Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Prevalence and Risk Factors for COPD in an Urbanizing Rural Area in Western China: A Cross-Sectional Study
Authors Zhang X, Lei Z, Wu Y, Song Y , Wu X, Yang B, Fan J, Feng S, Wu L, Li L, Dai Q, Zeng Z, Feng M, Zhang T
Received 6 December 2022
Accepted for publication 27 March 2023
Published 4 April 2023 Volume 2023:18 Pages 459—468
DOI https://doi.org/10.2147/COPD.S400213
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Xiaolong Zhang,1,* Zhiyin Lei,2,* Ying Wu,3 Yuanyuan Song,4 Xiaoling Wu,3,5 Bo Yang,2 Jianmei Fan,2 Shixu Feng,2 Liping Wu,2 Lingyan Li,2 Qin Dai,2 Zhen Zeng,2 Mei Feng,3 Tingting Zhang3
1Department of Thoracic Surgery, West China Hospital, Sichuan University, Chengdu, Sichuan Province, People’s Republic of China; 2Department of Respiratory and Critical Care Medicine, Jiajia Central Health Center of Chengdu Eastern New Area, Chengdu Eastern New Area, Sichuan Province, People’s Republic of China; 3Department of Respiratory and Critical Care Medicine, West China Hospital, Sichuan University/West China School of Nursing, Sichuan University, Chengdu, Sichuan Province, People’s Republic of China; 4Department of Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, Sichuan Province, People’s Republic of China; 5Department of Nursing, Sanya People’s Hospital/West China (Sanya) Hospital, Sichuan University, Sanya, Hainan Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ying Wu, Department of Respiratory and Critical Care Medicine, West China Hospital, Sichuan University/West China School of Nursing, Sichuan University, No. 37 Guoxue Alley, Chengdu, Sichuan Province, 610041, People’s Republic of China, Tel +86-18980606932, Email [email protected]
Purpose: To investigate the prevalence and risk factors for chronic obstructive pulmonary disease (COPD) in a rural area in western China with severe air pollution.
Patients and Methods: 10% of local residents aged 40 years and above were included using a convenience sampling method. This was a cross-sectional study. A self-designed questionnaire was used to collect participants’ demographic data. The screening program was comprised of two steps: First, a portable electronic spirometer was used for COPD screening. Those participants with FEV1/FVC ratio < 0.7 were then referred to a confirmatory pulmonary function (PF) test. COPD was confirmed according to the 2020 Global Initiative for Chronic Obstructive Lung Disease criteria.
Results: A total of 4577 participants aged 40 years old or above were included in the final analysis. Examination with a mobile spirometer identified 1159 individuals for confirmatory testing; after that, of the 1159 individuals, 889 were diagnosed with COPD by the confirmatory PF test. The prevalence of COPD among the target group was 19.4%. Older age, male sex (odds ratio [OR] = 1.537, 95% confidence interval [CI] 1.246– 1.894), smoking history (OR = 1.338, 95% CI 1.069– 1.675), family history of respiratory disease (OR = 1.625, 95% CI 1.350– 1.957), education level (OR = 0.735, 95% CI 0.617– 0.876), overweight (OR = 0.614, 95% CI 0.517– 0.730) and obesity (OR = 0.572, 95% CI 0.449– 0.721) were identified as independent factors associated with COPD. The screening program helped earlier detection of COPD in 719 participants.
Conclusion: COPD was highly prevalent in the rural area studied. Rural residents who were older, current or ever-smokers, male and those who had a lower education level were more vulnerable to developing COPD. The COPD screening program may be helpful for earlier disease detection in rural health-care settings.
Keywords: chronic obstructive pulmonary disease, prevalence, primary care, pulmonary function test, risk factors
Introduction
The World Health Organization (WHO) has declared chronic obstructive pulmonary disease (COPD) to be the third leading cause of mortality worldwide, causing 3.23 million deaths in 2019.1 According to the latest national cross-sectional study,2 the prevalence of COPD in the population aged 40 years and older is 13.7%, which represents a roughly 67% increase when compared with the prevalence in the same age group 10 years ago. COPD has become the third leading cause of disability3 and is among the top three most prevalent chronic diseases in China.2
Owing to different levels of exposure to air pollution and other social economic factors, the prevalence of COPD varies considerably across China.4 Specifically, COPD prevalence is higher in rural areas2 in China where access to health services is limited and people there are more susceptible to health and social disadvantages. Chengdu city, an economic center in western China, is rapidly developing, and is often listed among the top 10 cities with the worst air quality in the world.5 The prevalence of COPD in Chengdu was reported to be 20.2%,6 which is substantially higher than the national average. Parts of Jianyang, a rural area to the east of Chengdu, has entered a stage of rapid urbanization. The region is representative of rapidly developing and industrializing rural areas in middle-income countries. However, there is a dearth of epidemiological data on the prevalence and risk factors for COPD in this typical rural area.
Therefore, we performed a COPD screening program in primary care settings in this part of Jianyang, which is now known as Chengdu Eastern New Area, China. Because of large population sizes and limited resources in this area, a two-step screening strategy was considered as a suitable screening program.
In the current study, we aimed to determine the prevalence and risk factors for COPD in the target population.
Materials and Methods
Population
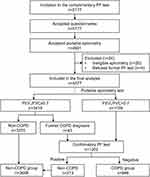
This cross-sectional survey was performed from May 2020 to December 2020. The target areas were Jiajia, Gaoming, Wumiao, Wuzhi and Laojun. These rural regions used to belong to Jianyang City, but according to the provincial economic development strategy, they have been classified as belonging to Chengdu city because of the geographic proximity to eastern Chengdu. The only secondary hospital located in Jiajia is responsible for all the rural areas mentioned above. These areas all together now are known as Chengdu Eastern New Area, experiencing a rapid process of urbanization and industrialization. This area comprises 75 villages and five districts. These villages and districts were all included in this survey. According to statistics provided by the local government, there are 95,357 local residents in the area, with 40,622 people aged 40 years or older. Sample size was calculated by PASS 21.0 (NCSS, LLC, Utah). A minimum sample size of 720 produces a two-sided 95% confidence interval (CI) with a width equal to 6% for our prespecified sample proportion of 20.2%6 using the Clopper-Pearson exact method.7,8 As a free health check program supported by local government, the program is designed to benefit more residents. Within time and budget constraints, the sample size was enlarged to 10% of the target population in total and equally in each village or district. During the study period, all individuals aged 40 years or older were invited to voluntarily undergo a free pulmonary function (PF) test using a portable spirometer performed at the primary care station in the individual’s village. If the sample size was not achieved at each location, we extended the program period. If the sample size was achieved, we completed the program as scheduled. The free health check program was publicized via an official poster on villages’ and districts’ bulletin boards as well as online announcement via Wechat group message sent by a primary social worker who organized and ran the online chat group for each community. Each online chatting group included at least one representative from each family, thus ensuring that every local family informed. Then the research team waited for participants to voluntarily and randomly attend. Ethical approval was obtained from the ethics committee of the first author’s hospital (No. 2019-065) and an informed consent form was provided in the first part of the questionnaire. If individuals agreed to participate in the survey, they could check the “I agree” box and continue completing the survey. Respondents aged 40 years or older from participating cites were included. Individuals who were unable to undergo spirometry or who had contraindications for spirometry or previous adverse events to Ventoline (salbutamol) were excluded from the study. Individuals who refused to complete the questionnaire or refused to undergo pulmonary function testing were also excluded. The entire screening process is shown in Figure 1.
COPD Definition and Participant Data
COPD was defined as a ratio of forced exhalation volume in one second to forced vital capacity (FEV1/FVC) of less than 0.7 in pulmonary function testing post bronchodilation (BD) according to The Global Initiative for Chronic Obstructive Lung Disease Report 2020 (GOLD 2020).9 GOLD9 also categorizes disease severity according to the value of FEV1 predicted: GOLD stage I (mild), ≥80% predicted; GOLD stage II (moderate), ≥50% to <80% predicted; GOLD stage III (severe), ≥30% to <50% predicted; and GOLD stage IV (very severe), <30% predicted. A self-designed questionnaire was used to collect participants’ demographic data and variables of interest in the study. The main items included education level, personal disease history, family respiratory disease history, occupational exposure, smoking history, cooking environment, heating method and livestock ownership. Participants were asked to complete the questionnaire by themselves. For participants who could not read or write, research nurses or family members helped them to complete the survey.
Pulmonary Function Testing
The screening steps were comprised of two steps: First, screening pulmonary function testing was performed with a portable spirometer (X1, Xeek, Co., Ltd, Xiamen, China) among local participants in primary care settings. The spirometer was calibrated once a day using a 3-L syringe. Nurses who performed pulmonary function testing were trained in a pulmonary function laboratory in a tertiary hospital. Pulmonary function tests were carried out from 8:00 to 12:00 and from 14:00 to 18:00. Spirometry outcomes included FEV1, FVC, and peak expiratory flow.
Second, those participants with negative results (FEV1/ FVC <0.70) identified by the portable spirometer were sent for a confirmatory pulmonary function test at a secondary hospital within 1 week after the free health check. A Jaeger spirometer (MasterScreen™ Pneumo, Jaeger, German) was used. Bronchodilation was performed via administration of 100 μg Ventoline (GlaxoSmithKline, Australia) per puff, and each participant received four puffs in total. Patients underwent pulmonary function testing 15–20 minutes after inhalation.
The Mobile Spirometer
This spirometer is a pressure differential flow sensor based instrument. In a lab test,10 its waveforms were found to be highly consistent with the ATS standard waveforms and Jaeger spirometer (MasterScreenTM Pneumo, Jaeger, Germany) in 10 different situations. All of the test errors were within a ±3% interval, meeting the accuracy requirements of both Calibration Specification for the Pulmonary Function Measuring Instrument11 and ATS/ERS standard.12 This test10 also included 980 healthy volunteers to evaluate the agreement between this spirometer and Jaeger spirometer. The Pearson’s correlation analysis showed that the r value of FVC, peak expiratory flow rate (PEF), FEV1 and FEV1/FVC between the portable spirometer and Jaeger spirometer were 0.984, 0.985, 0.982 and 0.941, respectively. Consistency boundary analysis showed that the consistency intervals for FVC, PEF, FEV1 and FEV1/FVC were (−0.313, 0.260)L, (−0.253, 0.243)L, (−0.587, 0.717)L/s, (−4.655, 5.472)%, respectively. More than 95% of participants’ results were located within the consistency boundaries. The results showed that the portable spirometer share sufficient agreement13 with the Jaeger spirometer (MasterScreen™ Pneumo, Jaeger, Germany) results.
Statistical Analysis
Age and Pulmonary function results were treated as continuous variables. For non-COPD participants who did not undergo post-BD PF test, we used pre-BD PF results for statistical analysis. For continuous variables with a normal distribution, the differences between variables were tested using an independent samples t-test; otherwise, the Mann–Whitney U-test was used. The remaining variables were treated as categorical or binary variables. The chi-square test was used for binary and categorical variables. For categorical variables with tendency, the Cochran–Armitage trend test was used. Potential risk factors for COPD were determined using a logistic regression model for univariate and multivariable analyses. Univariate analysis was used to evaluate the rough association between independent variables. Variables exhibiting a P value <0.25 in the univariate analysis were included in subsequent age and sex adjusted logistic regression and full-adjusted multivariable analysis. The overall prevalence of COPD was calculated by dividing the total number of confirmed cases across all sites by the total number of tested individuals during the study period. All statistical tests were performed with IBM SPSS version 24.0 (IBM Corp, Armonk, NY, USA). A P value <0.05 indicated a significant difference.
Results
From May 2020 to December 2020, 5117 participants were included in the study. Eight nurses finished the COPD screening program for all the participants in 44 days distributed across 7 months. After excluding participants who failed to meet the inclusion criteria and those without an eligible pulmonary function test, we included 4577 participants in the final analysis (1703 men and 2874 women, ratio 0.4:0.6). The average age of the total population was 60.4 years, ranging from 40 to 96 years. Mobile spirometry with a cut-off value of <0.7 identified 1159 individuals requiring confirmatory pulmonary function testing. Of these, 313 (27.0%) were positive (without COPD) after confirmatory testing. We also identified 3418 individuals who did not have COPD; 43 of these individuals had previously been medically diagnosed with COPD according to a self-reported disease history. We treated these 43 participants as potential COPD cases regardless of the results of mobile spirometry and continued confirmatory pulmonary function testing. Finally, 889 participants were diagnosed with COPD, in which 170 participants had been formally diagnosed with COPD prior to our program, and the screening program enabled earlier confirmation in the other 719 patients. The proportion of the 889 COPD participants in GOLD stages I, II, III and IV were 50.3% (447/889), 37.1% (330/889),10.7% (95/889), and 1.9% (17/889), respectively. For the 719 newly diagnosed patients, 83 (11.5%) out of them were in stage III or IV COPD. Meanwhile, the other 3688 participants were classified as the non-COPD group. The entire screening process is shown in Figure 1.
According to the diagnostic criteria for COPD in the GOLD 2020,9 the prevalence of COPD among people aged 40 or older in this area was 19.4% (889/4577). The general characteristics and risk factors of participants are listed in Table 1, grouped according to the COPD diagnosis. The ratio of FEV1/FVC of the COPD group was (0.55 ± 0.07), while that for the non-COPD group was (0.73±0.06), P < 0.001. The average age in the COPD group was significantly older than that in the non-COPD group (66.24 ± 8.97 years vs 59.63 ± 9.31 years, P < 0.001), and the COPD group had lower BMI values (P < 0.001). Men exhibited a higher prevalence of COPD than women (26.6% vs 15.2%, P < 0.001). There were more farmers (69.7% vs 65.7%, P < 0.05) in the COPD group compared with the non-COPD group. A family history of chronic respiratory disease was more frequent in the COPD group (P < 0.001). Regarding indoor air pollution, clean energy (gas and electricity) was more commonly used in the non-COPD group (P < 0.001). The distribution of COPD varied according to education level (P < 0.001), BMI (P < 0.001), smoking history (P < 0.001), and use of a kitchen ventilation system (P < 0.001). There were no differences in occupational exposure, passive smoking, heating method, and livestock ownership between the two groups.
 |
Table 1 General Characteristics and Risk Factors Between COPD and Non-COPD Subjects |
The univariate analysis identified age, sex, occupation, education level, body mass index, smoking history, cooking fuel, family history of chronic respiratory diseases and kitchen ventilation as significant variables (Figure 2). The results of multivariable analysis (Figure 2) revealed that the prevalence of COPD increased with age. Men were 53.7% more likely to have COPD than women (odds ratio [OR] = 1.537, 95% CI 1.246–1.894, P < 0.001). Compared with never smokers, the former or current smokers were 1.338 times more likely to have COPD (OR = 1.338, 95% CI 1.069–1.675, P = 0.011). Individuals with a family history of chronic respiratory disease were 62.5% more likely to have COPD than individuals with no family history of chronic respiratory disease (OR = 1.625, 95% CI 1.350–1.957, P < 0.001). People who completed middle school and those with higher levels of education were 26.5% less likely to have COPD than those with lower education levels (OR = 0.735, 95% CI 0.617–0.876, P = 0.001). BMI showed the following protective association with COPD: overweight individuals (OR = 0.614, 95% CI 0.517–0.730, P < 0.001) and obese individuals (OR = 0.572 95% CI 0.449–0.721, P < 0.001).
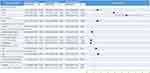 |
Figure 2 The association between risk factors and COPD via logistic regression modeling. Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; ref, reference. |
Discussion
This study was conducted in order to determine the prevalence and risk factors of COPD among local residents aged 40 years or older in a rapidly developing rural area of western China. Our study findings provide meaningful insight and knowledge regarding the prevalence of COPD in areas where industrialization and urbanization are rapidly progressing.
The prevalence of pulmonary function-identified COPD in the current study population was 19.4%. Yan et al14 reported that the prevalence of COPD was 12.4% among those aged 40 years and older in a well-developed city in eastern China, whereas a nationwide survey2 reported a prevalence of 13.7% in the same age group. Although the study methods were not entirely comparable, a rough comparison indicated that COPD in western rural areas is highly prevalent in China. Ambient air pollution has become a major public health crisis in China during the rapid process of industrialization and urbanization.15,16 Exposure to particulate matter 2.5 (PM2.5) is strongly associated with COPD occurrence.17 In western China, outdoor air is more severely polluted but the socioeconomic level is lower than that in eastern regions, partially affecting the epidemiology of COPD. These substantial changes in living and working environments in rural areas also lead to a reconsideration of risk factors for COPD. Older age, male sex, smoking history, lower education level, and family respiratory disease history were independent risk factors for COPD, while overweight and obesity were protective factors in our study.
Age is a well-recognized risk factor9 for chronic disease including COPD. As shown in the results, the association between age and prevalence of COPD increased with age in the population aged 40 years and above for each additional decade of age. This result is in line with those of previous studies.2,14
Our results showed that smoking was associated with a 1.6 times higher prevalence of COPD. Smoking has been confirmed as a direct cause of COPD.9 Additionally, a higher prevalence of COPD was found in men in our study in accordance with previous studies in China.2,18 This phenomenon has been explained by the higher likelihood of smoking among men.9 However, some studies in both developed and developing countries have found a similar prevalence in men and women,19,20 partially caused by passive smoking21 and biomass fuel exposure.22 In our study, exposure to passive smoking was comparable between the COPD and non-COPD groups. The use of biomass fuel (like firewood, coal and crop residues) and kitchen ventilation were significantly related to the prevalence of COPD after adjusting for age and sex. However, after full adjustment for multivariable analysis, the relationships between these factors and the prevalence of COPD became non-significant. The influence of ambient air pollution may mask the effect of biomass smoke on these residents.2
Many previous studies have established that a low level of education is associated with a higher prevalence of COPD.2,23 The current study showed that individuals who did not finish at least Nine Year Compulsory Education Program were more likely to have COPD. Education is also an index of socioeconomic24 status, which reflects access to health-care services. Populations living in rural areas of western China have poorer access to health care and limited health-related knowledge about this disease. This can lead to delayed disease detection and increased occurrence and exacerbation of COPD. In China, the public’s awareness of COPD lags far behind awareness of hypertension or diabetes.25 Education programs for prevention and diagnosis of COPD in rural areas are needed.
The current results revealed an increased prevalence of COPD among individuals with a family history of chronic respiratory disease. This result is in accordance with the findings of a previous survey26 in China. Family history of disease reflects both shared environmental exposures and inherited genetic susceptibilities. One confirmed genetic risk factor9 for COPD is severe α-1-antitrypsin deficiency (AATD) which has barely been reported in Chinese population. Zhou et al26 argued that the association between COPD and family history of respiratory disease can be traced back to the living environment where those COPD patients (who reported family history of lung disease) and their parents lived during their childhood. Living environment air pollution is a potential contributor to particles accumulation in the lungs of children, affecting the onset of lung disease as they grow older.
Unlike reports from many other studies in different countries27, being a farmer was not found to be an independent risk factor for COPD in our study. In previous studies, farmers were found to have a greater likelihood of being exposed to microorganisms and dust while farming or caring for livestock.27 However, in our study, similar occupational exposure rates were found in the COPD and non-COPD groups (62.7% VS 65.1%, P = 0.163) despite the greater number of farmers in the COPD group (69.7% VS 65.5%, P = 0.014) (Table 1).
In our study, both overweight (24–27.9 kg/m2) and obesity (≥28 kg/m2) were protective factors for COPD. The result is in accordance with the findings of previous studies.28–30 This protective effect could be explained as overweight/obese patients would have higher muscle mass that cannot be simply described by BMI, but can be assessed using the Fat Free Mass (FFM) index.31 For further understanding of the protective mechanisms of body weight, FFM can be a more persuasive indicator32 for loss of muscle compared with BMI, which requires further investigation.
The US Preventive Service Task Force33 (USPSTF) did not recommend screening for COPD routinely because there was no evidence showing improved clinical outcomes. However, this position is controversial.34,35 Some evidence36,37 indicates that COPD screening program may help the earlier detection of patients with severe or very severe airflow obstruction. The screening program in the current study enabled earlier COPD confirmation in the 719 patients. 11.5% of the patients were diagnosed as GOLD stage III and IV COPD. Unlike glucose testing or blood pressure monitoring which are accessible in most pharmacies, pulmonary function tests are barely available for the Chinese population.2 A mobile spirometry device can enable both primary care practitioners and rural residents to be more involved in the early detection of COPD. Eight nurses conducted spirometry testing for over 4601 participants in this limited time period in the current study. This COPD screening strategy in rural areas is inexpensive and rapid, making it suitable for use in low- and middle-income countries with a large potential population of individuals with COPD.
Limitations
The current study involved several limitations. First, we did not recruit participants with a random sampling method; and study participants voluntarily attended the health check. This may have led to a lack of data from some individuals who were unable to travel or paid less attention to their health; these groups are usually considered to have poorer health status or behaviors.31 This could have potentially resulted in an underestimation of the prevalence of COPD. Conversely, it is also possible that the prevalence was overestimated because individuals with symptoms were more likely to participate. To minimize the influence of missing data, we did our best to ensure that all eligible individuals were informed about the health check program via local official announcements through both online messages and traditional posters. The health check was conducted in the primary care station in each village or district to ensure equal involvement of participants aged 40 years and older based on approximately 10% of the target population at each research site. As a result, participants from a wide age range (40 to 96 years old) and different research sites were included. Second, different spirometers were used in the two screening steps. However, evidence10 indicated that the two kinds of spirometers shared sufficient agreement in test results. Using only the Jaeger spirometer at a secondary hospital would impede the involvement of participants in remote areas and it may not be possible for every primary care station to have its own complex pulmonary function test equipment. The adoption of a mobile spirometer may enable greater participation. These methods were more closer to the clinical practice in the real world.
Conclusion
The findings of the current study revealed a high prevalence of COPD in typical rural areas of western China. Rural residents who were older, current or ever smokers, male and with a lower education level were more vulnerable to developing COPD. During the process of urbanization, risk factors for COPD can change within any given target group. COPD screening programs can help with earlier disease detection in primary care settings. Such programs are convenient and accessible to the population in rural areas.
Ethical Approval
This project was approved by the Ethics Committee on Biomedical Research West China Hospital of Sichuan University (Ethical No. 2019-065). The study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
For continuous support, assistance, cooperation, and participation, we thank the local committees in the villages and districts in Jianyang, Sichuan Province, China. We thank Benjamin Knight, MSc., from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
Funding
This work was supported by West China Nursing Discipline Development Special Fund Project, Sichuan University (Grant NO. HXHL19034); 1-3-5 Project for Disciplines of Excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (Grant NO. 2018HXFH031); Chengdu Municipal Health Commission (Grant NO.2020116); Science and Technology of Sichuan Province (Grant NO. 2021YFS0130). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
1. WHO’s Global Health Estimates. The top 10 causes of death. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
2. Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717. doi:10.1016/S0140-6736(18)30841-9
3. IHME. What causes the most death and disability combined? Available from: http://www.healthdata.org/china.
4. Zhu B, Wang Y, Ming J, Wen C, Zhang L. Disease burden of COPD in China: a systematic review. Int J Chron Obstruct Pulmon Dis. 2018;13:1353–1364. doi:10.2147/COPD.S161555
5. IQAir. LIVE AQI city ranking; 2021. Available from: https://www.iqair.cn/cn/world-air-quality-ranking.
6. Pan Z, Dichens AP, Chi C, et al. Study to evaluate the effectiveness and cost-effectiveness of different screening strategies for identifying undiagnosed COPD among residents (≥40 years) in four cities in China: protocol for a multicentre cross-sectional study on behalf of the Breathe Well group. BMJ Open. 2020;10:e035738. doi:10.1136/bmjopen-2019-035738
7. Fleiss JL, Leivin B, Paik M. Statistical Methods for Rates and Proportions.
8. Newcombe R. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17(8):857–872. doi:10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e
9. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2020. Available from: https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf.
10. Zhou L, Jiang Y, Du C, et al. Development of an internet-of-things based portable spirometer and the validation of its accuracy (in Chinese). Int J Respir. 2019;39(2):113–118. doi:10.3760/cma.j.issn.1673-436X.2019.02.007
11. China Methology Association, ATC 17. Calibration specification for the pulmonary function measuring instrument. In: General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Beijing, China: China Methology Association; 2009.
12. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805
13. Gerbase M, Dupuis-Lozeron E, Schindler C, Keidel D, Künzli N. Agreement between spirometers a challenge in the follow-up of patients and populations? Respiration. 2013;85(6):505–514. doi:10.1159/000346649
14. Yan X, Xu L, Shi B, Wang H, Xu X, Xu G. Epidemiology and risk factors of chronic obstructive pulmonary disease in Suzhou: a population-based cross-sectional study. J Thorac Dis. 2020;12(10):5347–5356. doi:10.21037/jtd-20-1616
15. He M, Zeng X, Zhang K, Kinney PL. Fine particulate matter concentrations in urban Chinese cities, 2005–2016: a systematic review. Int J Environ Res Public Health. 2017;14:191. doi:10.3390/ijerph14020191
16. Guan WJ, Zheng XY, Chung KF, Zhong NS. Impact of air pollution on the burden of chronic respiratory diseases in China: time for urgent action. Lancet. 2016;388(10054):1939–1951. doi:10.1016/S0140-6736(16)31597-5
17. Liu S, Zhou Y, Liu S, et al. Association between exposure to ambient particulate matter and chronic obstructive pulmonary disease: results from a cross-sectional study in China. Thorax. 2017;72(9):788–795. doi:10.1136/thoraxjnl-2016-208910
18. Zhong N, Wang C, Yao W, et al. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med. 2007;176(8):753–760. doi:10.1164/rccm.200612-1749OC
19. Foreman MG, Zhang L, Murphy J, et al. Early-onset chronic obstructive pulmonary disease is associated with female sex, maternal factors, and African American race in the COPD gene study. Am J Respir Crit Care Med. 2011;184(4):414–420. doi:10.1164/rccm.201011-1928OC
20. Landis SH, Mannino H, Mannino DM, Menezes AM, Davis JK. Continuing to confront COPD international patient survey: methods, COPD prevalence, and disease burden in 2012–2013. Int J Chron Obstruct Pulmon Dis. 2014;9:597–611. doi:10.2147/COPD.S61854
21. Hagstad S, Bjerg A, Ekerljung L, et al. Passive smoking exposure is associated with increased risk of COPD in never smokers. Chest. 2014;145(6):1298–1304. doi:10.1378/chest.13-1349
22. Sana A, Somda S, Meda N, Bouland C. Chronic obstructive pulmonary disease associated with biomass fuel use in women: a systematic review and meta-analysis. BMJ Open Respir Res. 2018;5(1):e000246. doi:10.1136/bmjresp-2017-000246
23. Bird Y, Moraros J, Mahmood R, Esmaeelzadeh S, Soe N. Prevalence and associated factors of COPD among aboriginal peoples in Canada: a cross- sectional study. Int J COPD. 2017;12:1915–1922. doi:10.2147/COPD.S138304
24. Grigsby M, Siddharthan T, Chowdhury MA, et al. Socioeconomic status and COPD among low- and middle-income countries. Int J Chron Obstruct Pulmon Dis. 2016;11:2497–2507. doi:10.2147/COPD.S111145
25. Fan JW, Wang N, Fang LW, Feng YJ, Wang BH. Awareness of knowledge about chronic obstructive pulmonary disease and related factors in residents aged 40 years and older in China, 2014. Chin J Epidemiol. 2018;39(5):586–592. doi:10.3760/cma.j.issn.0254-6450.2018.05.009
26. Zhou Y, Wang C, Yao W, et al. COPD in Chinese nonsmokers. Eur Respir J. 2009;33:509–518. doi:10.1183/09031936.00084408
27. Guillien A, Puyraveau M, Soumagne T, et al. Prevalence and risk factors for COPD in farmers: a cross-sectional controlled study. Eur Respir J. 2016;47(1):95–103. doi:10.1183/13993003.00153-2015
28. Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronicobstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(6):1856–1861. doi:10.1164/ajrccm.160.6.9902115
29. Guo Y, Zhang T, Wang Z, et al. Body mass index and mortality in chronic obstructive pulmonary disease: a dose-response meta-analysis. Medicine. 2016;95(28):e4225. doi:10.1097/MD.0000000000004225
30. Brigham EP, Anderson JA, Brook RD, et al. Challenging the obesity paradox: extreme obesity and COPD mortality in the SUMMIT trial. ERJ Open Res. 2021;7(3):00902–2020. doi:10.1183/23120541.00902-2020
31. Hanson C, LeVan T. Obesity and chronic obstructive pulmonary disease: recent knowledge and future directions. Curr Opin Pulm Med. 2017;23(2):149–153. doi:10.1097/MCP.0000000000000354
32. Vestbo J, Prescott E, Almdal T, et al. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen city heart study. Am J Respir Crit Care Med. 2006;173(1):79–83. doi:10.1164/rccm.200506-969OC
33. Siu AL, Bibbins-Domingo K, Grossman DC; US Preventive Task Force. Screening for chronic obstructive pulmonary disease: US preventive services task force recommendation statement. JAMA. 2016;315(13):1372–1377. doi:10.1001/jama.2016.2638
34. Bhatt SP, O’Conner GT. Screening for chronic obstructive pulmonary disease challenges and opportunities. JAMA. 2022;19(8):1267–1274. doi:10.1001/jama.2022.3823
35. Fortis S. Should we consider screening spirometry in individuals who are “asymptomatic”? Ann Am Thorac Soc. 2022;19(8):1268–1269. doi:10.1513/AnnalsATS.202205-374ED
36. Bednarek M, Maciejewski J, Wozniak M, Kuca P, Zielinski J. Prevalence, severity and un-derdiagnosis of COPD in the primary care setting. Thorax. 2008;63(5):402–407. doi:10.1136/thx.2007.085456
37. Mapel DW, Dalal AA, Blanchette CM, Petersen H, Ferguson G. Severity of COPD at initial spirometry-confirmed diagnosis: data from medical charts and administrative claims. Int J Chron Obstruct Pulmon Dis. 2016;6:573–581. doi:10.2147/COPD.S16975
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.