Back to Journals » Infection and Drug Resistance » Volume 16
Prevalence and Trends of Carbapenem-Resistant Pseudomonas aeruginosa and Acinetobacter Species Isolated from Clinical Specimens at the Ethiopian Public Health Institute, Addis Ababa, Ethiopia: A Retrospective Analysis
Authors Abdeta A , Negeri AA, Beyene D, Adamu E, Fekede E, Fentaw S, Tesfaye M, Wakoya GK
Received 5 January 2023
Accepted for publication 6 March 2023
Published 11 March 2023 Volume 2023:16 Pages 1381—1390
DOI https://doi.org/10.2147/IDR.S403360
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Abera Abdeta,1 Abebe Aseffa Negeri,1 Degefu Beyene,1 Etsehiwot Adamu,1 Ebissa Fekede,1 Surafel Fentaw,1 Mheret Tesfaye,1 Getu Kusa Wakoya2
1National Clinical Bacteriology and Mycology Reference Laboratory, Ethiopian Public Health Institute, Addis Ababa, Ethiopia; 2Department of Internal Medicine, Madda Walabu University, Oromia, Ethiopia
Correspondence: Abera Abdeta, 1242, Tel +251911566420, Email [email protected]
Purpose: Carbapenem-resistant Acinetobacter species and P. aeruginosa are the leading cause of nosocomial infections. Therefore, the objective of this study was to analyze the prevalence, antimicrobial susceptibility profile, and trends of carbapenem-resistant P. aeruginosa and Acinetobacter species isolated from clinical specimens.
Patients and Methods: This retrospective study included data from Ethiopian Public Health Institute from 2017 to 2021. BD phoenix M50, Vitek 2 compact, and conventional identification methods were used to identify the organisms. The Kirby-Bauer disc diffusion, BD phoenix M50, and Vitek 2 compact methods were used to determine the antimicrobial susceptibility profiles of the isolates. Chi-square for linear trends using Epi Info was employed to test the significance of carbapenem resistance trends over time. The p-values of ≤ 0.05 were considered statistically significant.
Results: Following data cleaning, 7110 reports were used. Out of this, (N=185, 2.6%) and (N=142, 2%), Acinetobacter species and P. aeruginosa were isolated, respectively. Twenty-four Acinetobacter species and fourteen P. aeruginosa species were omitted because carbapenem antimicrobial agents were not tested for them. The overall prevalence of carbapenem-resistant Acinetobacter species and P. aeruginosa were 61% and 22%, respectively. The prevalence of carbapenem-resistant Acinetobacter species increased significantly from 50% in 2017 to 76.2% in 2021 (p=0.013). The trend of carbapenem-resistant P. aeruginosa was fluctuating (p=0.99). Carbapenem-resistant Acinetobacter had a lower resistance rate to amikacin (44%) and tobramycin (55%); similarly, carbapenem-resistant P. aeruginosa had a lower resistance rate to amikacin (27%) and tobramycin (47%).
Conclusion: This study revealed a high prevalence of carbapenem-resistant Acinetobacter species and P. aeruginosa, both of which showed better sensitivity to amikacin and tobramycin. Furthermore, Acinetobacter species showed a statistically significant increasing trend in carbapenem resistance.
Keywords: carbapenem resistance, trend analysis, Acinetobacter species, Pseudomonas aeruginosa
Introduction
Gram-negative bacteria including Pseudomonas aeruginosa (P. aeruginosa) and Acinetobacter species are non-fermentative bacteria that are ubiquitous in hospital environments and have emerged as one of the most bothersome pathogens in health-care settings.1 These bacteria are commonly responsible for the following hospital-acquired infections (HAI): ventilator-associated pneumonia, central line-associated bloodstream infections, and catheter-associated urinary tract infections, particularly in patients admitted to the intensive care unit.1–3 Healthcare-acquired infections, are infections that patients acquire while receiving treatment at a health-care facility after 48-h post-admission and are most commonly associated with invasive medical devices and surgical procedures.3
Treating HAIs caused by P. aeruginosa and Acinetobacter species is a challenge as these bacteria are mostly resistant to the commonly used antibiotics.4 These bacteria are intrinsically resistant to a wide range of antimicrobial agents and, more importantly, they can develop or acquire multiple antimicrobial resistance mechanisms, and their tendency to survive for prolonged periods under a wide range of environmental settings makes them a frequent cause of nosocomial infections.5–7 Previous antibiotic use, previous colonization, mechanical ventilation; previous intensive care unit stays, dialysis, catheterization, and hospitalization were the main risk factors for acquiring carbapenem-resistant Acinetobacter species and P. aeruginosa.8 Thus, these pathogens can become easily resistant to almost all available antimicrobial agents.6,9,10
The majority of P. aeruginosa and Acinetobacter species are resistant to the widely available and affordable antibiotics.4 Carbapenems are currently the most reliable last resort treatment option available for infections caused by multidrug-resistant P. aeruginosa and Acinetobacter species. These antibiotics are exceptionally stable against most beta-lactamases enzymes that inactivate beta-lactams due to the presence of a carbapenem along with the beta-lactam ring that gives them a unique molecular structure.11 However, carbapenem resistance among P. aeruginosa and Acinetobacter species is a persistent issue in healthcare, and resistance to carbapenems, particularly when caused by transferable genes that encode for carbapenemase, spreads quickly and severely restricts the number of treatments available.11 Other resistance mechanisms of P. aeruginosa and Acinetobacter species include efflux pumps and target-site or outer membrane alteration. Resistance to multiple antibiotics is typically caused by the interaction of multiple mechanisms in a single isolate or by the action of a single potent resistance mechanism.12 Due to the difficulty in obtaining accurate data, the burden of HAIs is unknown globally; however, the numbers in low- and middle-income countries are probably high.13,14 Despite the lack of adequate data on the burden of health-care-associated infections caused by carbapenem-resistant Acinetobacter species and P. aeruginosa in Ethiopia, a few studies reported prevalence rates ranging from 8.4% to 23% of infections caused by these pathogens.15–18 These bacterial pathogens frequently cause bloodstream, urinary tract, and surgical sites, as well as wound infections.15–18 Nordmann P and Poirel L reported that the global prevalence of carbapenem-resistant non-fermenters was greater than 60%.19 A systematic review of studies from East Africa reported pooled prevalence of carbapenem resistant Acinetobacter baumanii (23%) and P. aeruginosa (17%), respectively.20 However, different studies from Ethiopia reported the prevalence of P. aeruginosa and Acinetobacter species, with a carbapenem resistance ranging from 0% to 45.5% and 9.9% to 56.4%, respectively.16–18,21–25 Most of the studies that have been conducted in Ethiopia on carbapenem-resistant Acinetobacter species and P. aeruginosa analyzed fewer isolates from single hospitals. Therefore, the current study aimed to analyze the five-year prevalence, antimicrobial resistance patterns, and trends of carbapenem-resistant P. aeruginosa and Acinetobacter species isolated from different clinical specimens collected from different health facilities in Ethiopia. The findings from the current study will provide more information about the burden of carbapenem resistance among these pathogens, since data analyzed was five-year data from National Reference Laboratory.
Materials and Methods
Study Site and Design
This retrospective analysis of carbapenem resistance among P. aeruginosa and Acinetobacter species consists of data from the National Clinical Bacteriology and Mycology Reference Laboratory, Ethiopian Public Health Institute from 2017 to 2021. The laboratory was accredited in 2017 by the Ethiopian accreditation service per the requirements of the international standard ISO 15189:2012. It serves as a national diagnostic referral and research laboratory.
Specimen Collection
Specimens were collected from patients who came to and were admitted to various health facilities in Ethiopia. The specimens were collected from different body sites by trained health professionals into appropriate specimen containers for routine diagnostic purposes. Patient demographic data, type of specimens, time of collection, types of tests required, patient location, and other information were recorded in the standardized bacteriology request form by clinicians. The specimens were transported to the laboratory using the Triple Packaging system. In cases of specimen processing delays, appropriate transport media were used. The specimens were collected from different wards such as the intensive care unit, surgical, medical, and burn unit, etc. of the health facilities, and included both male and female patients of all age groups.
Specimen Processing and Bacterial Identification
Specimens that met the laboratory’s acceptance criteria were received and inoculated onto appropriate culture media and incubated at the appropriate temperature and time.26 The identification of Acinetobacter species and P. aeruginosa was achieved using one of the following methods available at the time of testing: Vitek 2 compact (BioMerieux, USA), BD phoenix M50 (Becton, Dickinson, USA), and conventional biochemical tests. For identification using conventional biochemical tests, a macroscopic colony characterization (color, size, shape, and texture) supported with gram staining followed by biochemical tests (Triple sugar iron agar, lysine iron agar, sulfide indole motility, Simmons Citrate Agar, urea agar, and oxidase) was used.26 The biochemical and culture media used by the laboratory from 2017 to 2021 were from the following manufacturers: (Oxoid Ltd., Basingstoke, Hampshire, England), (Liofilchem, Roseto degli Abruzzi, Italy), (Hardy diagnostics, Santa Maria, California, and Springboro, Ohio, United States), (Biomark, Dalviwadi, Dhairi Pune, Maharashtra, India), (Accumixx, Verna, Goa, India), and (HIMEDIA, Mumbai, Maharashtra, India).
Antimicrobial Susceptibility Testing
For antimicrobial susceptibility testing of these organisms, either of the following methods available at the time of testing was used: the Kirby-Bauer disk diffusion method on the Muller Hinton agar, Vitek 2 compact, and BD phoenix M50 systems. The most recent version of the Clinical and Laboratory Standards Institute (CLSI) M100 available during the study period was utilized to interpret the antimicrobial susceptibility testing results.
For P. aeruginosa, the following antimicrobial agents were reported as per CLSI M100:7 Piperacillin/tazobactam, piperacillin, ceftazidime, gentamicin, tobramycin, ciprofloxacin, amikacin, meropenem, imipenem, and cefepime.
For Acinetobacter species, the following antimicrobial agents were reported as per CLSI M100:7 Piperacillin/tazobactam, piperacillin, trimethoprim/sulfamethoxazole, ceftazidime, ceftriaxone, tetracycline, gentamicin, tobramycin, ciprofloxacin, amikacin, meropenem, imipenem, and cefepime. The antimicrobial disks for Kirby Bauer disk diffusion antimicrobial susceptibility testing were from the following manufacturers: (Oxoid Ltd., Basingstoke, Hampshire, England), (Liofilchem, Roseto degli Abruzzi, Italy) and (Hardy diagnostics, Santa Maria, California, and Springboro, Ohio, United States). For antimicrobial susceptibility testing by VITEK 2® Compact, we used the following cards: VITEK 2 AST-GN86, VITEK 2 AST-GN72, and VITEK 2 AST-GN67. The BD PhoenixTM NMIC/ID-431 panel was used for antimicrobial susceptibility testing using the BD Phoenix M50. Carbapenem resistance was defined as resistance to imipenem and/or meropenem.
Data Extraction
Data were extracted from the WHONET software and laboratory logbooks. The following details were obtained: types of specimens submitted, date of specimen collection, and received at the laboratory, patients’ demography (age and sex), organism identified with identification method used, antimicrobial susceptibility testing results, and referring health facilities. Data on the culture media and antibiotic disk origin used were extracted from the media preparation logbook and antibiotic quality control records, respectively. Only the first isolates from the patient were included to prevent bias from repeated culture.
Quality Control (QC)
American-type culture collection (ATCC) strains, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, and Staphylococcus aureus ATCC 25923 were used to check the quality of all the antimicrobial disks used as per clinical and laboratory standards institute (CLSI) M100 Table 4A 1–2.7 Quality control of culture and biochemical media was done as per laboratory standard operating procedures and manufacturers’ recommendations.
Data Analysis
Descriptive analysis was used for prevalence and rates. Resistance rates for each antimicrobial agent and the co-resistance profile of carbapenem-resistant isolates to other antimicrobial agents were analyzed using WHONET software. Carbapenem resistance trends were calculated yearly, and trends were assessed from year to year over 5 years for both isolates. Chi-square for trends using Epi Info Centers for Disease Control and Prevention was employed to test the significance of carbapenem resistance trends over time. The p-values of ≤0.05 were considered statistically significant.
Ethical Approval
Consent was waived due to the retrospective nature of the work by the institutional review board of the Ethiopian Public Health Institute (EPHI-IRB) with approval number EPHI-IRB-413-2021. To maintain confidentiality, patient names and other personal identifiers were anonymized from the data, and unique identification numbers were utilized to identify data. Therefore, the study was conducted following the Declaration of Helsinki.
Results
Seven thousand, one hundred ninety-nine (7199) clinical specimens obtained during 2017–2021 were used. Out of this (N=89) records were excluded due to missing information in Age, Sex, referring health facility, and types of specimens submitted. Records of (N=7110) Specimens with complete information were used to analyze the prevalence of Acinetobacter species and P. aeruginosa. The prevalence of Acinetobacter species and P. aeruginosa were (N=185/7110, 2.6%) and (N=142/7110, 2%), respectively. Twenty-four (24) and fourteen (14) Acinetobacter species and P. aeruginosa were excluded since carbapenem was not tested against them. Finally, we analyzed 161 Acinetobacter species and 128 Pseudomonas aeruginosa data Figure 1. Out of this, (N=98/161,61%) Acinetobacter species and (N=28/128, 22%) P. aeruginosa were resistant to carbapenem Figure 1.
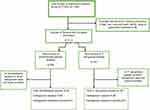 |
Figure 1 Data cleaning and analysis flowchart. Abbreviation: N, Total number. |
A total of 126 carbapenem-resistant were recovered from both isolates. Out of 126 carbapenem-resistant isolates, the highest number was recovered from Urine (N=40, 32%) and Pus (N=39, 31%). The remaining 37% of carbapenem-resistant isolates were recovered from sputum, blood, cerebrospinal fluid, tracheal aspirate, and ear discharge Figure 2.
 |
Figure 2 Carbapenem-resistant Acinetobacter species and P. aeruginosa distribution among different specimens. |
Patient Characteristics
Out of 126 carbapenem-resistant isolates, 66% and 34% were recovered from specimens collected from male and female patients, respectively. Twenty-eight (28) percent of the carbapenem-resistant isolates were isolated from specimens collected from people between the ages of 21 and 30. Out of 126 carbapenem-resistant isolates, 51.6% were isolated from specimens referred from AaBET hospital. Thirty (30) and twenty-seven (27) percent of the carbapenem-resistant isolates were recovered from specimens collected from the intensive care unit and emergency wards, respectively Table 1.
 |
Table 1 Distribution of Carbapenem-Resistant Isolates by Patient Demography, Health Facilities, and Wards |
Overall Antimicrobial Resistance Profile
During 2017–2021 the Acinetobacter species showed the highest resistance to ceftazidime (94.6%), ceftriaxone (94.1%), and cefepime (90%) with the lowest resistance being observed against amikacin (31.5%), tobramycin (51.7%), imipenem (59%), and meropenem (61%) Table 2. However, P. aeruginosa demonstrated relatively lower antimicrobial resistance percentages, with the lowest resistance to amikacin (10.3%), tobramycin (11.8%), gentamycin (12.9%), imipenem (16%), and meropenem (22%) and the highest resistance to cefepime (42.1%) and ceftazidime (35.4%) Table 3.
 |
Table 2 Overall Antimicrobial Susceptibility Profile of Acinetobacter Species Isolates During 2017–2021 |
 |
Table 3 Overall Antimicrobial Susceptibility Profile of P. aeruginosa Isolates During 2017–2021 |
Co-Resistance Profile of Carbapenem-Resistant Isolates to Other Antimicrobial Agents
The resistance profile of carbapenem-resistant isolates to other antimicrobial agents is as follows: carbapenem-resistant Acinetobacter species was 100% resistant to Piperacillin, Ceftazidime, ceftriaxone, and cefepime, whereas it was least resistant to amikacin (44%), tobramycin (55%), and tetracycline (67%) Table 4. Carbapenem resistant P. aeruginosa was 100% resistant to piperacillin, piperacillin/tazobactam, ceftazidime, and cefepime and least resistant to amikacin (27%), tobramycin (47%), gentamicin (55%), and ciprofloxacin (58%) Table 4.
 |
Table 4 Co-Resistance Profile of Carbapenem-Resistant Acinetobacter Species and P. aeruginosa to Other Antimicrobial Agents |
Trends of Carbapenem Resistance
The prevalence of carbapenem-resistant Acinetobacter species was 50% in 2017 and reached a peak of 76.2% in 2021 (p=0.013). The trend of carbapenem-resistant P. aeruginosa was fluctuating between 2017 and 2021. It was 30.3% in 2017 but dropped to 8.8% in 2018, increased to 24.2% in 2019, and reached a peak of 33.3% in 2020, but little dropped again to 22.7% in 2021 Table 5.
 |
Table 5 Carbapenem Resistance Trend of Acinetobacter Species and P. aeruginosa (2017–2021) |
Discussion
In this retrospective study, we analyzed the five-year antimicrobial resistance profiles of P. aeruginosa and Acinetobacter species with an emphasis on the prevalence and trends of carbapenem resistance at the Ethiopian Public Health Institute, Addis Ababa, Ethiopia.
The prevalence of carbapenem-resistant Acinetobacter species in the current study was 61%, which is consistent with the findings of the study from Pretoria, South Africa (63%),27 and Jimma, Ethiopia (56.4%),25 however, it is higher than the findings of studies from Lusaka, Zambia (18.2%),28 Switzerland (9.2%),29 North-East, Ethiopia (34.5%),18 Sidama, Ethiopia (9.9%),22 North Gondar, Ethiopia (20.77%),24 Northwest Ethiopia (33.3%),17 and Dessie, Ethiopia (43.8).16 The variation could be attributed to differences in study design, the number of isolates analyzed, the types of specimens considered, and the amount of data analyzed, as some studies only analyzed fewer data, while others analyzed a large amount of national antimicrobial resistance data, geographical differences, and antibiotic prescription policy differences. The high prevalence of carbapenem-resistant Acinetobacter species observed in our study could be attributed to improper carbapenem antibiotic prescribing in health-care facilities and the absence of strong antibiotic stewardship initiatives, which can guide antibiotic prescription practices.
In this study, the overall prevalence of carbapenem-resistant P. aeruginosa was 22%, which is lower compared to the study from Dessie Comprehensive Specialized Hospital, Ethiopia (41.3%),18 Jimma, Ethiopia (30%),25 and Felegehiwot referral hospital, Ethiopia (45.5%).17 The observed variation could be attributed to the fact that they only analyzed 46, 10, and 11 P. aeruginosa isolates, respectively, and additionally the differences in study design since these studies used specimens collected from single health facilities. However, it was higher compared to the study from Sidama, Ethiopia (7.8%),22 Asella, Ethiopia (0%),23 Dessie, Ethiopia (16.7%), and16 Taiwan (10.2%).30 The observed variations may result from variations in the number of isolates analyzed, and differences in antibiotic prescription policies.
Trends of Carbapenem Resistance
In this study, we reported statistically significant increasing trends of carbapenem-resistant Acinetobacter species, it was increased from 50% in 2017 to 76.2% in 2021 (p=0.013). Our findings are in line with the findings of the study from the Ethiopian Public Health Institute, Ethiopia, which reported a significant increase of meropenem-resistant Acinetobacter species from 12.5% in 2014 to 62% in 2018,21 and a study from southern China, which reported a significant rise of carbapenem-resistant Acinetobacter baumannii from 18% in 2012 to 60% in 2019.31 This could be due to the improper use of carbapenem antibiotics for empiric therapy, which could be due to the lack of a well-established microbiology laboratory capable of providing a reliable culture and antimicrobial susceptibility test service. Additionally, rising carbapenem resistance may be associated with a lack of strong antibiotic stewardship efforts that can guide clinicians’ antibiotic prescription practices. However, our findings are in disagreement with the findings of US studies that reported decreasing trends of carbapenem-resistant Acinetobacter species,32 as well as Germany’s national Antimicrobial Resistance Surveillance System report, which reported significantly decreasing trends of carbapenem-resistant Acinetobacter species from 7.6% in 2014 to 3.5% in 2018, p ≤ 0.001.33 The possible reason for the difference might be due to the difference of antibiotic stewardship efforts, infection prevention, and control practices and the differences in study design since the later study used a large amount of national antimicrobial resistance data.
In the current study, the trends of carbapenem-resistant P. aeruginosa were not statistically significant (p=0.99). Consistent with the findings of the study from Beirut, Lebanon.34 However, this contradicts the findings of Taiwan Surveillance of Antimicrobial Resistance (TSAR) from 2000 to 2010, which reported a statistically significant increase in carbapenem-resistant P. aeruginosa (p=0.007).30 The observed difference could be attributed to differences in study design, as well as geographical differences.
Overall Antimicrobial Resistance
During the study period, Acinetobacter species demonstrated the highest resistance to piperacillin/tazobactam (85.5%), extended-spectrum cephalosporins (ceftazidime 94.6%, ceftriaxone 94.1%, and cefepime 90%) and lowest resistance to aminoglycosides (amikacin 31.5%, and tobramycin 51.7%) and carbapenems (imipenem 59%) Table 2. Although the prevalence of resistance is higher in our study, the result is partially in agreement with studies from Sidama, Ethiopia,22 and Northeast Ethiopia,16,18 which reported the highest resistance to extended-spectrum cephalosporins and piperacillin/tazobactam and lowest resistance to aminoglycosides and carbapenems.
Pseudomonas aeruginosa showed the highest resistance to cefepime (42.1%), ceftazidime (35.4%), and ciprofloxacin (24.8%) during the study period, and the lowest resistance to amikacin (10.3%), tobramycin (11.7%), and gentamicin (12.9%) Table 3. Our findings are in line with the findings of studies from Ethiopia; Sidama, Ethiopia,22 and Northeast Ethiopia16,18 which reported higher resistance to cephalosporins (ceftazidime and cefepime) and lower resistance to aminoglycosides (amikacin, gentamicin, and tobramycin). The high prevalence of extended-spectrum cephalosporin resistance observed against these organisms could be attributed to the overprescription of these antibiotics in Ethiopia, even for minor infections.
Limitations
Due to the retrospective nature of our work, we did not analyze underlying patient health conditions and risk factors for carbapenem resistance. Furthermore, we did not use a Polymerase chain reaction to detect genes responsible for carbapenem resistance.
Conclusion
In this study, we reported a high prevalence (61%) and statistically significant increasing trends of carbapenem-resistant Acinetobacter species (p=0.013) between 2017 and 2021. However, the trend of carbapenem-resistant P. aeruginosa was not statistically significant. Both carbapenem-resistant Acinetobacter and P. aeruginosa had a lower resistance rate to aminoglycosides such as amikacin and tobramycin. Moreover, to reduce infections caused by these bacteria, strengthening antimicrobial surveillance, antibiotic stewardship, and infection control at the health facility level is important.
Acknowledgments
We would like to thank the Ethiopian Public Health Institute’s National Clinical Bacteriology and Mycology Reference Laboratory for allowing us to conduct this research.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mehrad B, Clark NM, Zhanel GG, Lynch JP. Antimicrobial resistance in hospital-acquired gram-negative bacterial infections. Chest. 2015;147(5):1413–1421. doi:10.1378/chest.14-2171
2. Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria types of infections NIH public access. N Engl J Med. 2010;362(19):1804–1813. doi:10.1056/NEJMra0904124.Hospital-Acquired
3. Sartelli M, Mckimm J, Bakar MA, Abu Bakar MB. Health care-associated infections – an overview. Infect Drug Resist. 2018;11:2321–2333. doi:10.2147/IDR.S177247
4. Garnacho-Montero J, Amaya-Villar R. Multiresistant Acinetobacter baumannii infections: epidemiology and management. Curr Opin Infect Dis. 2010;23(4):332–339. doi:10.1097/QCO.0b013e32833ae38b
5. Karageorgopoulos DE, Falagas ME. Current control and treatment of multidrug-resistant Acinetobacter baumannii infections. Lancet Infect Dis. 2008;8(12):751–762. doi:10.1016/S1473-3099(08)70279-2
6. Bonomo RA, Szabo D. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin Infect Dis. 2006;43(SUPPL. 2):49–56. doi:10.1086/504477
7. Clinical and Laboratory Standards Institute (CLSI). M100 Performance Standards for Antimicrobial Susceptibility Testing. Vol. M100-Ed32. Clinical and Laboratory Standards Institute (CLSI); 2021.
8. Palacios-Baena ZR, Giannella M, Manissero D, et al. Risk factors for carbapenem-resistant Gram-negative bacterial infections: a systematic review. Clin Microbiol Infect. 2021;27(2):228–235. doi:10.1016/j.cmi.2020.10.016
9. Livermore DM. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin Infect Dis. 2002;34(5):634–640. doi:10.1086/338782
10. Grossi P, Gasperina DD. Treatment of Pseudomonas aeruginosa infection in critically ill patients. Expert Rev Anti Infect Ther. 2006;4(4):639–662. doi:10.1586/14787210.4.4.639
11. Meletis G. Carbapenem resistance: overview of the problem and future perspectives. Ther Adv Infect Dis. 2016;3(1):15–21. doi:10.1177/2049936115621709
12. Zavascki AP, Carvalhaes CG, Picão RC, Gales AC. Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii: resistance mechanisms and implications for therapy. Expert Rev Anti Infect Ther. 2010;8(1):71–93. doi:10.1586/eri.09.108
13. Zaidi AKM, Huskins WC, Thaver D, Bhutta ZA, Abbas Z, Goldmann DA. Hospital-acquired neonatal infections in developing countries. Lancet. 2005;365(9465):1175–1188. doi:10.1016/S0140-6736(05)71881-X
14. Allegranzi B, Nejad SB, Combescure C, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011;377(9761):228–241. doi:10.1016/S0140-6736(10)61458-4
15. Bitew A, Adane A, Abdeta A. Bacteriological spectrum, extended ‑ spectrum β ‑ lactamase production and antimicrobial resistance pattern among patients with bloodstream infection in Addis Ababa. Sci Rep. 2023;13(1):1–11. doi:10.1038/s41598-023-29337-x
16. Mekonnen H, Seid A, Fenta GM, Gebrecherkos T, Hasnain SE. Antimicrobial resistance profiles and associated factors of Acinetobacter and Pseudomonas aeruginosa nosocomial infection among patients admitted at Dessie comprehensive specialized Hospital, NorthEast Ethiopia. A cross-sectional study. PLoS One. 2021;16:1–17. doi:10.1371/journal.pone.0257272
17. Motbainor H, Bereded F, Mulu W. Multi-drug resistance of blood stream, urinary tract and surgical site nosocomial infections of Acinetobacter baumannii and Pseudomonas aeruginosa among patients hospitalized at Felegehiwot referral hospital, Northwest Ethiopia: a cross-sectional study. BMC Infect Dis. 2020;20(1):1–11. doi:10.1186/s12879-020-4811-8
18. Tilahun M, Gedefie A, Bisetegn H, Debash H. Emergence of high prevalence of extended-spectrum beta-lactamase and carbapenemase producing Acinetobacter species and Pseudomonas aeruginosa among hospitalized patients at Dessie comprehensive specialized hospital, North-East Ethiopia. Infect Drug Resist. 2022;15:895–911. doi:10.2147/IDR.S358116
19. Nordmann P, Poirel L. Epidemiology and diagnostics of carbapenem resistance in gram-negative bacteria. Clin Infect Dis. 2019;69(Suppl 7):S521–S528. doi:10.1093/cid/ciz824
20. Ssekatawa K, Byarugaba DK, Wampande E, Ejobi F. A systematic review: the current status of carbapenem resistance in East Africa. BMC Res Notes. 2018;11(1):1–9. doi:10.1186/s13104-018-3738-2
21. Ayenew Z, Tigabu E, Syoum E, Ebrahim S, Assefa D, Tsige E. Multidrug resistance pattern of Acinetobacter species isolated from clinical specimens referred to the Ethiopian Public Health Institute: 2014 to 2018 trend anaylsis. PLoS One. 2021;16:1–12. doi:10.1371/journal.pone.0250896
22. Alemayehu T, Asnake S, Tadesse B, et al. Phenotypic detection of carbapenem-resistant gram-negative bacilli from a clinical specimen in sidama, Ethiopia: a cross-sectional study. Infect Drug Resist. 2021;14:369–380. doi:10.2147/IDR.S289763
23. Tufa TB, Mackenzie CR, Orth HM, et al. Prevalence and characterization of antimicrobial resistance among gram-negative bacteria isolated from febrile hospitalized patients in central Ethiopia. Antimicrob Resist Infect Control. 2022;11(1):1–12. doi:10.1186/s13756-022-01053-7
24. Abda EM, Adugna Z, Assefa A. Elevated level of imipenem-resistant gram-negative bacteria isolated from patients attending health centers in North Gondar, Ethiopia. Infect Drug Resist. 2020;13:4509–4517. doi:10.2147/IDR.S287700
25. Sewunet T, Asrat D, Woldeamanuel Y, Aseffa A, Giske CG. Molecular epidemiology and antimicrobial susceptibility of Pseudomonas spp. and Acinetobacter spp. from clinical samples at Jimma Medical Center, Ethiopia. Front Microbiol. 2022;13. doi:10.3389/fmicb.2022.951857
26. Amy LL. Clinical Microbiology Procedures Handbook.
27. Kock MM, Bellomo AN, Storm N, Ehlers MM. Prevalence of carbapenem resistance genes in Acinetobacter baumannii isolated from clinical specimens obtained from an academic hospital in South Africa. South Afr J Epidemiol Infect. 2013;28(1):28–32. doi:10.1080/10158782.2013.11441516
28. Kaluba CK, Samutela MT, Kapesa C, et al. Carbapenem resistance in Pseudomonas aeruginosa and Acinetobacter species at a large tertiary referral hospital in Lusaka, Zambia. Sci Afr. 2021;13:e00908. doi:10.1016/j.sciaf.2021.e00908
29. Ramette A, Kronenberg A, Burnens A, et al. Prevalence of carbapenem-resistant Acinetobacter baumannii from 2005 to 2016 in Switzerland. BMC Infect Dis. 2018;18(1):1–6. doi:10.1186/s12879-018-3061-5
30. Lin KY, Lauderdale TL, Wang JT, Chang SC. Carbapenem-resistant Pseudomonas aeruginosa in Taiwan: prevalence, risk factors, and impact on outcome of infections. J Microbiol Immunol Infect. 2016;49(1):52–59. doi:10.1016/j.jmii.2014.01.005
31. Liang C, Zhang X, Zhou L, Meng G, Zhong L, Peng P. Trends and correlation between antibacterial consumption and carbapenem resistance in gram-negative bacteria in a tertiary hospital in China from 2012 to 2019. BMC Infect Dis. 2021;21(1):1–9. doi:10.1186/s12879-021-06140-5
32. Gupta V, Ye G, Olesky M, Lawrence K, Murray J, Yu K. Trends in resistant Enterobacteriaceae and Acinetobacter species in hospitalized patients in the United States: 2013–2017. BMC Infect Dis. 2019;19(1):1–9. doi:10.1186/s12879-019-4387-3
33. Said D, Willrich N, Ayobami O, Noll I, Eckmanns T, Markwart R. The epidemiology of carbapenem resistance in Acinetobacter baumannii complex in Germany (2014–2018): an analysis of data from the national Antimicrobial Resistance Surveillance system. Antimicrob Resist Infect Control. 2021;10(1):1–13. doi:10.1186/s13756-021-00909-8
34. Chamieh A, El-Hajj G, Zmerli O, Afif C, Azar E. Carbapenem resistant organisms: a 9-year surveillance and trends at Saint George University Medical Center. J Infect Public Health. 2020;13(12):2101–2106. doi:10.1016/j.jiph.2019.02.019
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
