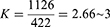Back to Journals » Infection and Drug Resistance » Volume 16
Prevalence, Antibiotic Susceptibility Pattern, and Associated Factors of Enteric Bacterial Pathogens Among HIV Infected Patients with Diarrhea Attending the ART Clinic of Dilla University Referral Hospital, Southern Ethiopia
Authors Mitiku A, Solomon Z , Gidisa B, Gebeyhu K, Tewabe H , Shenkute D , Kassa M, Gize A
Received 12 April 2023
Accepted for publication 21 June 2023
Published 29 June 2023 Volume 2023:16 Pages 4227—4236
DOI https://doi.org/10.2147/IDR.S410759
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Asaye Mitiku,1 Zerihin Solomon,1 Berhanu Gidisa,1 Kasie Gebeyhu,1 Haymanot Tewabe,2 Demissew Shenkute,3 Melkayehu Kassa,4 Addisu Gize4
1Department of Medical Laboratory Science, College of Medicine and Health Sciences, Dilla University, Dilla, Ethiopia; 2Department of Medical Laboratory Science, College of Medicine and Health Sciences, Debre Markos University, Debre Markos, Ethiopia; 3Department of Medical Laboratory Science, College of Health Sciences, Debre Birhan University, Debre Berhan, Ethiopia; 4Department of Microbiology, St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia
Correspondence: Asaye Mitiku, Email [email protected]
Background: In people with human immunodeficiency virus infection, diarrhea is reportedly associated with significant morbidity and mortality. Therefore, the aim of this study was to determine the prevalence, antibiotic susceptibility pattern, and associated factors of enteric bacterial pathogens among HIV infected patients with diarrhea attending the antiretroviral treatment (ART) clinic of Dilla University Referral Hospital, southern Ethiopia.
Methods: This institutional-based cross-sectional study was conducted on 422 study participants attending at ART clinic of Dilla University Referral Hospital from March to August 2022. Demographic and clinical data were collected by using a semi-structured questionnaire. Stool specimens were inoculated on selective media like Butzller’s medium and Xylose Lysine Deoxycholate (XLD) agar. Antimicrobial resistance pattern was assessed by using Kirby-Bauer disk diffusion techniques. Adjusted odds ratio (AOR) and 95% Confidence Interval (CI) was used to determine the presence of association.
Results: A total of 422 adult patients were enrolled in this study, 51.7% were females. The mean age of the study participants was 27.4 (± 15.6 SD) years. The overall prevalence of enteric pathogens was 14.7% (95% CI=11.4– 18.2). Shigella spp was the most prevalent organism. Being a farmer (AOR=5.1; 95% CI=1.4– 19.1; p< 0.015), the habit of hand washing after toilet (AOR=1.9; 95% CI=1.02– 3.47; p< 0.04), low CD4 cell count of < 200 cells (AOR=2.22; 95% CI=1.15– 4.27; p< 0.02), and longer duration of diarrhea (AOR=2.68; 95% CI=1.23– 5.85; p< 0.01) were statistically associated. In total, 98.4% of enteric bacterial isolates were sensitive for Meropenem, whereas 82.5% were resistant against Ampicillin. Multidrug resistance was detected in 49.2% of enteric bacteria.
Conclusion: We found that enteric bacteria are common causative agents of diarrhea in immune-compromised patients. The high rate of drug resistance calls for escalating antimicrobial susceptibility testing before prescribing antimicrobial agent.
Keywords: enteric pathogen, human immunodeficiency virus, diarrhea
Introduction
The virus HIV targets the immune system of the body.1 HIV is a virus that targets cells that aid in the body’s ability to fight infection, making a person more susceptible to contracting other illnesses and infections.2 It is disseminated through contact with specific body fluids of an HIV-positive person.2 AIDS caused by HIV is the most important public health problem and, according to a WHO estimate, 38.4 million (33.9–43.8 million) individuals worldwide live with HIV at the end of 2021.3 UNAIDS report on the global AIDS epidemic shows 650,000 (510,000–860,000) individuals passed away in 2021 as a result of AIDS-related illnesses and 12.6 million of the 38 million HIV-positive persons did not receive life-saving therapy.4
Since the beginning of the epidemic, 84.2 million (64.0–113.0 million) persons have contracted HIV, and 1.5 million (1.1–2.0 million) people became newly-infected with HIV in 2021.5 Worldwide, there were 36.7 million persons living with HIV, of whom 28.7 million persons had access to antiretroviral medicine at the end of December 2021, an increase of 7.8 million from 2010. Moreover, there were 1.8 million new HIV infections and 1 million deaths from AIDS.6 According to the most recent UNAIDS figures, young women and girls from Sub-Saharan Africa (SSA), between the ages of 15 and 24, continue to be at high risk of contracting HIV, and 75% of all HIV/AIDS deaths worldwide.7,8 Because Ethiopia is one of the SSA countries, the prevalence of HIV/AIDS is no different. It is estimated that HIV/AIDS is present in 11% of people of all ages.9
The management of other non-HIV associated diseases including bacterial infections in HIV patients has become increasingly important.10 Diarrhea is an important indication of HIV infection and is a significant cause of morbidity and mortality in later phases of AIDS, irrespective of exposure to antiretrovirals.11 It is the second leading cause of death and is responsible for killing around 525,000 patients every year and nearly 60% of these are immune suppressed (HIV/AIDS) infected patients.12 The frequency of bacterial infection most commonly caused by enteric bacteria is an opportunistic infection, it is gradually spreading among HIV-positive people.13 There is evidence that bacterial infection is more common as HIV disease progresses.14
The most prevalent bacterial pathogens are enteric ones, including E.coli, Shigella, Campylobacter, and Salmonella species (especially enteric serotypes), are prevalent and campylobacter infections alone occurrence is 40-times higher among AIDS patients than non-infected individuals.15 To address this problem, ongoing research activity is mandatory, especially in low socioeconomic countries like Ethiopia. The aim of this research was to determine the prevalence, antimicrobial susceptibility, and associated factors of enteric bacteria among HIV-positive patients.
Materials and Methods
Study Design and Study Area
The study was carried out at the Dilla University Referral Hospital, which is situated in the Southern Nations Nationalities and Peoples’ Region in the Gedeo Zone of Dilla Town. Dilla is located 360 km from Ethiopia’s capital Addis Ababa. It is located in the Kola agro-ecological zone at an elevation of 1,400 km above sea level, From March to August 2022, a hospital-based cross-sectional study was carried out on all HIV-positive people who had diarrhea and went to the ART clinic at Dilla University Referral Hospital. All HIV-positive people over the age of 18 who agreed to participate in the study met the inclusion criteria. All HIV patients who were critically ill and unable to produce stool samples, as well as HIV patients who had recently antibiotic therapy for diarrhea within the 2 weeks prior to their visit to the ART clinic at Dilla University Referral Hospital were excluded.
Sample Size and Sampling Technique
A single population proportion calculation was used to determine the sample size to obtain the total number of study participants. Owing to the lack of prior research that fully addressed the study’s purpose in Ethiopia, the 50% population proportion calculation was adopted with considering a confidence interval of 95% (z =1.96) and a 5% marginal error (d =0.05), the sample size was calculated to be 384. After addition of a 5% non-response rate was applied, the final sample size became 422.
We have applied a systematic sampling technique to get a representative sample size. The sampling interval was computed by dividing the total number of the target population by the sample size, as stated in the most recent annual report.
Since the value of K was 4, samples were taken every kth value.
Data Collection and Laboratory Processing
Written consent was obtained from each study participant prior to the collection of data and samples. A total of 422 HIV-positive diarrheic patients who were receiving ART at the Dilla University Referral hospital were surveyed using a structured questionnaire to collect their socio-demographic, clinical, and associated risk variables. To get an adequate amount of samples, each participant was given instructions to collect 5 g of loose or watery stool. Thus, following the required recommendations, a freshly passed stool sample was collected using a clean, wooden applicator stick and a leak-proof, wide mouth, sterile, screw-capped plastic container (FL Medical, Italy) that were pre-labeled with the date, time, and identification code.
The gathered samples were taken to the Dilla University Microbiology and Parasitology laboratory, kept in a refrigerated box, and processed there within 1 hour of collection.
Bacterial Cultivation and Identification
All freshly passed stools were cultured directly in various media using calibrated wire loops (0.01 mL) in Selenite F enrichment broth, Xylose Lysine Deoxycholate (XLD) agar, and MacConkey and Butzller’s medium. The streaked culture plates were then incubated at 37°C for 18–24 hours. A culture media with mixed colony was sub-culture for further isolation of a single colony. The unique look of Salmonella and Shigella species on XLD agar allowed investigators to monitor their growth (Shigella: red colonies, Salmonella: red with a black center). A variety of biochemical tests, including the lysine decarboxylase (LDC) test, indole, urease, citrate, motility test, and Kligler iron agar (KIA) test were employed to further examine the suspected colonies. Campylobacter Supplement-I (Blaser-Wang) (FD006) and campylobacter agar base with 10% sterile sheep blood were utilized for the isolation of Campylobacter spp. Microaerophilic conditions with 5–10% O2 and 10% CO2 concentrations were used to incubate agar plates for 24–48 hours at 42°C.16 The quality control was performed using an established reference strain of Campylobacter jejuni (ATCC 700819).
Antimicrobial Susceptibility Testing
The Clinical Laboratory Standards Kirby Bauer disc diffusion technique was used to conduct an antimicrobial susceptibility test.17 From three-to-five samples, pure culture was transferred and gently stirred until it creates a uniform suspension in a tube containing 5 mL of sterile normal saline (0.85% NaCl). The suspension’s turbidity was adjusted to have an optical density of 0.5 McFarland. The inoculated plates were allowed to dry for 3–5 minutes at room temperature. Following guidelines from the Clinical Laboratory Standard Institute, the resistance was measured and the diameter of the zone of inhibition surrounding the disc was measured to the closest millimeter. Ampicillin (AMP, 10 g), Chloramphenicol (CAF, 30 g), Ciprofloxacin (CI, 5 g), Co-trimoxazole (COT, 25 g), Erythromycin (ERY, 15 g), and Meropenem (10 g) were employed at the appropriate concentrations. These antimicrobial medication discs were chosen based on CLSI recommendations and taking into account their accessibility in the study area location.
Data Processing and Analysis
SPSS version 25 was used to analyze the data and we used descriptive statistics. To determine the relationship between the independent and outcome variable, bivariate and multivariate logistic regression models were performed. The multivariate logistic regression model was further examined to control the potential confounding variables using the variables with a p-value 0.25 in the bivariate logistic regression model. To assess the importance of the outcome predictors, crude odds ratio and an adjusted odd ratio (AOR) were utilized. For statistical significance, a p-value <0.05 was used.
Operational Definition
Diarrhea: diarrhea is defined as the passage of three or more loose or liquid stools per day (or more frequent passage than is normal for the individual).
Acute diarrhea is a common problem that typically lasts 1 or 2 days and goes away on its own.
Chronic diarrhea: lasts longer than 2 weeks and less than 4 weeks.
Ethical Consideration
The Dilla University Research and Ethics Review Committee granted its approval for the study under the specific protocol number duirb/012/22-01. In addition, each participant gave their written consent for the study before it started. The consent form was requested from study participants, who agreed to participate. All unique data security requirements were met while maintaining confidentiality. Physicians were informed of the laboratory examination results, and participants received their results and necessary therapy promptly. The study was conducted in accordance with the Declaration of Helsinki.
Results
Sociodemographic Characteristics
A total of 422 HIV-positive study participants were enrolled in this study. Out of the 422 HIV-infected individuals, 51.7% (n=218) were female. Most of these people (40.0%) were over 44 years old. Table 1 lists the participants’ specific sociodemographic details.
 |
Table 1 Sociodemographic Characteristics of HIV-Infected Patients with Diarrhea |
Clinical Profiles and Environmental Characteristics
About 64% (n=270) of the study participants did not have domestic animals in their home, while 46.2% (n=195) of them had the habit of hand washing after toilet utilization and before meals. From all study participants, 29.6% (n=125) and 71.5% (n=302) had a history of hospitalization within the last 12 months and used tap water as a source of water, respectively. More than half of the participants (51.9%; n=219) had a number of CD4 cell counts above 200 cells, whereas nearly two thirds of them were visiting the health care center with acute diarrhea (Table 2).
 |
Table 2 Frequency of HIV-Infected Patients with Diarrhea Who Have Certain Clinical and Environmental Characteristics |
Prevalence and Diversity of Etiologic Agents of Diarrhea
In total, 63 different enteric bacterial strains were found in HIV-positive people who developed diarrhea. The overall incidence of enteric bacterial isolates among HIV-infected people was found to be 14.9% (n=63) (95% CI=11.4–18.2). Among the isolates, Shigella spp were the most prevalent bacterial pathogens discovered, accounting for nearly half of the total isolates, that is 49.2% (n=31), followed by Salmonella spp (34.9%; n=22), and the remaining portion was Campylobacter spp (15.8%; n=10). All cultures were observed with mono-bacterial infections. Overall, compared to HIV seropositive individuals with CD4 counts greater than 200 cells/L, HIV seropositive individuals with CD4 counts less than 200 cells/L had the majority of isolated pathogens, that is aetiologic agents of diarrhea.
Factors Associated with Enteric Bacteria Among HIV Patients
By using a p-value cut-off of 0.25 in bivariate analysis, all variables, including socio-demographic features, were evaluated to see if they were factors or not for the prevalence of enteric bacteria. Bivariate logistic regressions used to analyze various sociodemographic data, including sex, educational status, monthly income, and residence did not not show a significant association with the prevalence of enteric bacteria, whereas age group (35–44), occupation (farmers), habit of hand washing after toilet and before meals, existence of domestic animals, number of CD4 cell count, and duration of diarrhea were shown to have a statistically significant association with the intestinal bacterial abundance and were enrolled to multivariable logistic regression analysis for final confirmation of statistical significance (Table 3).
From those various factors assessed by bivariate logistic regression analysis, being a farmer (p=0.015), hand washing after toilet and before meals (p=0.043), number of CD4 cells below 200 cells (p=0.017), and duration of diarrhea (p=0.013) were statistically significant for the prevalence of enteric bacteria. However, there was no statistically significant relationship between the sociodemographic variable and other environmental characteristics with the dependent variable (Table 3).
Antimicrobial Resistance Pattern of Enteric Bacteria
All the isolated enteric pathogens were tested for susceptibility pattern of selected antibiotics. Antimicrobials used in this study were Ampicillin, Chloramphenicol, Ciprofloxacin, Erythromycin, Co-trimoxazole, and Meropenem.
The overall enteric isolates showed a different resistance pattern to commonly prescribed antibiotics for the treatment of diarrhea. The highest resistance was observed in Ampicillin (82.5%) followed by Chloramphenicol (74.6%), while Meropenem had the highest sensitivity rate (98.4%). Co-trimoxazole was the most effective drug next to against enteric bacteria, and 85.7% of isolated were sensitive against Co-trimoxazole. Ciprofloxacin was the second most effective drug next to co-trimoxazole for treatment of enteric pathogen among HIV-infected patients with a total of 60.3% sensitivity.
Among the total number of Shigella spp, 90.3% were sensitive to co-trimoxazole, Ciprofloxacin (54%), Erythromycin (96.7%), and Meropenem (96.8%), but 61% were resistant against Ampicillin. Campylobacter was 80% sensitive to Co-trimoxazole and 100% resistant to Ampicillin. Another significant discovery of this research is that the highest multidrug resistance was noticed in Salmonella spp with a resistance rate of 63.6% followed by Campylobacter spp (50%) (Table 4 and Figure 1).
 |
Figure 1 Antimicrobial resistance rate against all isolated enteric bacterial pathogens. |
Discussion
Up to 60% of those with HIV experience three or more loose or watery bowel motions every day, making diarrhea a typical issue for them. In this study, enteric bacteria such as Salmonella, Shigella, and Campylobacter were assessed for the prevalence and predictive variables in HIV-infected diarrhea patients undergoing ART at Dilla University Referral Hospital. The 422 HIV-infected study participants produced a total of 63 intestinal bacterial pathogens. There was no single study participant infected by two or more enteric bacteria.
This study determined the total prevalence of enteric bacterial infection was 14.9% (95% CI=11.4–18.2). This result was consistent with a previously done study in Cambodia (12.5%).18 From the total prevalence of enteric bacterial pathogen, nearly 50% was accounted for by Shigella spp followed by Salmonella spp, that accounts for 34.9%, and the remaining was Campylobacter spp, at 15.8%. The finding of this research was relatively higher than studies done in Arba Minch, Ethiopia (8.3%),19 Dessie town, Ethiopia (7.1%),20 Osun State, Nigeria (6.6%),21 and Rajasthan state in India (16%).22 Whereas the overall prevalence of this study was lower than studies conducted in Nigeria (21.4%),22 India at Maulana Azad Medical College (20%).23 This disagreement rate could be explained by variations in sample sizes (this research has higher sample size), patients clinical condition, socioeconomic, geographical variation, and variation in prevention and control of opportunistic infections.
In the current study, Shigella species was detected in 7.3% of HIV infected study participants. This result was less significant than the research from Kampala, Uganda with a prevalence report of 11.1%.24 However, this finding was higher than from previously performed studies in Dessie, Ethiopia (1.8%),20 Gondar, northwest Ethiopia (3.7%),25 and Nigeria (5.3%).21 The possible justification may be due to sample size variation, the nature of the public water supply, and awareness about prevention and control of enteric pathogen. Meanwhile salmonella species was the second most prevalent enteric bacteria, isolated from 5.2% of total study participants, and Campylobacter spp was the least isolated etiologic agent of diarrhea in HIV infected patients, detected in 2.4%.
This study indicated a statistically significant correlation between intestinal bacterial infections and specific factors, including occupation (being a farmer), habit of hand washing, duration of diarrhea, and number of CD4 counts cells/μL. Those study participants who did not practice hand washing before a meal and after toilet (AOR=1.88; 95% CI=1.02–3.47; p=0.04) were 1.8 times more likely to develop bacterial infection compare to those had the habit of properly hand washing. Meanwhile, as well known HIV positive respondents with low CD4 cell counts <200 cells/μL (AOR=2.22; 95% CI=1.15–4.27; p=0.02) were 2.22 times more susceptible for enteric bacterial infection. This discovery was corroborated in India,26 Korea,27 and Arba Minch, Ethiopia.19 This similarity may be due to the fact that deficiency of cell immune response is the reason for prevalence of enteric etiology of diarrhea.
Moreover, as is predictable, having chronic diarrhea had a nearly 3-fold significant association with enteric bacterial infection (AOR=2.68; 95% CI=1.23–5.85; p=0.01). Another statistically significant variable was occupation (being farmer) (AOR=5.1; 95% CI=1.4–19.1; p=0.015). This was maintained with previously existing data documented in Ethiopia.28
Treatment of illnesses brought on by enteric bacterial pathogens is complicated by the principal clinical issue of rising antimicrobial resistance. The important analysis of this study was antimicrobial profile against all isolated enteric pathogens. Determining antimicrobial susceptibility is very crucial for effective treatment of not only for immune compromised patients but also the general population to get efficient therapy. The highest resistance was seen against Ampicillin (82.5%). The results of this study concur with a related investigation done in other parts of the globe, including Arba Minch, Ethiopia,19 Dessie, Ethiopia,20 and Nigeria.21 The possible explanations for the agreement could be due to the similarity in types of isolated bacteria that were enteric bacteria and drug resistance is new emerging found in a hasty increase, which leads to a scarcity of antibiotic options for treatment of infection.
Meropenem was the most potent antimicrobial drug with highest sensitivity rate (96.8%) against all isolated etiologic agents of diarrhea on study participants followed by co-trimoxazole (85.7%) and Erythromycin (84.1%). This finding was in agreement with earlier research done in Arba Minch, Ethiopia.19
Regarding the drug resistance profile of Shigella spp, 90.3% of isolates were sensitive to co-trimoxazole, Meropenem (96.8%), Ciprofloxacin (54.0%), and Erythromycin (93.5%), whereas 70.9% were resistant to Ampicillin. This result was not in agreement with findings of previously documented data that revealed 100% resistance against Ampicillin and Erythromycin in Arba Minch, Ethiopia,19 Northwest Ethiopia,25 Dessie, Ethiopia,20 and Ile Ife, Nigeria.21 This difference may be due to resistance being likely to have developed due to the unlimited, repeated, and inappropriate usage of antimicrobials in those study areas.
In the present study, our result revealed that 49.2% of the total isolated enteric bacterial pathogens were multidrug resistant. Of the total multidrug resistant findings, 63.6% was accounted for by Salmonella spp followed by Campylobacter spp (50%) and Shigella spp (38.7%). This finding is the testimony to clinicians caring for HIV-infected patients with diarrhea who are extremely concerned about the rise of drug-resistant bacteria, a major concern in this study area.
Conclusion
The study stresses the predominance of enteric bacterial infections as the cause of diarrhea in patients who are HIV-positive. The high prevalence of enteric bacteria warrants the urgent need of intervention to minimize the death of immune compromised patients due to diarrhea caused by them. Antimicrobial resistance of enteric bacterial infections is still a serious issue that contributes to higher rates of morbidity and death, as well as a higher risk of complications among HIV-infected patients. Being a farmer, not having the habit of hand washing after toilet and before meals, low number of CD4 cell count, and having chronic diarrhea were factors enhancing the prevalence of enteric bacteria among HIV positive patients. Antibiotic treatment is recommended based on the susceptibility rate of the etiologic agent of diarrhea.
Ethical Approval
This study was ethically approved by the Institutional Review Board of the College of Medicine and Health Sciences, Dilla University (Ref. duirb/0120/22-01). Before data and sample collections, written consent was obtained from all the participants and the importance of the study was briefly explained. Strict confidentiality was maintained during the interview process, and privacy was kept during data processing and report writing. The study was conducted in accordance with the Declaration of Helsinki.
Acknowledgment
The authors would like to give thanks to Dilla University Referral Hospital Laboratory personnel for all of their hard work and collaboration on this laboratory project work. We thank each and every study participant for their participation and for providing written consent. Finally, we would like to recognize the data collectors and questionnaire translator for their contribution to the achievements of this paper.
Funding
This research work was fully covered with a budget funded by Dilla University. The funder was not involved in any of the data collection, analysis, or interpretation.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Duggal S, Chugh TD, Duggal AK. HIV and malnutrition: effects on immune system. Clin Dev Immunol. 2012;2012:1–8. doi:10.1155/2012/784740
2. Jee BD, Uttal DH, Spiegel A, Diamond J. Expert–novice differences in mental models of viruses, vaccines, and the causes of infectious disease. Public Underst Sci. 2015;24(2):241–256. doi:10.1177/0963662513496954
3. Girum T, Wasie A, Worku A. Trend of HIV/AIDS for the last 26 years and predicting achievement of the 90-90-90 HIV prevention targets by 2020 in Ethiopia: a time series analysis. BMC Infect Dis. 2018;18(1):1–10. doi:10.1186/s12879-018-3214-6
4. Ruszel K, Piecewicz-Szczęsna H. The epidemiological situation of morbidity and mortality on HIV/AIDS cases in Poland and globally in recent years. J Educ Health Sport. 2020;10(8):189–198. doi:10.12775/JEHS.2020.10.08.022
5. Momenyan S, Kavousi A, Poorolajal J, Momenyan N. Spatial inequalities and predictors of HIV/AIDS mortality risk in Hamadan, Iran: a retrospective cohort study. Epidemiol Health. 2018;40:e2018038. doi:10.4178/epih.e2018038
6. Del Rio C. The global HIV epidemic: what the pathologist needs to know. In: Seminars in Diagnostic Pathology. Elsevier; 2017.
7. Sullivan PS, Siegler AJ. What will it take to meet UNAIDS targets for preexposure prophylaxis users? Curr Opin Infect Dis. 2022;35(1):1–8. doi:10.1097/QCO.0000000000000809
8. Adejumo OA, Malee KM, Ryscavage P, Hunter SJ, Taiwo BO. Contemporary issues on the epidemiology and antiretroviral adherence of HIV‐infected adolescents in sub‐Saharan Africa: a narrative review. J Int AIDS Soc. 2015;18(1):20049. doi:10.7448/IAS.18.1.20049
9. Azagew AW, Woreta HK, Tilahun AD, Anlay DZ. High prevalence of pain among adult HIV-infected patients at University of Gondar Hospital, Northwest Ethiopia. J Pain Res. 2017;10:2461. doi:10.2147/JPR.S141189
10. Witt DJ, Craven DE, McCabe WR. Bacterial infections in adult patients with the acquired immune deficiency syndrome (AIDS) and AIDS-related complex. Am J Med. 1987;82(5):900–906. doi:10.1016/0002-9343(87)90150-1
11. Panarelli NC. Infectious diseases of the upper gastrointestinal tract. Histopathology. 2021;78(1):70–87. doi:10.1111/his.14243
12. Troeger C, Blacker BF, Khalil IA, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1211–1228. doi:10.1016/S1473-3099(18)30362-1
13. Ford N, Shubber Z, Meintjes G, et al. Causes of hospital admission among people living with HIV worldwide: a systematic review and meta-analysis. Lancet HIV. 2015;2(10):e438–e44. doi:10.1016/S2352-3018(15)00137-X
14. Alvarez N, Aguilar-Jimenez W, Rugeles MT. The potential protective role of vitamin D supplementation on HIV-1 infection. Front Immunol. 2019;10:2291. doi:10.3389/fimmu.2019.02291
15. Geissler AL, Bustos Carrillo F, Swanson K, et al. Increasing Campylobacter infections, outbreaks, and antimicrobial resistance in the United States, 2004–2012. Clin Infect Dis. 2017;65(10):1624–1631. doi:10.1093/cid/cix624
16. Monica C. District Laboratory Practice in Tropical Countries. Cambridge University Press; 2006.
17. Humphries RM, Abbott AN, Hindler JA. Understanding and addressing CLSI breakpoint revisions: a primer for clinical laboratories. J Clin Microbiol. 2019;57(6):e00203–19. doi:10.1128/JCM.00203-19
18. Chhin S, Harwell JI, Bell JD, et al. Etiology of chronic diarrhea in antiretroviral-naive patients with HIV infection admitted to Norodom Sihanouk Hospital, Phnom Penh, Cambodia. Clin Infect Dis. 2006;43(7):925–932. doi:10.1086/507531
19. Ayele A, Tadesse D, Manilal A, Yohanes T, Seid M, Mekuria MS. Prevalence of enteric bacteria and enteroparasites in human immunodeficiency virus-infected individuals with diarrhoea attending antiretroviral treatment clinic, Arba Minch General Hospital, southern Ethiopia. N Microbes N Infect. 2020;38:100789. doi:10.1016/j.nmni.2020.100789
20. Belay A, Ashagrie M, Seyoum B, Alemu M, Tsegaye A. Prevalence of enteric pathogens, intestinal parasites and resistance profile of bacterial isolates among HIV infected and non-infected diarrheic patients in Dessie Town, Northeast Ethiopia. PLoS One. 2020;15(12):e0243479. doi:10.1371/journal.pone.0243479
21. Adekunle OT, Olopade B, Hassan-Olajokun R, Olaniran O, Afolayan D, Shittu O. Prevalence and antimicrobial susceptibility patterns of Salmonella and Shigella in HIV patients in Ile-Ife, Osun State. Int J Biomed Adv Res. 2015;6(5):413–417.
22. Vyas N, Pathan N, Aziz A. Enteric pathogens in HIV-positive patients with diarrhoea and their correlation with CD4+ T-lymphocyte counts. Trop Parasitol. 2012;2(1):29. doi:10.4103/2229-5070.97236
23. Uppal B, Kashyap B, Bhalla P. Enteric pathogens in HIV/AIDS from a tertiary care hospital. Indian J Commun Med. 2009;34(3):237. doi:10.4103/0970-0218.55291
24. Musiime V, Kalyesubula I, Kaddu-Mulindwa D, Byarugaba J. Enteric bacterial pathogens in HIV-infected children with acute diarrhea in Mulago referral and teaching hospital, Kampala, Uganda. J Int Assoc Physicians AIDS Care. 2009;8(3):185–190. doi:10.1177/1545109709333082
25. Andualem B, Kassu A, Diro E, Moges F, Gedefaw M. The prevalence and antimicrobial responses of Shigella isolates in HIV-1 infected and uninfected adult diarrhoea patients in North West Ethiopia. Ethiop J Health Dev. 2006;20(2):99–105.
26. Jha AK, Uppal B, Chadha S, et al. Clinical and microbiological profile of HIV/AIDS cases with diarrhea in North India. J Pathog. 2012;2012:1–7. doi:10.1155/2012/971958
27. Khalil S, Mirdha BR, Sinha S, et al. Intestinal parasitosis in relation to anti-retroviral therapy, CD4+ T-cell count and diarrhea in HIV patients. Korean J Parasitol. 2015;53(6):705. doi:10.3347/kjp.2015.53.6.705
28. Kebede A, Aragie S, Shimelis T. The common enteric bacterial pathogens and their antimicrobial susceptibility pattern among HIV-infected individuals attending the antiretroviral therapy clinic of Hawassa university hospital, southern Ethiopia. Antimicrob Resist Infect Control. 2017;6(1):1–7. doi:10.1186/s13756-017-0288-7
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.