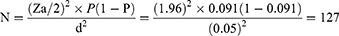Back to Journals » Infection and Drug Resistance » Volume 16
Prevalence, Antibiotic Susceptibility Profile and Associated Factors of Group A Streptococcal pharyngitis Among Pediatric Patients with Acute Pharyngitis in Gondar, Northwest Ethiopia
Authors Tadesse M, Hailu Y , Biset S , Ferede G, Gelaw B
Received 22 December 2022
Accepted for publication 16 March 2023
Published 22 March 2023 Volume 2023:16 Pages 1637—1648
DOI https://doi.org/10.2147/IDR.S402292
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Molla Tadesse,1 Yohanes Hailu,2 Sirak Biset,3 Getachew Ferede,3 Baye Gelaw3
1Department of Medical Microbiology, Jigjiga University, Jigjiga, Ethiopia; 2Department of Pediatrics and Child Health, School of Medicine, University of Gondar, Gondar, Ethiopia; 3Department of Medical Microbiology, School of BioMedical and Laboratory Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Sirak Biset, Tel +251-911-598-568, Email [email protected]
Background: Streptococcus pyogenes (S. pyogenes) or group A streptococcus is a common cause of bacterial pharyngitis in children. Since it is difficult to distinguish between viral and bacterial pharyngitis using solely signs and symptoms, culture-based diagnosis and treatment are critical for avoiding serious complications. Therefore, this study aimed to determine the prevalence, antimicrobial susceptibility patterns, and associated factors of S. pyogenes among pediatric patients with acute pharyngitis.
Methods: A hospital-based cross-sectional study was conducted at the University of Gondar Comprehensive Specialized Hospital from April to June 2021. Standard microbiological procedures were used to collect and process throat swabs and to isolate and identify S. pyogenes. The disc diffusion method was used for antimicrobial susceptibility testing (AST).
Results: A total of 215 children with acute pharyngitis were included in this study. Of these, 23 (10.7%) were culture positive for S. pyogenes. The presence of an inflamed tonsil, tonsillar exudate, scalariform rash, and dysphagia were associated with streptococcal pharyngitis. Children aged 5 to 15 were more susceptible to streptococcal throat infection than younger children. Penicillin, vancomycin, chloramphenicol, clindamycin, and ceftriaxone were effective against 100%, 95.7%, 95.7%, 91%, and 87% of isolates, respectively. In contrast, 56.5%, 39.1%, and 30.4% of isolates showed at least reduced susceptibility to tetracycline, erythromycin, and azithromycin, respectively.
Conclusion: Streptococcus pyogenes is responsible for 10.7% of acute pharyngitis cases among pediatric patients in the study area. Although all isolates remain sensitive to penicillin, many showed reduced susceptibility to tetracycline and macrolides. Therefore, prior to antibiotic prescription, screening children with acute pharyngitis for S. pyogenes and testing the antibiotic susceptibility of isolates is recommended.
Keywords: bacterial pharyngitis, S. pyogenes, group A streptococcus, antibiotic resistance, Ethiopia
Two Letters to the Editor have been received and published for this article
Introduction
Streptococcus pyogenes (S. pyogenes) or group A streptococcus (GAS) is a Gram positive, β-hemolytic bacterium usually found in the skin and mucous membranes of the human host.1–3 GAS possesses a plethora of virulence factors such as proteins that contribute to its invasiveness in humans.4,5 It causes a wide range of diseases from superficial to invasive infections, and immune mediated post-infectious sequelae.6–10 Acute pharyngitis, which is often caused by viruses, is one of the most common complaints that physicians encounter in the ambulatory care setting, accounting for around 12 million visits annually.11 This infection is common in school-age children, peaking during winter and early spring, and primarily spreads through direct contact with nasal secretions or saliva from infected patients in crowded settings.3,10,12–14 GAS is responsible for most of the bacterial pharyngitis (strep throat) cases (~15–30%) among children aged 5 to 15 years.9,15,16 Those with strep throat may experience a sudden onset of fever, sore throat, tonsillar exudates, and cervical adenopathy without the typical viral infection symptoms of new-onset rhinitis, laryngitis, or cough.14,17,18
Group A streptococcus is an important cause of morbidity and mortality worldwide,19 causing about 288 million episodes of sore throat among children aged 5–14 years, resulting in 0.1 million disability-adjusted life years (DALYs) each year.20 Infections due to GAS are common in developing countries, where poverty, overcrowding, and limited access to medical care are prevalent.21–23 Since these infections are less common and prevalent in developed countries, they have received insufficient global attention, making it difficult to reduce their burden in resource-limited settings.24 As a result, chronic immune-mediated and other invasive diseases due to GAS are still a significant cause of morbidity and mortality in many developing countries, particularly in sub-Saharan Africa.25
Effective antibiotic treatment of GAS pharyngitis is needed to prevent serious complications, including immune-mediated diseases (acute rheumatic fever (ARF), can result in rheumatic heart disease (RHD), and acute post-streptococcal glomerulonephritis (PSGN), can result in chronic kidney disease (CKD)) and other invasive diseases (such as toxic shock syndrome, necrotizing fasciitis, endocarditis, and others).10,14,26 However, it is challenging for physicians to distinguish between sore throats of viral and bacterial origin clinically. As a result, most antibiotics are prescribed wrongly (particularly in resource-limited settings), which leads to side effects and contributes to the development of antibiotic resistance.9,27,28
In developing countries such as Ethiopia, where empirical therapy is frequently used due to lack of well-equipped diagnostic facilities, the emergence of drug resistance, consumption of expensive agents, and drug toxicity are all concerns.29 Furthermore, there are only a few reports on the epidemiology and clinical aspects of acute bacterial pharyngitis in susceptible populations in Ethiopia.30,31 Therefore, determining the prevalence, antimicrobial susceptibility pattern (ASP), and associated factors of GAS among pediatric patients with acute pharyngitis is needed.
Methods
Study Area, Design, and Period
A hospital-based cross-sectional study was conducted from April to June 2021 among strep throat-suspected pediatric patients at the University of Gondar Comprehensive Specialized Hospital (UoGCSH), Gondar. This hospital is the only specialized hospital in Gondar town and one of the biggest teaching hospitals in the Amhara region. The hospital provides outpatient and inpatient services for around seven million residents in North Gondar and its surrounding areas. This hospital offers curative, rehabilitative, educational, and promotional services. It has more than 500 beds and different health service-providing departments, including pediatric department.
Population
All patients with signs and symptoms of acute pharyngitis who visited the pediatric outpatient department during the study period were recruited.
Sample Size and Sampling Procedure
The sample size was calculated using a single population proportion formula with 5% expected margins of error, 95% confidence interval (Za/2 = 1.96) and 9.1% prevalence from a study conducted in Bahir Dar, Ethiopia.31
Where, N - minimum sample size required for the study, d – margin of error, and P – prevalence. A convenient sampling technique was used to select the study participants among patients presenting with signs and symptoms of acute pharyngitis in the outpatient pediatric department.
Inclusion and Exclusion Criteria
Children aged 1–15 years with acute pharyngitis at the UoGCSH were eligible. However, those who had used antibiotics in the two weeks prior to data collection day were excluded. This was done to avoid the impact of recent antibiotic use on bacterial identification.
Variables
Dependent: Prevalence of S. pyogenes and antibiotic susceptibility patterns; Independent: Socio-demographic and clinical characteristics.
Definition of Terms
MDR: is defined as acquired non-susceptibility to at least one agent in three or more antimicrobial categories.32 Acute pharyngitis: is defined by the rapid onset of sore throat and pharyngeal inflammation (with or without exudate) in less than a week.33
Data Collection and Processing
All data were collected after taking written informed assent and consent from children and parents/guardians, respectively. A structured and pre-tested questionnaire was used to collect socio-demographic, environmental, behavioral, housing related, and clinical data. The data were collected by trained pediatric clinical officers. At each data collection point, sufficient explanation about the aim of the research was given to the parents or study participants.
Sample Collection, Transportation, and Processing
A pediatrician collected a throat swab by rubbing a sterile cotton swab tip against both tonsils and the posterior pharyngeal wall and moving the swab without touching the teeth, gums, or tongue. The swab was placed immediately in Amies transport medium (Oxoid, England) and transported to the UoGCSH Bacteriology Laboratory, where they were processed within 2 hours of collection.34,35 Then, the throat swab was inoculated onto 5% sheep’s blood agar plates (BAP) (Oxoid Ltd, England) and incubated for 24 hours at 37 °C in a candle jar.36 Cultured plates negative for β-hemolytic colonies were incubated for an additional 24 hours for slow growers. The GAS isolate was identified using standard microbiological techniques, such as hemolytic activity with small colony characteristics on BAP, Gram-positive, catalase-negative, and Bacitracin Disc (0.04-U) sensitive.37 AST was performed by using the disc diffusion method, following the guidelines established by the Clinical Laboratory and Standard Institute (CLSI).37 The suspension was prepared from pure S. pyogenes colonies mixed with normal saline in a sterile glass test tube, which was then matched with 0.5 McFarland standards (which carry 108CFU/mL). Using a sterile cotton swab, the suspension was uniformly applied to Mueller–Hinton agar (MHA) (Oxoid, Basingstoke, and Hampshire, England) that had been supplemented with 5% sheep blood. Soon after, antibiotic discs were placed on the inoculated plate, and the plates were incubated at 37 °C in a candle jar overnight. The antibiotic discs were chosen in accordance with CLSI standards and prescription trends. The following drug discs were tested: erythromycin (15μg), azithromycin (15μg), tetracycline (30μg), chloramphenicol (30μg), clindamycin (2μg), vancomycin (30μg), ceftriaxone (30μg) and penicillin (10μg). These antibiotic discs were from BD, BBLTM Company, USA Product. The diameter of the zone of inhibition was interpreted as sensitive, intermediate, and resistant, according to the principles established by CLSI.37
Quality Control
A pre-tested and structured questionnaire was used to collect information on the study subjects. Standard Operating Procedures (SOPs) were strictly followed during all the stages of sample collection and laboratory work, including culture media sterility and performance testing.38 Quality control of the culture media was tested using Streptococcus pneumoniae ATCC 49619.37 Positive control involves observing the BAP for the appearance of translucent or opaque white to gray–colored colonies surrounded by a zone of beta hemolysis and a zone of no colony growth around the disk. For bacitracin test, S. pyogenes ATCC 19615 was used as positive control and Streptococcus agalactiae ATCC 13813 as a negative control. Antibiotic susceptibility inhibition zone was interpreted based on the CLSI M100 guideline as resistance, intermediate, and sensitive.37
Data Processing and Analysis
Data were coded and entered into Epi Data version 7.2 and transferred to IBM SPSS statistics version 25 (IBM Corp, Armonk, NY, USA) for analysis. Descriptive statistics were used to summarize the characteristics of the study population. Chi-square test was used to test the association between possible sign and symptoms of acute pharyngitis and culture confirmed GAS pharyngitis. Bivariate and multivariable logistic regression analysis was also done to identify the association between possible associated factors and GAS pharyngitis.
Results
Socio-Demographic Characteristics
A total of 215 study participants were enrolled. The male-to-female ratio was 1.34:1, and 123 (57.2%) were male patients. The mean age of the study participants was 3.75 years (± 2.6 SD), and 167 (77.7%) were below the age of 5 years. Most of the children 202 (94%) were urban dwellers, and 165 (76.7%) had a household size of <5 people (Table 1).
 |
Table 1 Socio-Demographic and Clinical Characteristics of Pediatric Patients with Acute Pharyngitis at the UoGCSH, Gondar, Ethiopia, 2021 |
Prevalence of Group a Streptococci (GAS)
The overall prevalence of S. pyogenes was 23/215 (10.7%) (95%; CI = 7.1–15.4), with 11/81 (12%) of the isolates were from female subjects. The prevalence of S. pyogenes among children aged between 5 and 15 years was 9/48 (18.8%). The prevalence was also higher among subjects with history of hospital visit 18/139 (12.9%) and history of hospital admission 3/15 (20%) than their counterparts (Table 2).
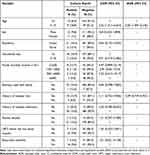 |
Table 2 Bi-Variable and Multi-Variable Analysis of Factors Associated with the Presence of GAS Among Children with Acute Pharyngitis at the UoGCSH, Gondar, Ethiopia, 2021 |
Factors Associated with GAS Infection
Independent variables having a p-value of <0.2 during the bivariate analysis were subjected to multivariable analysis. According to this, age (COR = 2.52, 95% CI = 1.017–6.254) and history of hospital visit (COR = 2.11, 95% CI = 0.752–5.936) were entered into the multivariable analysis. Finally, age (AOR = 2.50, 95% CI = 1.004–6.246, p-value = 0.049) was the only variable significantly associated with S. pyogenes pharyngitis (Table 2).
Symptoms and Signs
Among the study subjects, 204 (94.9%), 196 (91.6%), 157 (73.0%), 149 (69.3%), and 131 (60.9%) had symptoms and signs: enlarged tonsil, sore throat, fever, headache, and inflamed tonsil, respectively. Even though 22 (95.65%), 21 (91.2%), 18 (78.3%), and 16 (69.6%) of GAS positive patients had enlarged tonsil, sore throat, fever, and headache, respectively, the association was not significant. However, inflamed tonsil 19 (82.6%), tonsillopharyngeal exudate 17 (73.9%), scalariform rash 16 (69.6%), and dysphagia 15 (65.2%) were associated with the presence of GAS pharyngitis (Table 3).
 |
Table 3 Prevalence of Signs and Symptoms in Patients with Group A Streptococcus (GAS) Pharyngitis at the UoGCSH, Gondar, Ethiopia, 2021 |
Antimicrobial Susceptibility Profile of GAS
We used eight commonly prescribed antibiotics to identify the ASP of S. pyogenes isolates. According to the results of ASP of these isolates, the overall susceptible, intermediate, and resistance rate was 80.43%, 11.41%, and 8.15%, respectively. All isolates were susceptible to penicillin, 95.7% to vancomycin and chloramphenicol, 91% to clindamycin, and 87% to ceftriaxone. In contrast, 56.5%, 39.1%, and 30.4% of the isolates showed at least reduced susceptibility to tetracycline, erythromycin, and azithromycin, respectively. None of the isolates in this study were MDR (Table 4).
 |
Table 4 Antibiotic Susceptibility Patterns of S. pyogenes Isolates from Pediatric Patients with Acute Pharyngitis at the UoGCSH, Gondar, Ethiopia, 2021 |
Discussion
Group A streptococcus causes a variety of clinical conditions in humans, ranging from pharyngitis to severe invasive infections, mainly through adhesion and invasion of host mucosal surface epithelial cells of the oral and nasal cavities.5 Although quick and accurate diagnosis along with proper treatment of streptococcal pharyngitis are critical in preventing suppurative and non-suppurative complications, antibiotic resistant isolates have been reported.14 One reason for this is the overuse of antibiotics by physicians in the treatment of pharyngitis, which is more common in low-income countries.39,40 This highlights the need of understanding the prevalence and antimicrobial susceptibility pattern of GAS to prevent the emergence and spread of resistant strains.
The prevalence of S. pyogenes infection in this study was 10.7% (95%; CI = 7.1–15.4), which is comparable with the study results from other parts of Ethiopia such as Bahir Dar 9.1%31 and Jimma 11.3%.41 It is also similar to studies from different countries such as Turkey 7.5%,42 Indonesia 7.9%,43 Morocco 9.1%,44 and Iran 11.8%.45 However, it was higher than reports from Morocco 6.2%,46 India 5.5%,47 Romania 4%,48 Northern India 2.8%,49 and Iran 2.5%.50 On the other hand, it was lower than estimates from Pakistan 25.3%,51 India 28.4%,52 Iran 30%,53 and Yemen 41.5%.54 The different prevalence of GAS pharyngitis in different countries may be due to the differences in social determinants such as socioeconomic status of a population and crowded living conditions.23,55 The age of the study participants can also have an impact on prevalence; for instance, in this study, there were a significant number of participants under the age of 5, who are less likely positive for bacterial pharyngitis than school-aged children.56
The prevalence of GAS pharyngitis in this study was slightly higher among females (12%) than male (9.8%) children, but this difference was not statistically significant. According to a review paper, females are more commonly affected with upper respiratory tract infections (URTIs), and males with lower-RTIs. However, most RTIs are more severe in males than in females, and these disparate results could be explained by anatomical, lifestyle, behavioral, and socioeconomic variations between males and females.57 The prevalence of bacterial pharyngitis in this study was significantly higher in children aged 5–15 years than younger children. This is comparable with a 2010 meta-analysis report56 and 2012 guidelines from the Infectious Diseases Society of America.14 These reports indicate that children 5–15 years of age are more likely to have GAS pharyngitis than younger children.
Bacterial culture is the gold standard for GAS pharyngitis diagnosis. However, it takes up to 48 hours and requires standard facilities. Therefore, it may be difficult to use in primary care practice, especially in the developing world. Even though clinical manifestations have low diagnostic value by themselves, assessing their impact could help physicians to diagnose GAS infections.52,58 In the current study, clinical manifestations such as the presence of tonsillar exudate, scarlatiniform rash, dysphagia, and inflamed tonsil were associated with GAS pharyngitis. In the resource-limited countries where there is no culture facility, these clinical manifestations can be useful to diagnose GAS pharyngitis and at the same time to reduce the over prescription of antibiotics.59,60
Empirical antimicrobial treatment of children with sore throats is common, especially in developing countries, resulting in considerable overtreatment of non-streptococcal pharyngitis, which leads to emerging antibiotic resistance.61 As a result, utilizing a more specific and sensitive test, such as a throat culture, to differentiate between viral and GAS pharyngitis as well as performing AST before treatment is substantial.14,62 In our study, all the GAS isolates were susceptible to penicillin, which is also reported by several studies worldwide.30,31,41,45,46 Hence, given its narrow spectrum, low cost, and efficacy in preventing strep throat complications such as RHD and PSGN, penicillin remains the drug of choice for treating GAS pharyngitis.17 Our findings reveal that vancomycin 4.3% and ceftriaxone 13% have reduced susceptibility against GAS causing pharyngitis. A higher resistance rate to these antibiotics (35.7% and 35.5%, respectively) was reported from Bahir Dar, Ethiopia.31 There are reports that 1st generation cephalosporins have cross-allergy with penicillins, mainly due to side chain similarity. However, with 2nd and 3rd generation cephalosporins, this cross-allergy has been reported negligible, opening the door to the safe use of selected cephalosporins to treat infections in patients who are allergic to penicillin.63,64 S. pyogenes reduced susceptibility to clindamycin 8.6% was also revealed in this study, which was lower than studies reported in Morocco 9.1%46 and Bahir Dar 50%.31
Although macrolides, lincosamides, and streptogramins are recommended as alternative antibiotics in GAS infected patients who are allergic to β-lactams, some strains possess resistance mechanisms to these antibiotics.14 In this study, GAS isolates showed reduced susceptibility (showing intermediate or resistant patterns) to erythromycin 39.1% and azithromycin 30.4%. Other studies confirm that there is an increasing prevalence of macrolide-resistance of GAS isolates worldwide.65,66 S. pyogenes can acquire erm (erythromycin ribosome methylase) gene, which mediates ribosomal modification, and mef (macrolide efflux) gene, which encodes a drug efflux pump. These genes can also be encoded on mobile genetic elements, favoring lateral transfer of resistance.17,67–69 The resistance rate of macrolides may also be associated with M-protein serotypes of S. pyogenes. There are emm types that can express the macrolide-lincosamide-streptogramin B resistance phenotype such as emm89 strains, which usually possesses erm (B) gene, and emm1 and emm44/61 strains, which can possess mef (A) genes.70 Thus, AST is suggested before choosing macrolides or lincosamides as an alternative treatment for penicillin-allergic patients.
The isolates also showed 95.7% susceptibility to chloramphenicol, which is consistent with reports from other studies in Ethiopia.30,71 The acquisition of chloramphenicol O-acetyltransferase (CAT) enzymes or the presence of active efflux mediated by specific transporters is highly associated with chloramphenicol resistance. Target modifications are also possible through point mutations or cfr (chloramphenicol–florfenicol-resistance)-mediated methylation in 23S rRNA (ribosomal ribonucleic acid).72,73 Chloramphenicol can be used for penicillin allergic patients with serious infections if other less dangerous antimicrobials are ineffective, not tolerated, or contraindicated. Using this antibiotic, especially for minor infections, is not advised due to its serious adverse effects such as bone marrow toxicity and grey baby syndrome.74 It was also demonstrated that 56.5% of the S. pyogenes isolates showed reduced susceptibility to tetracycline. This finding is consistent with previous reports from Ethiopia, Jimma 52%41 and Hawassa 57.1%.71 Tetracycline resistance may be due to drug inactivation, active efflux, and ribosomal protection mechanisms, which are associated with the presence of tet (M), tet (K), or tet (L) genes in the bacteria.8,75 In general, the high level of tetracycline resistance reported in our study might be attributed by many factors including over and misuse of antimicrobials in the study area where there is weak regulatory practice and inadequate bacteriological surveillance.76
Limitations
Since this cross-sectional study was conducted over a short period of time, the prevalence of S. pyogenes may have been influenced by seasonal or environmental factors. Furthermore, confirming the GAS isolates with other phenotypic tests such as PYR test or by using PCR methods was not performed due to unavailability of resources during the study period. Minimum inhibitory concentration (MIC) test, which can evaluate AST better than disc diffusion test, was not used in this study.
Conclusion and Recommendations
Group A streptococcus was found in 10.7% of pediatric patients with acute pharyngitis, which is consistent with previous reports from Ethiopia. Clinical manifestations of strep throat such as tonsillar exudate, inflamed tonsils, difficulty swallowing, and a scarlatiniform rash, which have been mentioned in guidelines and previous articles, are also reported in this study. Penicillin remains the drug of choice for treating GAS pharyngitis in the study area, but tetracycline and macrolides have a high resistance rate. Screening children with acute pharyngitis for S. pyogenes and evaluating the ASP of isolates is recommended on a regular basis. Large-scale studies throughout the year with better laboratory detection methods are advocated for.
Abbreviations
AOR, Adjusted odds ratio; ARF, Acute rheumatic fever; ASP, Antibiotic susceptibility pattern; AST, Antibiotic susceptibility testing; ATCC, American Type Culture Collection; BAP, Blood Agar Plate; CFU, Colony Forming Unit; CI, Confidence interval; CKD, chronic kidney disease; CLSI, Clinical Laboratory and Standard Institute; COR, Crude odds ratio; GAS, Group A streptococcus; MHA, Muller Hinton Agar; MDR, Multi-drug resistant; PSGN, post-streptococcal glomerulonephritis; RHD, rheumatic heart disease; SOP, Standard operating procedure; SPSS, Statistical Package for the Social Sciences; UoGCSH, University of Gondar Comprehensive Specialized Hospital.
Data Sharing Statement
All data generated or analyzed during this study were included in this article. Data that support the findings of this study are also available from the corresponding author upon reasonable request.
Ethical Approval and Consent to Participate
Before the commencement of the study, we obtained ethical clearance (Ref. No/SBMLS/2723/Feb 2021) from the UoG, School of Biomedical and Laboratory Sciences ethical review committee, and an official letter of co-operations was provided to the UoGCSH. Before data collection, we explained the study objectives to the children’s parent or caregiver. Strict confidentiality was maintained throughout the study period and used only for the study purpose. We conducted the study following the Declaration of Helsinki.77
Acknowledgments
We would like to thank the Department of Medical Microbiology, University of Gondar. We would like to acknowledge all the children, parents, and the University of Gondar Comprehensive Specialized Hospital pediatric staff members who collaborated with us in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Sidrah K, Pradeep V. Streptococcus Pyogenes - StatPearls - NCBI Bookshelf. Stat Pearls; 2022.
2. Lancefield RC. A micro precipitin-technic for classifying hemolytic streptococci, and improved methods for producing antisera. Proc Soc Exp Biol Med. 1938;38:4.
3. Arnold JC, Nizet V. Pharyngitis. In: Long SS, Proper CG, Fischer M, editors. Principles and Practice of Pediatric Infectious Diseases. Elsevier; 2018.
4. Jasim SA, Hatem ZA, Mohammed ZA. Virulence factors and clinical features of Streptococcus pyogenes: overview. Ann Rom Soc Cell Biol. 2021;25:1.
5. Terao Y. The virulence factors and pathogenic mechanisms of Streptococcus pyogenes. J Oral Biosci. 2012;54(2):96–100.
6. Darenberg J, Henriques-Normark B, Lepp T, Tegmark-Wisell K, Tegnell A, Widgren K. Increased incidence of invasive group A streptococcal infections in Sweden, January 2012–February 2013. Eurosurveillance. 2013;18:14.
7. Ibrahim J, Eisen JA, Jospin G, Coil DA, Khazen G, Tokajian S. Genome analysis of Streptococcus pyogenes associated with pharyngitis and skin infections. PLoS One. 2016;11(12):e0168177.
8. Bennett JE, Dolin R, Blaser MJ, editors, Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Philadelphia, PA: Elsevier; 2020:5407.
9. Wessels MR. Streptococcal Pharyngitis. N Engl J Med. 2011;364(7):648–655.
10. Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13(3):470–511.
11. Ashurst JV, Edgerley-Gibb L. Streptococcal Pharyngitis. N Eng J Med. 2011;364(7):648–655.
12. Renner B, Mueller CA, Shephard A. Environmental and non-infectious factors in the aetiology of pharyngitis (sore throat). Inflamm Res. 2012;61(10):1041–1052.
13. Olafsdottir LB, Erlendsdóttir H, Melo-Cristino J, et al. Invasive infections due to Streptococcus pyogenes: seasonal variation of severity and clinical characteristics, Iceland, 1975 to 2012. Eurosurveillance. 2014;19:17.
14. Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group a streptococcal pharyngitis: 2012 update by the infectious diseases society of America. Clin Infect Dis. 2012;55(10):e86–102.
15. Gunnarsson RK, Holm SE, Söderström M. The prevalence of beta-haemolytic streptococci in throat specimens from healthy children and adults: implications for the clinical value of throat cultures. Scand J Prim Health Care. 1997;15(3):149–155.
16. Al-Hasnawi EAF, Abbas OK, Al-Taie W. Detection and evaluation of Streptococcus pyogenes (group A) as a superior infectious agent of acute pharyngitis among school age children. Ann Trop Med Public Heal. 2020;23(7):562–569.
17. Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus Pyogenes: Basic Biology to Clinical Manifestations. University of Oklahoma Health Sciences Center; 2016:467–476.
18. Wolford RW, Goyal A, Belgam Syed SY, Schaefer TJ. Pharyngitis. StatPearls; 2022.
19. Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5(11):685–694.
20. Miller KM, Carapetis JR, Van Beneden CA, et al. The global burden of sore throat and group A Streptococcus pharyngitis: a systematic review and meta-analysis. eClinicalMedicine. 2022;48:101458.
21. Ryan ET, Hill DR, Solomon T, Aronson NE, Endy TP. Hunter’s Tropical Medicine and Emerging Infectious Disease.
22. Bennett J, Moreland NJ, Zhang J, et al. Risk factors for group A streptococcal pharyngitis and skin infections: a case control study. Lancet Reg Heal. 2022;26:100507.
23. Avire NJ, Whiley H, Ross K. A review of Streptococcus pyogenes: public health risk factors, prevention and control. Pathogens. 2021;10(2):248.
24. Good MF. Streptococcus: an organism causing diseases beyond neglect. PLoS Negl Trop Dis. 2020;14(5):e0008095.
25. Gemechu T, Mahmoud H, Parry EH, Phillips DI, Yacoub MH. Community-based prevalence study of rheumatic heart disease in rural Ethiopia. Eur J Prev Cardiol. 2017;24(7):717–723.
26. Martin JM. Pharyngitis and streptococcal throat infections. Pediatr Ann. 2010;39:1.
27. Gunnarsson MS, Sundvall PD, Gunnarsson R. In primary health care, never prescribe antibiotics to patients suspected of having an uncomplicated sore throat caused by group A beta-haemolytic streptococci without first confirming the presence of this bacterium. Scand J Infect Dis. 2012;44(12):915–921.
28. McCaig LF, Besser RE, Hughes JM. Trends in antimicrobial prescribing rates for children and adolescents. JAMA. 2002;287(23):3096.
29. Pfaller MA, Carvalhaes CG, Smith CJ, Diekema DJ, Castanheira M. Bacterial and fungal pathogens isolated from patients with bloodstream infection: frequency of occurrence and antimicrobial susceptibility patterns from the SENTRY antimicrobial surveillance program (2012–2017). Diagn Microbiol Infect Dis. 2020;97(2):115016.
30. Barsenga S, Mitiku H, Tesfa T, Shume T. Throat carriage rate, associated factors, and antimicrobial susceptibility pattern of group A Streptococcus among healthy school children in Jigjiga City, Eastern Ethiopia. BMC Pediatr. 2022;22(1):227.
31. Kebede D, Admas A, Mekonnen D. Prevalence and antibiotics susceptibility profiles of Streptococcus pyogenes among pediatric patients with acute pharyngitis at Felege Hiwot Comprehensive Specialized Hospital, Northwest Ethiopia. BMC Microbiol. 2021;21(1):135.
32. Magiorakos A-P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281.
33. Flores AR, Caserta MT. Pharyngitis. In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Elsevier; 2015:753–759.e2.
34. McDonald M, Towers R, Fagan P, et al. Recovering streptococci from the throat, a practical alternative to direct plating in remote tropical communities. J Clin Microbiol. 2006;44(2):547–552.
35. World Health Organization. Guidelines for the Collection of Clinical Specimens During Field Investigation of Outbreaks. World Health Organization; 2000.
36. Cheesbrough M. District Laboratory Practice in Tropical Countries: Part 2.
37. CLSI. Performance Standards for Antimicrobial Susceptibility Testing: CLSI Supplement M100.
38. Basu S, Pal A, Desai P. Quality control of culture media in a microbiology laboratory. Indian J Med Microbiol. 2005;23(3):159.
39. Ahmed M, Hassan M, Eida M, Metwally L. Evaluation of appropriateness of antibiotic use and validation of the McIsaac-modified Centor score for group a beta hemolytic streptococcal acute pharyngitis in Suez Canal area. Suez Canal Univ Med J. 2015;18(2):117–124.
40. Mustafa Z, Ghaffari M. Diagnostic methods, clinical guidelines, and antibiotic treatment for group a Streptococcal pharyngitis: a narrative review. Front Cell Infect Microbiol. 2020;15:10.
41. Tesfaw G, Kibru G, Mekonnen D, Abdissa A. Prevalence of group A β-haemolytic Streptococcus among children with pharyngitis in Jimma town, Southwest Ethiopia. Egypt J Ear Nose Throat Allied Sci. 2015;16(1):35–40.
42. Altun M, Mericli Yapıcı B. Detection of group a beta hemolytic streptococci species, emm, and exotoxin genes isolated from patients with tonsillopharyngitis. Curr Microbiol. 2020;77(9):2064–2070.
43. Malino IY, Utama DL, Soenarto Y. McIsaac criteria for diagnosis of acute group-A β-hemolytic streptococcal pharyngitis. Paediatr Indones. 2016;53(5):258.
44. Benouda A, Sibile S, Ziane Y, Elouennass M, Dahani K, Hassani A. Place de Streptococcus pyogenes dans les angines au Maroc et état actuel de sa sensibilité aux antibiotiques. Pathol Biol. 2009;57(1):76–80.
45. Sherkatolabbasieh H, Firouzi M, Shafizadeh S, Amiri I. Antibiotic susceptibility evaluation of bacterial agents causing infection in children with acute tonsillopharyngitis. Infect Disord. 2021;21:6.
46. Himri S, Oumokhtar B, Atmani S, et al. Prevalence of group a streptococci in Moroccan children with pharyngitis and emm type distribution. Arch Pediatr Infect Dis. 2021;9:4.
47. Khandekar A, Dangre-Mudey G. Tackling rheumatic heart disease: prevalence and antibiogram of Streptococcus pyogenes in cases of paediatric pharyngitis. J Clin Diagn Res. 2019;2019:1.
48. Bobia AA, Blaj OA, Oancea D, et al. The prevalence of beta hemolytic Streptococcus in a children’s tertiary care hospital in Timisoara. Cent Eur J Clin Res. 2019;2(1):73–78.
49. Kumar R, Vohra H, Chakraborty A, et al. Epidemiology of group a streptococcal pharyngitis & impetigo: a cross-sectional & follow up study in a rural community of northern India. Indian J Med Res. 2009;130:6.
50. Khosravi AD, Ebrahimifard N, Shamsizadeh A, Shoja S. Isolation of Streptococcus pyogenes from children with pharyngitis and emm type analysis. J Chin Med Assoc. 2016;79(5):276–280.
51. Rathi SK, Ahmed R. Pakistan prevalence survey in acute pharyngitis. J Pak Med Assoc. 2014;64:8.
52. Bhalla K, Bhardwaj P, Gupta A, Mehra S, Nehra D, Nanda S. Role of epidemiological risk factors in improving the clinical diagnosis of streptococcal sore throat in pediatric clinical practice. J Fam Med Prim Care. 2019;8(10):3130.
53. Sayyahfar S, Fahimzad A, Naddaf A, Tavassoli S. Antibiotic susceptibility evaluation of group a Streptococcus isolated from children with pharyngitis: a study from Iran. Infect Chemother. 2015;47(4):225.
54. Ba-Saddik IA, Munibari AA, Alhilali AM, et al. Prevalence of Group A beta-haemolytic Streptococcus isolated from children with acute pharyngotonsillitis in Aden, Yemen. Trop Med Int Heal. 2014;19(4):431–439.
55. Coffey PM, Ralph AP, Krause VL. The role of social determinants of health in the risk and prevention of group A streptococcal infection, acute rheumatic fever and rheumatic heart disease: a systematic review. PLoS Negl Trop Dis. 2018;12(6):e0006577.
56. Shaikh N, Leonard E, Martin JM. Prevalence of streptococcal pharyngitis and streptococcal carriage in children: a meta-analysis. Pediatrics. 2010;126(3):e557–64.
57. Falagas ME, Mourtzoukou EG, Vardakas KZ. Sex differences in the incidence and severity of respiratory tract infections. Respir Med. 2007;101(9):1845–1863.
58. Jo SA, Ma SH, Kim S. Diagnostic impact of clinical manifestations of group A Streptococcal pharyngitis. Infect Chemother. 2021;53(3):553.
59. Palla AH, Khan RA, Gilani AH, Marra F. Over prescription of antibiotics for adult pharyngitis is prevalent in developing countries but can be reduced using McIsaac modification of Centor scores: a cross-sectional study. BMC Pulm Med. 2012;12(1):70.
60. Smeesters PR, Campos D, Van Melderen L, de Aguiar E, Vanderpas J, Vergison A. Pharyngitis in low-resources settings: a pragmatic clinical approach to reduce unnecessary antibiotic use. Pediatrics. 2006;118(6):e1607–11.
61. Teng CL, Tong SF, Khoo EM, et al. Antibiotics for URTI and UTI – prescribing in Malaysian primary care settings. Aust Fam Physician. 2011;40(5):325–329.
62. Regoli M, Chiappini E, Bonsignori F, Galli L, de Martino M. Update on the management of acute pharyngitis in children. Ital J Pediatr. 2011;37(1):10.
63. Pichichero ME, Casey JR. Safe use of selected cephalosporins in penicillin‐allergic patients: a meta‐analysis. Otolaryngol Neck Surg. 2007;136(3):340–347.
64. Romano A, Valluzzi RL, Caruso C, Maggioletti M, Quaratino D, Gaeta F. Cross-reactivity and tolerability of cephalosporins in patients with IgE-mediated hypersensitivity to penicillins. J Allergy Clin Immunol Pract. 2018;6(5):1662–1672.
65. Chuang P-K, Wang S-M, Lin H-C, et al. The trend of macrolide resistance and emm types of group A streptococci from children at a medical center in southern Taiwan. J Microbiol Immunol Infect. 2015;48(2):160–167.
66. Tsai W-C, Shen C-F, Lin Y-L, et al. Emergence of macrolide-resistant Streptococcus pyogenes emm12 in southern Taiwan from 2000 to 2019. J Microbiol Immunol Infect. 2021;54(6):1086–1093.
67. Silva-Costa C, Friães A, Ramirez M, Melo-Cristino J. Macrolide-resistant Streptococcus pyogenes: prevalence and treatment strategies. Expert Rev Anti Infect Ther. 2015;13(5):615–628.
68. DeMuri GP, Sterkel AK, Kubica PA, Duster MN, Reed KD, Wald ER. Macrolide and clindamycin resistance in group A streptococci isolated from children with pharyngitis. Pediatr Infect Dis J. 2017;36(3):342–344.
69. Leclercq R, Courvalin P. Bacterial resistance to macrolide, lincosamide, and streptogramin antibiotics by target modification. Antimicrob Agents Chemother. 1991;35:1.
70. Creti R, Imperi M, Baldassarri L, et al. Types, virulence factors, and antibiotic resistance of invasive Streptococcus pyogenes isolates from Italy: what has changed in 11 years? J Clin Microbiol. 2007;45(7):2249–2256.
71. Anja A, Beyene G, Mariam Z, Daka D. Asymptomatic pharyngeal carriage rate of Streptococcus pyogenes, its associated factors and antibiotic susceptibility pattern among school children in Hawassa town, southern Ethiopia. BMC Res Notes. 2019;12(1):564.
72. Kehrenberg C, Schwarz S, Jacobsen L, Hansen LH, Vester B. A new mechanism for chloramphenicol, florfenicol and clindamycin resistance: methylation of 23S ribosomal RNA at A2503. Mol Microbiol. 2005;57(4):1064–1073.
73. Schwarz S, Kehrenberg C, Doublet B, Cloeckaert A. Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiol Rev. 2004;28(5):519–542.
74. Oong GC, Tadi P. Chloramphenicol. In: StatPearls. StatPearls Publishing; 2022.
75. Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev. 2001;65(2):232–260.
76. Belachew SA, Hall L, Selvey LA. Non-prescription dispensing of antibiotic agents among community drug retail outlets in Sub-Saharan African countries: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2021;10(1):13.
77. World Medical Association. Declaration of Helsinki. JAMA. 2013;310(20):2191.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.