, 31.4% of the study participants had viral loads ≥1,000 copies/mL and we did not find any malaria-positive children (Table 1).
 |
Table 1 Socio-Demographic and Clinical Characteristics of HIV-Infected Children and Their Family/Caregivers Who Visited UoGCSH ART Clinic, Northwest Ethiopia, 2020 (N=255) |
Prevalence of Intestinal Parasitic Infection
The overall prevalence of IPI among HAART initiated children was 22.4% (57/255; 95% CI=17–28). The most prevalent type of parasite in this study was Entamoeba histolytica (35.1%), followed by 24.6% of Ascaris lumbricoides. Moreover, 14% of the study participants were infected by two concurrently intestinal parasites. Of them, Ascaris with Entamoeba histolytica and Hymenolepsis nana dual infection was found in 8.8% and 5.3% of HAART experienced children, respectively (Figure 1).
 |
Figure 1 Distribution of intestinal parasite detected among HAART experienced children attending at UOGCSH, Northwest Ethiopia, 2020. |
Associated Factors of Intestinal Parasites Among HAART Experienced Children
To determine the association, bivariable and multivariable logistic regression analysis was done. Based on the analysis, variables with a P-value less than 0.2 in bi-variable logistic regression were included in multivariable logistic regression. Hence, in multivariable logistic regression analysis the presence of OIs (AOR=2.09; 95% CI=1.81–5.43), being male (AOR=0.45; 95% CI=0.22–0.90), not eating under-cooked animal products (AOR=0.38; 95% CI=0.16–0.94), viral load rate >1,000 copies/mL, (AOR=1.80; 95% CI=1.67–4.19),and cytopenia (AOR=2.71; 95% CI=1.59–12.25) remained significantly associated with IPI among HAART experienced children (Table 2).
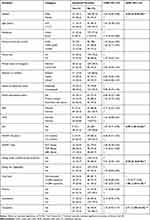 |
Table 2 Bi-Variable and Multi-Variable Logistic Regression Analysis for Associated Factors of IPI Among HAART experianced Children at UoGCSH, ART Clinic, Northwest Ethiopia, 2020 (N=255) |
Discussion
There was a high prevalence of both the helminths and the protozoan intestinal parasitic infestation, which is one of the leading causes of morbidity and mortality among HIV-infected children.1,22 The overall prevalence of intestinal parasites among HAART experienced children in the present study was 22.4% (95% CI=17–28%). This is in accordance with prevalences of 24.5% in the University of Gondar hospital, Ethiopia,23 19.2% in Nigeria,24 and 24.6% in Kinshasa.25 However, the prevalence is lower than studies conducted in Ethiopia (48%),26 Indonesia (57.1%),27 Egypt (94%),28 and Ovidius” University Constanta (94.9%).29 Moreover, our finding is higher than the result reported by Gebre et al19 (16.7%) in southern Ethiopia. The occurrence of this variation might be due to implementation of a periodic deworming program, variation in sample size, and the presence of multiple infection.
The prevalences of Entamoeba histolytic and Ascaris lumbricoides were 35.1% and 24.6%, respectively, which are higher than other intestinal parasites detected in this study. This is inconsistent with the study carried out in Kenya, Entamoeba histolytica (36.7%) being the common parasite,13 and the Democratic Republic of Sa˜o Tome´ and Principe, where Ascaris lumbricoides (27.6%) were the most prevalent parasite.30
The prevalence of cryptosporidium species infection is 3.5% in this study, comparable with a previous study among school-aged children in Western Uganda (4%).22 In the fact, Cryptosporidium species were considered as a significant pathogen and are more frequent in children with HIV who had persistent and chronic diarrhea. The result in this study showed less frequency as compared to the finding reported in Mozambique (12%) among diarrheic hospitalized children.31
The finding further revealed that 14% of study participants were infected by two intestinal parasite species and none of the children had polyparasitism. Ascaris with Entamoeba histolytica were the most prevalent coinfections (8.8%) among infected children, while the combination of Ascaris and Hymenolepsis nana dual infection was (5.3%). This is not in agreement with the finding in Malaysia suggesting that polyparasitism were 71.4% and concurrent infections with two parasite species were 54%.32 This observed difference may be due to environmental sanitation, socioeconomic, and cultural variation of the population. Additionally, most were likely associated with variation in diagnostic approaches, unlike the present study they were examined by using six different techniques (direct smear, formalin-ether sedimentation, Kato-Katz, Harada Mori, trichrome stain, and modified Ziehl Neelsen stain techniques) thereby increasing the detection rate.
The occurrence of parasitic infection was lower in male children (18.8%) than their female counterparts (26.6%) and the difference was statistically significant (AOR=0.45; 95% CI=0.22–0.90; P<0.01). This finding was in agreement with the study done by Abossie and Seid,33 and Khadka et al34 reported that the prevalence of parasitic infection was higher in girls than in boys regardless of significant association. But this in contrast to a study done by Gebretsadik et al.35 This is most likely to be influenced by cultural values that parents who give more priorities to the health of their sons than their daughters and females may come into contact with more contaminated water and food than males, which may increase the risk of exposure to parasites.
The burden of IPI was higher among the study participants who were eating under-cooked animal products. The habit of not eating under-cooked animal product decreased the risk of IPI by 62% (AOR=0.38; 95% CI=0.16–0.94). Many more studies have reported that having the habit of consuming uncooked meat might increase the risk of exposure to human helminths.26,36,37 Moreover, the odds of IPI among HAART initiated children that had concurrent OIs increased 2.09-fold as compared with children free from OI (AOR=2.09; 95% CI=1.81–5.43). This observation could be explained by the fact that immunocompromised children are prone to serious opportunistic infection including TB and pneumonia. Hence, these superimposed infections are occurring due to the extent of deterioration of the immune system and then could predict the prevalence of enteric parasites.27,38
The current study also revealed that children who had a viral load >1,000 copies/mL were 1.8-times more likely to be infected with intestinal parasites (AOR=1.80; 95% CI=1.67–4.19; P=0.01). Despite the absence of a significant association, this is in agreement with the study conducted by Fregonesi et al,39 which suggested that a high percentage of parasite-infected children were observed among children with high viral loads. It has been documented that high viral replication may deteriorate the immune function and thereby accelerate susceptibility to IPI.40–42
Furthermore, HAART experienced children who had cytopenia on their blood were 2.71-times more at risk of an increase in the prevalence of IPI (AOR=2.71; 95% CI=1.59–12.25; P=0.001). This finding is similar with a previous study conducted among school children in Egypt,43 and Southern Angola44 there was a strong association between anemia and IPI, and this was probably due to that lower blood cell counts such as cytopenia may be lowering the immunity to infections which leads to extend the intestinal parasite infection.
Limitations of the Study
The main limitation of the present study was it's single site and cross-sectional nature of study design, which is difficult to making true relationships between intestinal parasites and its associated factors, as it is a temporal association. The other limitation is also unable to perform advanced diagnostic technique including modified Ziehl Neelsen and trichrome stain techniques due to budget restrictions, which is sensitive enough to recognize protozoan intestinal parasites, especially in children.
Conclusion and Recommendation
This study found that the prevalence of IPI was relatively high, and it was noted that Entamoeba histolytica and Ascaris lumbricoides were more common among HAART initiated children. Factors such as gender, eating under-cooked animal products, the presence of OI, viral load >1,000 copies/mL, and cytopenia showed a significant association with the prevalence of IPI. Therefore, health education, prompt treatment, and regular deworming of children should be implemented to alleviate the burden of intestinal parasites in HIV-infected children. Moreover, the large-scale longitudinal study is recommended with the implementation of more sensitive diagnostic techniques that can be helpful for a better diagnosis of intestinal parasites.
Abbreviations
AIDS, acquired immunodeficiency syndrome; HAZ, height-for-age; HAART, highly active anti-retroviral therapy; HIV, human immunodeficiency virus; IPI, intestinal parasite infection; STHs, soil transmitted helminths; SSA, Sub-Saharan Africa; WHZ, weight-for-height; WAZ, weight-for-age; WHO, World Health Organization.
Data Sharing Statement
All relevant data are fully available without restriction within the manuscript.
Acknowledgment
The authors would like to acknowledge the study participants who invested their respective time and participated in this study. Also, heartful gratitude goes to the Department of Hematology and Immunohematology School of Biomedical and Laboratory Sciences, College of Medicine and Health science, University of Gondar for all necessary supports to this study.
Funding
No funding source.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Zemene T, Shiferaw MB. Prevalence of intestinal parasitic infections in children under the age of 5 years attending the Debre Birhan referral hospital, North Shoa, Ethiopia. BMC Res Notes. 2018;11(1):58. doi:10.1186/s13104-018-3166-3
2. Nkenfou CN, Nana CT, Payne VK, Sued O. Intestinal parasitic infections in HIV infected and non-infected patients in a low HIV prevalence region, West-Cameroon. PLoS One. 2013;8(2):2. doi:10.1371/journal.pone.0057914
3. HIV/AIDS JUNPo. UNAIDS Data 2019. Geneva: UNAIDS; 2019.
4. Awulachew E, Diriba K, Gemede A, Wudneh F. Prevalence of cryptosporidium species among HIV/AIDS patients in Sub Saharan Africa; systematic review and meta-analysis. J HIV Clin Sci Res. 2020;7(1):006–012. doi:10.17352/2455-3786.000030
5. Cerveja BZ, Tucuzo RM, Madureira AC, et al. Prevalence of intestinal parasites among HIV infected and HIV uninfected patients treated at the 1 de Maio Health Centre in Maputo, Mozambique. EC Microbiol. 2017;9(6):231.
6. Bangert M, Molyneux DH, Lindsay SW, Fitzpatrick C, Engels D. The cross-cutting contribution of the end of neglected tropical diseases to the sustainable development goals. Infect Dis Poverty. 2017;6(1):73. doi:10.1186/s40249-017-0288-0
7. Ojha SC, Jaide C, Jinawath N, Rotjanapan P, Baral P. Geohelminths: public health significance. J Infect Dev Ctries. 2014;8(01):005–016. doi:10.3855/jidc.3183
8. Hotez PJ, Fenwick A, Savioli L, Molyneux DH. Rescuing the bottom billion through control of neglected tropical diseases. Lancet. 2009;373(9674):1570–1575. doi:10.1016/S0140-6736(09)60233-6
9. Yiltok ES, Pam SD, Oguche S, Banwat EB, Yohanna S. Intestinal parasites and human immunodeficiency virus (HIV) status of children in Jos, Nigeria. J AIDS HIV Res. 2014;6(3):72–78. doi:10.5897/JAHR2013.0281
10. Mehraj V, Hatcher J, Akhtar S, Rafique G, Beg MA, Sutherland CJ. Prevalence and factors associated with intestinal parasitic infection among children in an urban slum of Karachi. PLoS One. 2008;3(11):11. doi:10.1371/journal.pone.0003680
11. Galgamuwa LS, Iddawela D, Dharmaratne SD. Intestinal protozoa infections, associated risk factors and clinical features among children in a low-income tea plantation community in Sri Lanka. Int J Community Med Public Health. 2016;3(9):2452–2458. doi:10.18203/2394-6040.ijcmph20163053
12. Cañete R, Díaz MM, García RA, Martinez PML, Ponce FM. Intestinal parasites in children from a day care centre in Matanzas City, Cuba. PLoS One. 2012;7(12):e51394.
13. Mbae CK, Nokes DJ, Mulinge E, Nyambura J, Waruru A, Kariuki S. Intestinal parasitic infections in children presenting with diarrhoea in outpatient and inpatient settings in an informal settlement of Nairobi, Kenya. BMC Infect Dis. 2013;13(1):243. doi:10.1186/1471-2334-13-243
14. Sitotaw B, Shiferaw W. Prevalence of intestinal parasitic infections and associated risk factors among the first-cycle primary schoolchildren in Sasiga District, Southwest Ethiopia. J Parasitol Res. 2020;2020.
15. Mengist HM, Taye B, Tsegaye A, Wormley FL
16. Sahiledengle B, Beker S, Girum Y. Prevalence and risk factors of intestinal parasites among primary school children in Shashamane town, southern Ethiopia. Can J Infect Dis Med Microbiolol. 2020;9(3):55–61.
17. Hailegebriel T. Prevalence of intestinal parasitic infections and associated risk factors among students at Dona Berber primary school, Bahir Dar, Ethiopia. BMC Infect Dis. 2017;17(1):362. doi:10.1186/s12879-017-2466-x
18. Tian L-G, Chen J-X, Wang T-P, et al. Co-infection of HIV and intestinal parasites in rural area of China. Parasit Vectors. 2012;5(1):36. doi:10.1186/1756-3305-5-36
19. Gebre B, Alemayehu T, Girma M, Ayalew F, Tadesse BT, Shemelis T. Cryptosporidiosis and other intestinal parasitic infections and concomitant threats among HIV-infected children in Southern Ethiopia receiving first-line antiretroviral therapy. HIV AIDS (Auckl). 2019;11:299.
20. Van Eijk AM, Brooks JT, Adcock PM, et al. Diarrhea in children less than two years of age with known HIV status in Kisumu, Kenya. Int J Infect Dis. 2010;14(3):e220–e225. doi:10.1016/j.ijid.2009.06.001
21. Koye DN, Ayele TA, Zeleke BM. Predictors of mortality among children on antiretroviral therapy at a referral hospital, Northwest Ethiopia: a retrospective follow up study. BMC pediatr. 2012;12(1):161. doi:10.1186/1471-2431-12-161
22. Ntulume I, Tibyangye J, Aliero AA, Banson BJ. Prevalence of intestinal protozoan infections and the associated risk factors among children in Bushenyi District, Western Uganda. Int J Trop Dis Heal. 2017;23(2):1–9. doi:10.9734/IJTDH/2017/33255
23. Enawgaw B, Alem M, Melku M, Addis Z, Terefe B, Yitayew G. Prevalence and associated risk factors of anemia among HIV infected children attending Gondar university hospital, Northwest Ethiopia: a cross sectional study. BMC Hematol. 2015;15(1):12. doi:10.1186/s12878-015-0032-6
24. Oyedeji OA, Adejuyigbe E, Oninla SO, Akindele AA, Adedokun SA, Agelebe E. Intestinal parasitoses in HIV infected children in a Nigerian Tertiary Hospital. J Clin Diagn Res. 2015;9(11):SC01.
25. Kangoy JM. Déterminants des parasitoses intestinales chez les enfants VIH-positifs à kinshasa determinants of intestinal parasitosis with HIV positive in Children, Kinshasa. Ann Afr Med. 2019;12(4):e3411.
26. Chelkeba L, Mekonnen Z, Alemu Y, Emana D. Epidemiology of intestinal parasitic infections in preschool and school-aged Ethiopian children: a systematic review and meta-analysis. BMC Public Health. 2020;20(1):117. doi:10.1186/s12889-020-8222-y
27. Idris NS, Dwipoerwantoro PG, Kurniawan A, Said M. Intestinal parasitic infection of immunocompromised children with diarrhoea: clinical profile and therapeutic response. J Infect Dev Ctries. 2010;4(05):309–317. doi:10.3855/jidc.275
28. Abdel-Hafeez EH, Ahmad AK, Ali BA, Moslam FA. Opportunistic parasites among immunosuppressed children in Minia District, Egypt. Korean J Parasitol. 2012;50(1):57. doi:10.3347/kjp.2012.50.1.57
29. Cambrea SC, Gorun E, Ilie MM, Halichidis S. Evolution of parasitic diseases in a collectivity of HIV positive children. ARS Medica Tomitana. 2013;19(4):202–205. doi:10.2478/arsm-2013-0036
30. Lobo ML, Augusto J, Antunes F, et al. Cryptosporidium spp., giardia duodenalis, enterocytozoon bieneusi and other intestinal parasites in young children in lobata province, democratic Republic of São Tomé and principe. PLoS One. 2014;9(5):e97708. doi:10.1371/journal.pone.0097708
31. Bauhofer AFL, Cossa-Moiane I, Marques S, et al. Intestinal protozoan infections among children 0–168 months with diarrhea in Mozambique: June 2014-January 2018. PLoS Negl Trop Dis. 2020;14(4):e0008195. doi:10.1371/journal.pntd.0008195
32. Al-Delaimy AK, Al-Mekhlafi HM, Nasr NA, et al. Epidemiology of intestinal polyparasitism among orang asli school children in rural Malaysia. PLoS Negl Trop Dis. 2014;8(8):e3074. doi:10.1371/journal.pntd.0003074
33. Abossie A, Seid M. Assessment of the prevalence of intestinal parasitosis and associated risk factors among primary school children in Chencha town, Southern Ethiopia. BMC Public Health. 2014;14(1):166. doi:10.1186/1471-2458-14-166
34. Khadka KS, Kaphle HP, Gurung K, Shah Y, Sigdel M. Study of intestinal parasitosis among school going children in Pokhara, Nepal. J Health Allied Sci. 2013;3(1):47–50. doi:10.37107/jhas.54
35. Gebretsadik D, Tesfaye M, Adamu A, Zewde G. Prevalence of intestinal parasitic infection and its associated factors among school children in two primary schools in Harbu Town, North East Ethiopia: Cross-Sectional Study. Pediatric Health Med Ther. 2020;11:179–188. doi:10.2147/PHMT.S252061
36. Feleke DG, Wage EK, Getachew T, Gedefie A. Intestinal parasitic infections and associated factors among street dwellers’ in Dessie town, North-East Ethiopia: a cross sectional study. BMC Res Notes. 2019;12(1):262. doi:10.1186/s13104-019-4302-4
37. Jejaw A, Zeynudin A, Zemene E, Belay T. Status of intestinal parasitic infections among residents of Jimma Town, Ethiopia. BMC Res Notes. 2014;7(1):502. doi:10.1186/1756-0500-7-502
38. Gupta K, Bala M, Deb M, Muralidhar S, Sharma D. Prevalence of intestinal parasitic infections in HIV-infected individuals and their relationship with immune status. Indian J Med Microbiol. 2013;31(2):161.
39. Fregonesi BM, Suzuki MN, Machado CS, et al. Emergent and re-emergent parasites in HIV-infected children: immunological and socio-environmental conditions that are involved in the transmission of Giardia spp. and Cryptosporidium spp. Rev Soc Bras Med Trop. 2015;48(6):753–758. doi:10.1590/0037-8682-0119-2015
40. Mulu A, Maier M, Liebert UG. Deworming of intestinal helminths reduces HIV-1 subtype C viremia in chronically co-infected individuals. Int J Infect Dis. 2013;17(10):e897–e901. doi:10.1016/j.ijid.2013.03.022
41. Nissapatorn V, Sawangjaroen N. Parasitic infections in HIV infected individuals: diagnostic & therapeutic challenges. Indian J Med Res. 2011;134(6):878. doi:10.4103/0971-5916.92633
42. Eligio-García L, Cano-Estrada A, Cruz C, Jiménez-Cardoso E. Association of viral load and CD4+ count with infection of intestinal emerging parasites in HIV patients. J AIDS HIV Res. 2016;7(12). doi:10.4172/2155-6113.1000644
43. Bayoumy A, Hassan K, Geneidy M, Metawea A. Impact of intestinal parasites on hematological parameters among school children in Gharbia Governorate, Egypt. J Egypt Soc Parasitol. 2018;48(1):157–164.
44. Oliveira D, Ferreira FS, Atouguia J, Fortes F, Guerra A, Centeno-Lima S. Infection by intestinal parasites, stunting and anemia in school-aged children from southern Angola. PLoS One. 2015;10(9):e0137327. doi:10.1371/journal.pone.0137327
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
