Back to Journals » Infection and Drug Resistance » Volume 16
Prevalence, Antibiotic Resistance and Associated Factors of Neisseria gonorrhoeae Among Patients Attending Non-Profitable Private Clinics in Mekelle, Tigrai, Ethiopia
Authors Kahsay AG , Mezgebo TA, Gebrekidan GB, Desta BL, Mihretu HG, Dejene TA
Received 20 April 2023
Accepted for publication 21 June 2023
Published 23 June 2023 Volume 2023:16 Pages 4065—4072
DOI https://doi.org/10.2147/IDR.S416344
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Atsebaha Gebrekidan Kahsay,1 Tadele Araya Mezgebo,1 Gebregziabher Berihu Gebrekidan,2 Birhane Lemlem Desta,3 Hagos Gidey Mihretu,3 Tsehaye Asmelash Dejene1
1Department of Medical Microbiology and Immunology, School of Medicine, College of Health Sciences, Mekelle University, Mekelle, Tigrai, Ethiopia; 2Department of Health Systems, School of Public Health, College of Health Sciences, Mekelle University, Mekelle, Tigrai, Ethiopia; 3Department of Gynecology and Obstetrics, College of Health Sciences, Mekelle University, Mekelle, Tigrai, Ethiopia
Correspondence: Atsebaha Gebrekidan Kahsay, Department of Medical Microbiology and Immunology, School of Medicine, College of Health Sciences Mekelle University, P. O. Box: 1871, Mekelle, Tigrai, Ethiopia, Email [email protected]
Background: Globally, Neisseria gonorrhoeae is the second most common cause of bacterial sexually transmitted diseases. The prominent predicament of this bacterium is its complications, non-susceptibility for many drugs, and aggravated transmission of other sexually transmitted infections. There is limited information about the prevalence, antibiotic resistance, and risk factors of N. gonorrhoeae in Tigrai, Ethiopia. Therefore, we aimed to determine the prevalence, antibiotic resistance, and risk factors of N. gonorrhoeae among patients attending non-profitable private clinics in Mekelle, Tigrai, Ethiopia.
Methods: A cross-sectional study among 229 patients was conducted from February to June 2018. The socio-demographic data and associated factors were gathered using structured questionnaire, and swabs were taken from urethra and cervix of males and females, respectively. Specimens were inoculated on standard bacteriological culture media and antibiotic susceptibility testing was performed using Kirby-Bauer disc diffusion technique following the Clinical and Laboratory Standard Institute. Data were analyzed using Statistical Package for Social Sciences Version 21. The level of significance at p-value < 0.05 was considered statistically significant.
Results: The overall prevalence of N. gonorrhoeae was 23 (10.04%). High prevalence rates of N. gonorrhoeae were observed in females, urban residents and married ones. N. gonorrhoeae had shown statistically significant association with HIV positive, previous history of STIs, shisha users, Khat (Catha edulis) users, condom non-users and having more than two sexual partners. All isolates showed resistance to penicillin followed by tetracycline 16 (69.6%) and ciprofloxacin 8 (34.8%). Four isolates (7.4%) exhibited resistance to azithromycin with no resistance to ceftriaxone. Twelve (52.2%) isolates showed multidrug resistance (MDR).
Conclusions: The prevalence of N. gonorrhoeae and drug resistance, including multidrug resistance, was high in the study. Multiple factors were associated with the acquisition of N. gonorrhoeae. Therefore, behavioral change and communication should be strengthened.
Keywords: antibiotic resistance, associated factors, Neisseria gonorrhoeae, nonprofitable private clinics, Tigrai
Introduction
Neisseria gonorrhoeae is one of the major causes of global public health problems and it is the second most common cause of bacterial sexually transmitted infections.1,2 World Health Organization (WHO) predicted that the global incidence rate of N. gonorrhoeae among adults in 2012 was 78 million. Of these, 11.4 million cases were shared by the African region.3 The incidence rates of the bacteria reported from Russia and Poland were 1.9 up to 23.5 per 100, 000 populations.4,5 Multiple sexual partners, sexually active age, unsafe sexual practice, lower socio-economic status, urban residency and substance use were described as predisposing factors and may increase the prevalence rate.6
The noticeable complications of N. gonorrhoeae are epididymitis, pelvic inflammatory disease, ectopic pregnancy, infertility, miscarriage and fetal deaths.2,7,8 Human Immunodeficiency Virus (HIV) infected persons are more susceptible to N. gonorrhoeae and pregnant mothers can transmit the bacteria to their newborn, and the newborn can acquire neonatal ophthalmia.9,10
Due to the lack of appropriate laboratory diagnostic techniques, the management of sexually transmitted infections in Ethiopia is based on syndromic approach.11 The syndromic approach management of STIs may lead to the emergence of multidrug-resistant N. gonorrhoeae in the region. The prevalence of N. gonorrhoeae among different groups of populations in Ethiopia ranges from 2.7% to 11.6%12–14 whilst the prevalence rate increased to 17.7% in HIV patients under antiretroviral treatment.15
The emergence of resistant N. gonorrhoeae to various antibiotics remains another principal public health threat. Today, the antibiotics that displayed non-susceptibility for N. gonorrhoeae are sulphonamides, penicillin, tetracycline, macrolides, fluoroquinolones, and early-generation cephalosporins.16–18 Hence, the WHO recommended that N. gonorrhoeae suspected patients should be treated using ceftriaxone plus azithromycin19 because countries in the WHO region reported that resistance rates for ceftriaxone, ciprofloxacin and azithromycin were significantly increased.20
In the study area, there was limited data about the prevalence, antibiotic resistance and associated risk factors of N. gonorrhoeae. Therefore, the study was conducted to determine the prevalence, antibiotic resistance and associated risk factors of N. gonorrhoeae among patients attending non-profitable private clinics in Mekelle, Tigrai, Ethiopia.
Methods and Materials
Study Design, Settings and Study Period
A cross-sectional study among patients attending non-profitable private clinics was conducted from February to June 2018 in Mekelle, Tigrai, Ethiopia. Mekelle is the capital city of Tigrai regional state and is located 784km north of Addis Ababa.
Sample Size and Sampling Technique
The sample size was calculated using single population proportion formula, taking the prevalence rate of N. gonorrhoeae 11.3%.14 Expected margin of error (d) was 0.04 and the level of confidence was 95%. Thus, two hundred twenty-nine consecutive patients with 10% contingency were included in our study.
Socio-Demographic and Risk Factors Data Collection
Once secured the informed consent, information about the risk factors and socio-demographic characteristics were collected using questionnaire by trained health professionals.
Urethral and Cervical Swab Samples Collection
Two urethral or endocervical swab samples were collected from each patient aseptically following Standard Operating Procedure (SOP). The urethral sample was collected using sterile rayon swab by gentle massaging of the urethra from above downward. For those who did not have noticeable pus, the rayon swabs were inserted approximately 2 cm into the urethra and rotated gently before withdrawing. The cervical canals of female participants were opened using a well-disinfected vaginal speculum and a sterile rayon swab was rotated against the wall of the endocervical canal several times for 20–30 seconds and withdrawn without touching the vaginal surface. The specimens were inoculated on Modified Thayer Martin agar in the sample collection site and put in the candle jar having 5–10% Co2 and transported to Mekelle University Medical Microbiology laboratory within 2 hours of collection and incubated immediately following the SOP.21
Isolation and Identification of Neisseria gonorrhoeae
The Modified Thayer Martin medium (OXOID, UK) plate contains vancomycin, colistin, nystatin and trimethoprim. The inoculated media were incubated at 37°C for 24–48 hrs. Then, N. gonorrhoeae was isolated and identified based on colony morphology, gram staining, oxidase test, and carbohydrate utilization test. Small raised, grey shiny colonies, gram-negative diplococci, oxidase positive and glucose fermentation were considered as N. gonorrhoeae.
Antibiotic Susceptibility Testing
From a pure culture, 3–5 selected colonies of N. gonorrhoeae were transferred to a tube with a straight wire and prepared suspension of 2.5 mL normal saline and incubated at 36.5°C until the turbidity of the suspension equal to 0.5McFarland standard. The bacteria were inoculated evenly over the entire surface of MTM agar using a sterile cotton swab. The susceptibility to the following antimicrobial agents (OXOID, UK) was assessed: penicillin (10 IU), tetracycline (30 μg), ciprofloxacin (5μg), ceftriaxone (30μg), spectinomycin (100μg), and azithromycin (15μg).
The criteria used to select the antimicrobial agents tested were based on their availability and frequent prescriptions for the management of gonococcal infection and the national list of medicines by the Food, Medicine and Health-Care Administration and Control Authority (FMHACA) of Ethiopia.11
Finally, antimicrobial susceptibility testing was performed for all isolates according to the criteria of Clinical and Laboratory Standard Institute (CLSI) by the modified Kirby-Bauer disk diffusion method on Thayer Martin medium plates.22
Data Quality Control
Five percent of the questionnaire was pre-tested for comprehensiveness, effectiveness, reliability and validity. Culture media sterility was ensured by incubating 5% of each batch of the prepared media at 37°C for 24 hrs. The performance of prepared media was also checked by inoculating with control strains N. gonorrhoeae ATCCTM 49226. Moreover, the data collectors were trained and demonstrated for two days. The investigators conducted strict supervision on a daily basis.
Data Analysis
Data related to N. gonorrhoeae were analyzed using SPSS version 21, and descriptive statistics were presented using tables and percentages. Crude odds ratio (COR) and adjusted odds ratio (AOR) at 95% level of confidence were calculated. A p-value less than 0.05 indicated that the association between dependent and independent variables existed.
Ethical Consideration
Ethical clearance was obtained from the Institutional Review Board (IRB) of Mekelle University, College of Health Sciences with reference number ERC 1195/2017. The study was carried out in accordance with relevant national, international and scientific guidelines’ along with our study was conducted in accordance with the Declaration of Helsinki. After briefing the objectives of the study and before collecting the data, informed consent and assent were collected from adult participants and minors’ guardians, respectively. Furthermore, the study participants tested culture positive for N. gonorrhoeae were consulted. The data and samples were kept confidential and used for the specified objectives only and finally, the specimens were discarded following the infection prevention guideline.
Results
Socio-Demographic Characteristics and Risk Factors
A total of 229 patients were participated in this study. Of which, the proportion of females, 25–34 years old, urban residences, secondary school level of education, married and employees were 128 (55.9%), 123 (53.7%), 190 (83%), 84 (36.7%), 139 (60.7%) and 76 (33.2%), respectively.
Nineteen (8.3%) of the study participants were HIV positive and twenty (8.7%) had previous history of sexually transmitted infections. The proportion of shisha users and Khat (Catha edulis) chewers were 13 (5.7%) and 26 (11.4%), respectively. There were also 36 (15.7%) participants who do not use condom during sexual intercourse and other 36 (15.7%) participants who had more than 2 sexual partners. About 90 (39.3%) of the participants had the habit of alcohol intake, Table 1.
 |
Table 1 Socio-Demographic Characteristics of Patients Attending Non-Profitable Private Clinics of Mekelle, Tigrai, Ethiopia |
Prevalence of N. gonorrhoeae
The overall prevalence of N. gonorrhoeae in the current study was 23 (10.04%). The prevalence of N. gonorrhoeae among females, 25–34 years old, urban residences, secondary school educational status, married and employee participants were 15 (6.6%), 16 (7%), 18 (7.9%), 9 (3.9%), 17 (7.4%) and 6 (2.6%), respectively. Similarly, the prevalence of N. gonorrhoeae among HIV positive persons, persons having previous history of STIs, shisha users, alcohol drinkers, Khat chewers, condom non users and having more than two sexual partners were 6 (2.6), 5 (2.2), 7 (3.1), 7 (3.1), 10 (4.4), 9 (3.9) and 6 (2.6), respectively, Table 1.
Associate Factors of N. gonorrhoeae
Our study analyzed the factors associated with N. gonorrhoeae using binary logistic regression. HIV-positivity, previous history of STIs, shisha usage, Khat users, condom non-users and having more than 2 sexual partners were significantly associated with the prevalence of N. gonorrhoeae, Table 2.
 |
Table 2 Univarate and Multivariate Analysis of Associated Factors of N. gonorrhoeae |
Antibiotic Susceptibility Testing of N. gonorrhoeae
Twenty-three isolates of N. gonorrhoeae were tested with six antibiotics. All isolates were resistant to penicillin, 69.6% isolates to tetracycline and 34.8% isolates to ciprofloxacin and 30.4% isolates to spectinomycin. No isolate showed resistance to ceftriaxone, Table 3.
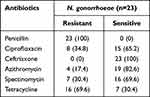 |
Table 3 Antibiotic Susceptibility Patterns of N. gonorrhoeae |
Multidrug-Resistant N. gonorrhoeae
Multidrug-resistance is defined as the resistance of an isolate in one or more than one agent to three or more classes of antibiotics. Based on the definition, the multidrug-resistant N. gonorrhoeae was encountered in 12 isolates (52.2%). Four isolates of N. gonorrhoeae showed resistance to ciprofloxacin, penicillin and tetracycline; three isolates to penicillin, spectinomycin and tetracycline; and one isolate to penicillin, azithromycin and spectinomycin. Three isolates showed resistance to ciprofloxacin, penicillin, azithromycin, and tetracycline. No N. gonorrhoeae isolate was identified as susceptible to all antibiotics tested in our study, Table 4.
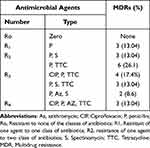 |
Table 4 Multidrug-Resistant N. gonorrhoeae |
Discussion
Neisseria gonorrhoeae is not only the cause of second leading bacterial sexually transmitted infection, but also it is resistant to the commonly used antibiotics as reported from different regions of the globe.16–18,20 Hence, this study described the prevalence, antibiotic resistance and associated factors among patients attending non-profitable clinics in Mekelle, Tigrai, Ethiopia.
The overall prevalence of culture-confirmed N. gonorrhoeae was 23 (10.04%), which was comparable with studies from Ethiopia13,14 and Mongolia.23 However, it was lower than reports from Ethiopia,15 Egypt,24 Nigeria25 and Mozambique,26 but higher than the finding reported from Ethiopia,12 Nigeria,27 Colombia,28 and the United States of America.29 The difference in prevalence may be due to the differences in sexual behavior of societies in different countries and knowledge and use of prevention methods for sexually transmitted infections. The prevalence of N. gonorrhoeae may also be varied in different countries because of other various reasons such as lack of laboratory diagnosis methods, the difference in the accuracy of laboratory methods, and the difference in the skill of professionals and N. gonorrhoeae had a statistically significant association with HIV positive, previous history of STIs, shisha users, Khat users, condom non-users and having more than two sexual partners. These associated factors may enhance the transmission of N. gonorrhoeae and are supported by other studies.14,30,31 The proportions of N. gonorrhoeae in females, 25–34 years old and urban residents were high, which was different from studies in Ethiopia.14,31
N. gonorrhoeae isolate in this study was 65% susceptible to ciprofloxacin, which is higher than a study in Ethiopia,31 but lower than another report from Ethiopia12 and Thailand.17,18 Likewise, about 70% are susceptible to spectinomycin which is lower than a report from Ethiopia.12 On the other side, four (17.4%) isolates of N. gonorrhoeae were resistant to azithromycin, which is higher than the study reported from Brazil.32 This may be due to syndromic approach of treatment which, in turn, is due to the lack of an antimicrobial susceptibility test. In our study, all isolates of N. gonorrhoeae remained susceptible to ceftriaxone which is different from other reports that showed resistance from Thailand17 and Spain.33 Hence, high multidrug resistant isolates of N. gonorrhoeae were observed in the study area.
This study may be contributed to the local policy makers in prevention and control of the infection and its drug resistance in the study area. It is also a regional input to the national and international scientists. However, there are some limitations, including the inability to determine the prevalence of other STIs because of the budgetary resources for acquiring chemicals and reagents. The study was also unable to determine resistance at the genetic level because of the lack of instrumentations and the necessary budgets.
Conclusions
High prevalence of N. gonorrhoeae isolates and drug resistance including multidrug resistance were observed in the study. These were associated with HIV positivity, having a previous history of STIs, shisha users, Khat users, condom non-users and having more than two sexual partners. Moreover, the highest rates of N. gonorrhoeae were identified in urban residences, married and employees. Furthermore, significant amount of N. gonorrhoeae isolates showed resistance to tetracycline, ciprofloxacin and spectinomycin. All of the isolates of N. gonorrhoeae showed resistance to penicillin, but no isolate was resistant to ceftriaxone. More than.
Abbreviations
CLSI, Clinical and Laboratory Standard Institutes; HIV, Human Immunodeficiency Virus; STI, Sexually Transmitted Infections; SOP, Standard Operating Procedures; SPSS, Statistical Package for Social Sciences; WHO, World Health Organizations.
Acknowledgment
We would like to forward our gratitude to administrative office of Tigray Health Bureau for giving letters of support and College of Health Sciences of Mekelle University for their collaboration in giving ethical clearance.
Author Contributions
All authors made a significant contribution in the conception, study design, execution, acquisition of data, or analysis, and interpretation. Moreover, all authors took part in drafting, revising or in critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
We declared that we have no competing interests.
References
1. World Health Organization (WHO). Global Action Plan to Control the Spread and Impact of Antimicrobial Resistance in Neisseria Gonorrhoeae. Geneva: World Health Organization; 2012.
2. Tapsall JW, Ndowa F, Lewis DA, Unemo M. Meeting the public health challenge of multidrug- and extensively drug-resistant Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 2009;7(7):821–834. doi:10.1586/eri.09.63
3. Newman L, Rowley J, Vander Hoorn S, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One. 2015;10(12):e0143304. doi:10.1371/0143304
4. Kubanov A, Vorobyev D, Chestkov A, et al. Molecular epidemiology of drug-resistant Neisseria gonorrhoeae in Russia (Current Status, 2015). BMC Infect Dis. 2016;16(1):389. doi:10.1186/s12879-016-1688-7
5. Mlynarczyk BB, Serwin AB, Golparian D, et al. Antimicrobial susceptibility/resistance and genetic characteristics of Neisseria gonorrhoeae isolates from Poland, 2010–2012. BMC Infect Dis. 2014;14(1):65. doi:10.1186/1471-2334-14-65
6. Workowski KA, Bolan GA; CDC. Sexually transmitted diseases treatment guidelines. Atlanta Morb Mortal Wkly Rep. 2014;64(3):3–180.
7. Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other STD to sexual transmission of HIV infection. Sex Transm Infect. 1999;75(1):3–17. doi:10.1136/sti.75.1.3
8. World Health Organization. Report on Global Sexually Transmitted Infection Surveillance 2013. Geneva: World Health Organization; 2014.
9. Gewirtzman A, Bobrick L, Conner K, Tyring SK. Epidemiology of sexually transmitted infections. Sex Transm Dis. 2011;2019:13–34.
10. Tapsall JW. Antimicrobial Resistance in Neisseria Gonorrhoeae. Sydney: Australia WHO collaborating centre for STD and HIV; 2001:1–48.
11. State Minister of Health of the Federal Democratic Republic of Ethiopia. National guidelines for the management of sexually transmitted infections using syndromic approach, Addis Ababa, Ethiopia; 2015.
12. Hailemariam M, Abebe T, Mihret A, Lambiyo T. Prevalence of N. gonorrhoeae and their antimicrobial susceptibility patterns among symptomatic women attending gynaecology outpatient department in Hawassa Referral Hospital, Hawassa, Ethiopia. Ethiop J Health Sci. 2013;23(1):10–17.
13. Tibebu M, Shibabaw A, Medhin G, Kassu A. Neisseria gonorrhoeae nonsusceptible to cephalosporins and quinolones in Northwest Ethiopia. BMC Infect Dis. 2013;13:415. doi:10.1186/1471-2334-13-415
14. Ali S, Sewunet T, Sahlemariam Z, Kibru G. Neisseria gonorrhoeae among suspects of sexually transmitted infection in Gambella hospital, Ethiopia: risk factors and drug resistance. BMC Res Notes. 2016;9(1):439. doi:10.1186/s13104-016-2247-4
15. Mitiku S, Mossie A, Fekadu S. Substance use and sexually transmitted infections among anti-retroviral treatment attendees in Jimma University Specialized Hospital, Jimma, Ethiopia. Ethiop J Health Sci. 2012;22(3):181–187.
16. Unemo M. Current and future antimicrobial treatment of gonorrhoea – the rapidly evolving Neisseria gonorrhoeae continues to challenge. BMC Infect Dis. 2015;15(1):364. doi:10.1186/s12879-015-1029-2
17. Kueakulpattana N, Wannigama DL, Luk-In S, et al. Multidrug-resistant Neisseria gonorrhoeae infection in heterosexual men with reduced susceptibility to ceftriaxone, first report in Thailand. Sci Rep. 2021;11(1):21659. doi:10.1038/s41598-021-00675-y
18. Nokchan N, Wongsurawat T, Jenjaroenpun P, Nitayanon P, Tribuddharat C, Rahman AS. Whole-genome sequence analysis of high-level penicillin-resistant strains and antimicrobial susceptibility of Neisseria gonorrhoeae clinical isolates from Thailand. PLoS One. 2022;17(7):e0271657. doi:10.1371/journal.pone.0271657
19. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWRRecomm Rep. 2015;64(RR–03):1–137.
20. Wi T, Lahra MM, Ndowa F, et al. Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action. PLoS Med. 2017;14(7):e1002344. doi:10.1371/journal.pmed.1002344
21. Cheesbrough M. District Laboratory Practice in Tropical Countries Part 2.
22. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. In: CLSI Supplements M100S.
23. Garland SM, Tabrizi SN, Chen S, Byambaa C, Davaajav K. Prevalence of sexually transmitted infections (Neisseria gonorrhoeae, chlamydia trachomatis, trichomonas vaginalis and human papillomavirus) in female attendees of a sexually transmitted diseases clinic in Ulaanbaatar. Mongolia Infect Dis Obstet Gynecol. 2001;9(3):143–146. doi:10.1155/S1064744901000254
24. Ali F, Aziz AA, Helmy MF, Mobdy AA, Darwish M. Prevalence of certain sexually transmitted diseases in Egypt. J Egypt Public Health Assoc. 1995;71(5–6):553–575.
25. Bersoff-Matcha SJ, Horgan MM, Fraser VJ, Mundy LM, Stoner BP. Sexually transmitted disease acquisition among women infected with human immunodeficiency virus type 1. J Infect Dis. 1998;178(4):1174–1177. doi:10.1086/515678
26. Zimba TF, Apalata T, Sturm WA, Moodley P. Aetiology of sexually transmitted infections in Maputo. Mozambique J Infect Dev Ctries. 2010;5(01):41–47. doi:10.3855/jidc.1179
27. Ángel-Müller E, Rodríguez A, Núñez-Forero LM, et al. The prevalence of and factors associated with C. Trachomatis, N. gonorrhoea, T. Vaginalis, C. Albicans infection, syphilis, HIV and bacterial vaginosis in females suffering lower genital tract infection symptoms in three healthcare attention sites in Bogotá, Colombia, 2010. Rev Colomb Obstet Ginecol. 2012;63(1):14–24.
28. Okonko IO, Okerentugba PO, Adejuwon AO, Onoh CC. Prevalence of sexually transmitted infections (STIs) among attendees of lead city university medical Centre in Ibadan, southwestern. Nigeria Arch Appl Sci Res. 2012;4(2):980–987.
29. Kent CK, Chaw JK, Wong W, et al. Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhoea. Detected in 2 clinical settings among men who have sex with men: san Francisco, California, 2003. Clin Infect Dis. 2005;41(1):67–74. doi:10.1086/430704
30. Taffa N, Bjune G, Sundby J, Gaustad P, Alestrøm A. Prevalence of gonococcal and chlamydial infections and sexual risk behavior among youth in Addis Ababa. Ethiopia Sex Transm Dis. 2002;29(12):828–833. doi:10.1097/00007435-200212000-00015
31. Yeshanew AG, Geremew RA. Neisseria gonorrhoeae and their antimicrobial susceptibility patterns among symptomatic patients from Gondar town, North West Ethiopia. Antimicrob Resist Infect Control. 2018;7(1):85. doi:10.1186/s13756-018-0376-3
32. Costa LMB, Pedroso ÊRP, Neto VV, Souza VCP, Teixeira MJB. Antimicrobial susceptibility of Neisseria gonorrhoeae isolates from patients attending public referral center for sexually transmitted diseases in Belo Horizonte, State of Minas Gerais, Brazil. Rev Soc Bras Med Trop. 2013;46(3):304–309. doi:10.1590/0037-8682-0009-2013
33. Herrero M, Broner S, Cruells A, et al. Epidemiology and antimicrobial resistance profile of Neisseria gonorrhoeae in Catalonia, Spain, 2016–2019. Eur J Clin Microbiol Infect Dis. 2023;42(7):883–893. doi:10.1007/s10096-023-04601
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
