Back to Journals » Infection and Drug Resistance » Volume 15
Prevalence and Predictors of Persistent Symptoms After Clearance of SARS-CoV-2 Infection: A Multicenter Study from Egypt
Authors Khalaf M, Alboraie M , Abdel-Gawad M , Abdelmalek M, Abu-Elfatth A, Abdelhamed W, Zaghloul M , ElDeeb R , Abdeltwab D, Abdelghani M, El-Raey F, Aboalam H, Badry A, Tharwat M, Afify S, Elwazzan D, Abdelmohsen AS , Fathy H , Wagih Shaltout S , Hetta HF , Bazeed SE
Received 21 December 2021
Accepted for publication 12 May 2022
Published 20 May 2022 Volume 2022:15 Pages 2575—2587
DOI https://doi.org/10.2147/IDR.S355064
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Marwa Khalaf,1 Mohamed Alboraie,2 Muhammad Abdel-Gawad,3 Mohamed Abdelmalek,4 Ahmed Abu-Elfatth,4 Walaa Abdelhamed,5 Mariam Zaghloul,6 Rabab ElDeeb,7 Doaa Abdeltwab,4 Mohamed Abdelghani,4 Fathiya El-Raey,8 Hani Aboalam,1 Azza Badry,9 Mina Tharwat,10 Shima Afify,11 Doaa Elwazzan,7 Ahmed Shawkat Abdelmohsen,4 Hayam Fathy,12 Shaker Wagih Shaltout,13 Helal F Hetta,14 Shamardan E Bazeed15
1Assiut Liver Center, Ministry of Health, Assiut, 71515, Egypt; 2Department of Internal Medicine, Al-Azhar University, Cairo, Egypt; 3Hepatology, Gastroenterology and Infectious Diseases Department, Al-Azhar University, Assiut, Egypt; 4Tropical Medicine and Gastroenterology Department, Assiut University, Assiut, Egypt; 5Tropical Medicine and Gastroenterology Department, Sohag University Hospital, Sohag, Egypt; 6Hepatology, Gastroenterology and Infectious Diseases Department, Kafr El-Sheikh University, Kafr El-Sheikh, Egypt; 7Tropical Medicine Department, Alexandria University, Alexandria, Egypt; 8Hepatogastroenterology and Infectious Diseases Department, Al-Azhar University, Damietta, Egypt; 9Epidemiologist, Infectious Disease Control Department Preventive Medicine Assiut Health Affairs Directorate, Assiut, Egypt; 10Tropical Medicine and Gastroenterology Department, Aswan University, Aswan, Egypt; 11Gastroenterology Department, National Hepatology and Tropical Medicine Research Institute, Cairo, Egypt; 12Internal Medicine, Hepatogastroenterology Unit, Assiut University, Assiut, Egypt; 13Tropical Medicine Department, Port Said University, Port Said, Egypt; 14Department of Medical Microbiology and Immunology, Faculty of Medicine, Assiut University, Assiut, 71515, Egypt; 15Tropical Medicine and Gastroenterology Department, South Valley University, Qena, Egypt
Correspondence: Marwa Khalaf, Assiut liver center, Ministry of health, Assiut, 71515, Egypt, Email [email protected] Helal F Hetta, Department of Medical Microbiology and Immunology, Faculty of Medicine, Assiut University, Assiut, 71515, Egypt, Email [email protected]
Background and Aim: Little is known about the persistence of symptoms after clearance of SARS-CoV-2 infection. Our study aimed to assess persistent symptoms in COVID-19 patients after clearance of SARS-CoV-2 infection.
Methodology: A multi-center survey was conducted on first wave COVID-19 patients with confirmed SARS-CoV-2 infection. Sociodemographic and clinical characteristics, including presenting symptoms and persistent symptoms after viral clearance and possible factors contributing to persistence of such symptoms, were collected using an online multicomponent questionnaire. Descriptive and inferential statistical analysis was performed to detect the most persisting symptoms and factors contributing to their persistence.
Results: Overall, 538 patients were enrolled. Mean age was 41.17 (±SD 14.84), 54.1% were males, and 18.6% were smokers. Hypertension and diabetes were the most reported co-morbidities. Mild symptoms were reported in 61.3% of patients, 51.3% were admitted to hospital and 6.5% were admitted to the intensive care unit. Our study identified 49 types of persisting symptoms. Fatigue (59.1%), sense of fever (46.5%), anorexia (24.3%) and diarrhea (24.3%) were the most commonly reported persisting symptoms followed by loss of taste and smell (22.3%), headache (21.4%), cough (20.8) and dyspnea (21%). The use of hydroxychloroquine, azithromycin and multivitamins were significantly associated with persistence of symptoms (OR = 8.03, 8.89 and 10.12, respectively).
Conclusion: Our study revealed that in COVID-19 recovered patients, many patients reported persistence of at least one symptom, particularly fatigue and sense of fever. Follow-up of patients after discharge from hospital is recommended until complete resolution of symptoms.
Keywords: coronavirus, COVID-19, SARS-CoV-2, fatigue, dyspnea
Introduction
In December 2019, a novel coronavirus (initially named 2019-nCov) was discovered to be responsible for outbreaks of an unusual series of viral pneumonia of unknown origin in Wuhan. It was later named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), because of the structural similarities with SARS-CoV, that caused the outbreak of SARS in 2003.1–3
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is an enveloped, single-stranded ribonucleic acid beta coronavirus. This highly contagious pathogen is transmitted by respiratory droplets and aerosols, direct contact of mucous membranes and probably the fecal–oral route.4–6
This viral infection primarily targets the respiratory system, and is usually presented by fever, cough, sore throat or shortness of breath as initial symptoms.7,8 Although some patients may be asymptomatic and they are likely to spread the infection, a group of them may develop symptoms and their condition may worsen.9–12
Pulmonary symptoms are the most frequently reported symptoms, however recent studies proved the presence of neurological and gastrointestinal manifestations among the SARS-CoV-2 infected patients.13,14
Although real-time reverse transcription-polymerase chain reaction (RT-PCR) assay is considered the first tool to make a definitive diagnosis of COVID-19, the high false negative results, low sensitivity and limited supplies might delay accurate diagnosis. Computed tomography (CT) has been reported as an important tool to identify and investigate suspected patients with COVID-19 at an early stage.15
Many patients with mild or severe SARS-CoV-2 do not make a full recovery and have a wide range of persistent symptoms for weeks or months after infection, often of a neurological, cognitive or psychiatric nature.16
A standardized case definition for post-COVID-19 syndrome is still being developed. The Centre for Disease Control (CDC) has formulated post-COVID-19 conditions to describe health issues that persist more than four weeks after being infected with COVID-19. The World Health Organization has also developed a clinical case definition of post-COVID-19 syndrome to include individuals with a history of probable or confirmed SARS-CoV-2 infection usually 3 months from the onset of infection with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis.17
The pathophysiological basis is not well understood, however immune reaction, inflammation, persistent viremia, relapse or reinfection are all suggested etiologies.16,18,19
To date physicians and researchers are still learning about the symptoms and signs of this novel virus. Many survivors may experience many morbidities and multiple manifestations requiring long-term monitoring. Hence the aim of this study was to determine the persistence of any symptoms or signs after clearance of SARS-CoV-2 in patients with COVID-19 infection during the first wave.
Methods
Patient Grouping and Selection Criteria
During the period between August 2020 and October 2020, a multicenter cross-sectional survey was done.
A list was made of all patients who had been discharged from quarantine hospitals after recovery from COVID-19 during the period from March to May 2020. Our patients fulfilled the criteria of the World Health Organization for discontinuation of quarantine which include that the patient should have no fever for 3 consecutive days, the test results should be negative for SARS-CoV-2, with improvement of other symptoms. A stratified sampling technique was used to select a random sample from this list.
The sample size was calculated by OpenEpi, Version 3, and open-source calculator. It was found to be 384 with CI 95% and error probability of 5%.
Data on specific symptoms, which may be correlated with COVID-19, were obtained using a standardized questionnaire which was adapted and administered by the researchers to the patients by visit or phone call.
The study tool included two sections, the first one was for demographic data (age, sex, governorate, and smoking), pre-existing comorbidities, medication used, date of initial diagnosis (first positive PCR test for SARS-CoV-2) and date of negative PCR for SARS-CoV-2. In addition there was a section on health-care management details (home isolation, hospital or ICU admission) including length of hospital stay, medication used, oxygen therapy and if used ventilation (invasive or non-invasive).
Patients were questioned about the presence or absence of symptoms during the acute phase of COVID-19 and if each symptom persisted at the time of the visit or phone call. Patients were asked about: sense of fever, skin rash, pruritus, bone aches, cough, dyspnea, sore throat, rhinorrhea, chest pain/tightness, palpitation, syncopal attacks, fatigue, muscle pain, joint pain/stiffness, anosmia, ageusia, headache, dizziness, numbness, diarrhea, nausea, vomiting, anorexia, abdominal pain, constipation, dyspepsia, dysphagia, jaundice, weight loss/gain, hematemesis, melena, visual changes, hearing changes, vertigo, low/high mood, poor sleep, agitation, self-harm, delusions, hallucinations, thought disorders, suicidal tendency, dysuria, hematuria, vaginal bleeding, abortion, puffy eyes, loss of libido, and erectile dysfunction. Date of appearance and date of resolution were reported. An open text field was added at the end of the symptoms collection sheet to add any other symptoms or possible complications of COVID-19 infection. Patients or the public were not involved in the design, conduct, reporting, or dissemination plans of our research.
Sample Size
The sample size was calculated using OpenEpi, Version 3, for proportion studies. Population size (number of reported COVID-19 patients in Egypt at the time of the study) (N): ~338,000, Hypothesized % frequency of post-COVID-19 symptoms in the population (p): 50% ± 5, confidence interval of 95%, and design effect (for cluster surveys-DEFF): 1.
The sample size was 384 with CI 95% and error probability of 5%. However, we included 538 cases.
Consent and Ethics Committee Approval
To achieve proper social distancing and to decrease risk of possible transmission of COVID-19, respondents were interviewed either by a visit in a non-COVID designated area or through a phone call. Paper use for documentation was also avoided. We explained to the respondents the objectives of the study and sent them an information sheet containing all details of the study to read before the interview. A written consent to participate in the study was obtained before administration of the questionnaires. This study was approved by the Damietta Faculty of Medicine – Al Azhar University Ethical committee – IRB 00012367.
Patient and Public Involvement
There was no direct patient involvement in this study.
Statistical Analysis
Descriptive data analysis was performed for categorical variables including frequencies and proportions. As appropriate, inferential statistics were performed between groups with the Chi square test or Kruskal–Wallis test. Differences within groups were evaluated with the Wilcoxon Signed Rank test. Multiple regression analysis was performed to predict the persistence of symptoms at follow-up. P value level of significance was set at 0.05. Data entry and analysis were completed using MS Excel 2017 and data analysed using SPSS Version 25.
Results
We started with 561 subjects, 23 were excluded either due to difficult communication or refusal of the patient to participate in the study. So, our study included 538 patients with confirmed SARS-CoV-2 infections. The study flow chart is shown in Figure 1. 54.1% were male. The mean age was 41.17 (±SD 14.84, range 5–87 years) and 18.6% were smokers. The most reported co-morbid conditions were diabetes mellitus in 17.1%, hypertension in 19.5% (5.2% were receiving ARBs and 7.6% were receiving ACEIs), COPD in 5.4%, chronic kidney disease in 1.1%, ischemic heart disease in 4.5% and immunosuppressive state in 0.4% (Table 1).
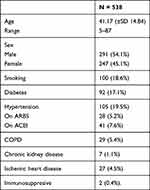 |
Table 1 Demographic and Clinical Characters of Studied Patients |
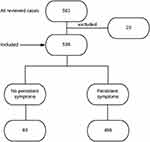 |
Figure 1 Study flow chart. |
Almost half of the studied patients (51.3%) were admitted to hospital with an average hospital stay of 13.58 (SD ± 6.40, range 4–37) days, 6.5% were admitted to ICU with an average ICU stay of 9.66 (SD ± 5.85, range 2–30) days. Symptoms were mild in 61.3%, moderate in 31% and severe in 7.6% of patients (Table 2).
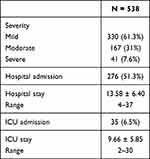 |
Table 2 Severity and Hospital Stay Characterization of Studied Patients |
Frequencies of medication used in treatment of the studied patients are presented in Figure 2. Most commonly reported symptoms persisting after viral cure were fatigue, cough, dyspnea, sore throat, loss of smell, anorexia, loss of taste, diarrhea, headache, low mood, abdominal pain, nausea, muscle pain, chest pain, joint pain and poor sleep (Figure 3). Although reported in the active stage of the disease, the following symptoms were not persistent after viral clearance: abortion (reported initially in 0.6%), puffy eyes (reported initially in 0.4%), hallucination (reported initially in 0.8%), thought disorders (reported initially in 0.2%), suicidal tendency (reported initially in 0.4%), self-harm (reported initially in 0.2%), facial droop (reported initially in 0.2%), photophobia (reported initially in 1%), dysarthria (reported initially in 0.6%), vomiting (reported initially in 12.3%), wheeze (reported initially in 2%), hemoptysis (reported initially in 0.4%) and rhinorrhea (reported initially in 5.8%). The symptoms reported initially and that persisted after viral cure are presented in Table 3. Factors associated with symptoms persistence were hospital admission, disease severity, treatment with hydroxychloroquine, steroid, anticoagulant, azithromycin, multivitamins and receiving oxygen therapy; the rest of the other factors were not associated with symptom persistence in univariate analysis (Table 4). Multivariate analysis showed that treatment with hydroxychloroquine, azithromycin and multivitamins were the only factors associated with symptom persistence (Table 5).
 |
Table 3 Symptoms Persisting After Clearance of SARS-CoV-2 Infection |
 |
Table 4 Factors Associated with Persistent Symptoms Persistence |
 |
Table 5 Multivariate Analysis for Predictors of Post-Covid-19 Persisting Symptoms |
 |
Figure 2 Frequencies of medication used in treatment of the studied patients. |
 |
Figure 3 Post Covid-19 acute and persistent symptoms. |
Discussion
Since the start of the COVID-19 pandemic, Egypt reported 337,487 confirmed cases and 228,583 were discharged after clearance of the virus.8 Interestingly, some of those patients presented to the outpatient clinics complaining of vague symptoms resembling the acute phase symptoms that triggered the concepts of incomplete recovery or persistence of COVID-19 infection. This is an Egyptian study for assessment of the post-discharge persistent symptoms after recovery from COVID-19 and possible long-term impact of COVID‐19 infection.
In our study, 84.6% of patients who recovered from COVID-19 have one or more persistent symptoms. Fatigue, cough, sense of fever and dyspnea were among the most common reported symptoms followed by sore throat, anorexia, loss of taste and smell, diarrhea, headache, and low mood.
The median duration to symptom resolution among those with persistent symptoms ranged from 1 to 83 days from the negative PCR test date, with the longest duration reported for vertigo (median = 82 days; 23–147 days) and numbness (median = 77 days; 1–126 days).
A telephone-based report from the USA investigating 274 symptomatic COVID-19 adult outpatients, found 2–3 weeks are needed by about 30% of contributors to get back to their usual state. Cough, fatigue and shortness of breath at the time of testing were the most persistent symptoms. The median duration for disappearance of symptoms ranged from 4–8 days from the test date. The longest duration was reported for anosmia (median = 8 days; IQR = 5–10.5 days) and loss of taste (median = 8 days; IQR = 4–10 days).20
Also, a single-center study from Rome included 143 hospitalized post-COVID-19 recovered patients who were assessed 60 days following infection. Surprisingly, only about 13% were completely free of any persistent symptoms. Meanwhile, 32% had at least one or two symptoms and 55% showed three or more persistent manifestations.21
A Facebook-based survey in the Netherlands and Belgium that included a large scale of COVID-19 patients either hospitalized or non-hospitalized, confirmed or suspected, showed that only 0.7% of the respondents were symptom-free 79 days after the infection. Fatigue and dyspnea were the most common symptoms, in both hospitalized and non-hospitalized patients.22
Interestingly, 58.5% of our patients with mild COVID-19 infection have one or more persistent symptoms which is consistent with anecdotal evidence, which stated that patients with the so-called “mild” COVID-19 may still complain about persistent symptoms, even weeks after the onset of symptoms.23,24
In agreement with our results, Davido et al.25 reported that most of the outpatients who experienced mild symptoms attributable to COVID-19 would further present with persistent symptoms, such as sense of fever, severe fatigue, chest tightness, palpitations, muscle aches, anxiety and headaches shortly after convalescence.
In our study, fatigue persisted in about 59.1% of participants for a median of 31 days. This was in accordance with data reported from France,25 Italy21 and UK.26 Fatigue was explained by dysautonomia that was reported in the ALBA COVID registry (2.5%),27, also endocrine disturbance with hypothalamus-pituitary-adrenal axis attenuation, reactive mood disorder such as depression or anxiety could be contributing factors for pathophysiology of post-COVID-19 fatigue syndrome.28,29
Similar results also reported from a single-center study in the UK that investigated 100 post-discharge COVID-19 patients showed that fatigue was the most commonly described symptom in both ICU and ward groups (72% and 60.3%, respectively).26
Contrary to our findings, a Chinese prospective cohort study of 131 COVID-19 patients in Wuhan found that by 3–4 weeks post-discharge 86% of patients were asymptomatic, only 1.5% had shortness of breath and 0% had fatigue. This could be attributed to the lower case severity of these patients with few co-morbidities. Moreover, underreporting could be expected due to the nature of this study focusing on evaluation of ongoing transmissibility, and participants were asked about the quarantine situation.30
Post viral infection fatigue syndrome was first described in Epstein–Barr virus (EBV) infection.31 In the previously experienced epidemics of SARS, H1N1 and Ebola, many patients with persistent fatigue were serious enough to be diagnosed as Myalgia Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). More than 50% of patients surviving SARS experienced fatigue during their recovery: 64% reported fatigue at 3 months, 54% at 6 months and 60% at 12 months.32
Fatigue and breathlessness are not uncommonly reported as persistent symptoms following community-acquired pneumonia and ICU admission, but the duration varies substantially.33,34 Hospitalized patients with community-acquired pneumonia in several studies were found to experience breathlessness and fatigue that usually resolved in 10–14 days from symptom onset.35
Among the persistent neuropsychiatric symptoms detected in our work were low mood (20.6%), poor sleep (12.6%) and poor concentration (4.8%). Our results are consistent with Garrigues et al.36 who found that after a mean of 110.9 days, the most frequently reported persistent post-COVID-19 symptoms were loss of memory, concentration and sleep disorders (34%, 28% and 30.8%, respectively). These results are in agreement with Srivastava et al. who reported that recovered COVID-19 patients suffered from a significant degree of depression and high rate of post-traumatic stress disorder (PTSD).37
Classic neurological disorder such as loss of taste, smell, headache, numbness and vertigo were present and persist in 22.9%, 21.7%, 21.4, 19% and 12%, respectively, these results were in accordance with results from a systematic review conducted in 2022 by Whittaker et al.38
These neurological disorders are attributed to endothelial injury and microangiopathy, which was described in brain biopsies of severe form of COVID-19.39 Also, severity of condition and PTSD could be co-factor in neuropsychiatric persistence symptoms,40 in children it seems similar to the late Kawasaki syndrome that was reported after COVID-19.41
In the present study, a sense of fever was detected in 250 (46.5%) patients and persisted for 20.68 ± 30.66 days (12 patients confirmed the presence of fever by measuring temperature) in contrast with the progressive decline observed in cases of influenza.
Ng et al. studied 142 patients with COVID-19 for persistence of fever. They observed that 12.7% had fever lasting more than 7 days (prolonged fever), and 9.9% had recurrence of fever lasting less than a day after defervescence after day 7 of illness (saddleback fever) that may be correlated to decreased levels of interleukin 1 alpha and increased levels of interferon gamma-induced protein 10 in their patients with prolonged fever.42
Moreover, it was found that COVID-19 patients may complain of low-grade fever during convalescence which was attributed to the incomplete recovery of their immunity at that stage which elucidates the recurrence of SARS-CoV-2 positivity that was noticed in many patients during convalescence.43
Among the non-respiratory manifestations that are of special concern in COVID-19 patients, were the gastrointestinal tract (GIT) symptoms. They may be solitary, they may become progressive during the course of the disease and they may occur early, which is completely different from the other coronaviruses.44
The most prevalent GI symptom in our study was anorexia which was detected in 131 patients (24.3%) as a persistent symptom after cure for 1–97 days. Moreover, 131 patients (24.3%) had diarrhea that continued after cure for 1–100 days. Diarrhea can be explained by the change in the intestinal permeability that is caused by the virus, leading to dysfunction of the enterocytes.45 That was in agreement with the Garrigues et al.36 study in which diarrhea persisted in 29 patients (24.2%).
In our study, abdominal pain was reported in 106 patients (18.7%) and 97 patients (18%) had persistent pain after cure for 1–104 days. In contrast, Kecler-Pietrzyk et al. reported anorexia, diarrhea and nausea among the common persistent symptoms, but abdominal pain was rare, particularly as the initial presenting complaint.46
Neither age nor presence of co-morbid conditions were associated with persistent symptoms in our study, whereas Tenforde et al.20 found that those with older age and chronic co-morbidities were associated with much prolonged disease.
In our work, it was found that hospital admission and the use of some drugs such as chloroquine, steroids, anticoagulants, azithromycin, multivitamins and oxygen therapy during acute COVID-19 phase, and severity of the disease were associated with persistence of symptoms. However, results of multivariate logistic regression analysis revealed that the use of chloroquine, azithromycin and multivitamins only were significantly associated with persistence of symptoms (Odds ratio 8.03, 8.89 and 10.12, respectively). This is also in accordance with results of other studies on post-viral/infectious syndromes47,48 and those with critically ill ICU (non-COVID) patients, who still suffer a variety of symptoms months after their hospitalization, what is also named post-ICU syndrome.49,50
Limitations of our study include the lack of information on symptom history before acute COVID-19 illness and being based on a single phone call interview that created an obstacle of contacting certain participants, such as those with dementia and/or learning difficulties. Also, the telephone-based survey is subjected to incomplete recall errors or recall bias. So, we recommend future interviews at monthly intervals for better characterization of symptoms progression of post‐COVID‐19 patients. Furthermore, patients who had a negative swab result and clinical-radiological criteria suggestive of COVID-19 were not included in this study. Our study had the advantage of obtaining detailed symptom severity inquiry. In addition, this is a multi-centre study with a relatively large number of patients.
Conclusions
The post-COVID-19 symptoms should be carefully addressed and evaluated carefully. Those patients could suddenly seek care for what might be considered a chronic fatigue syndrome. Persistent symptomatic post-COVID-19 patients should be managed by a multidisciplinary team including a psychologist, a pulmonologist, a neurologist and a specialist in physical medicine and rehabilitation in specialized post-COVID-19 clinics to optimize our health-care services.
Institutional Review Board Statement
The study was conducted in accordance with ethical guidelines of the 1975 Helsinki Declaration. This study was approved by the Damietta Faculty of Medicine – Al Azhar University Ethical committee – IRB 00012367. All participants were adults and all of them provided written informed consent before collection of samples. To achieve proper social distancing and to decrease risk of possible transmission of COVID-19, respondents were interviewed either by a visit in a non-COVID designated area or through a phone call. Paper use for documentation was also avoided. We explained to the respondents the objectives of the study and sent them an information sheet containing all details of the study to read before the interview. A written consent to participate in the study was obtained before administration of the questionnaires.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest.
References
1. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi:10.1038/s41586-020-2012-7
2. Abd Ellah NH, Gad SF, Muhammad K, Batiha EG, Hetta HF. Nanomedicine as a promising approach for diagnosis, treatment and prophylaxis against COVID-19. Nanomedicine. 2020;15(21):2085–2102. doi:10.2217/nnm-2020-0247
3. Abid SA, Muneer AA, Al-Kadmy IM, et al. Biosensors as a future diagnostic approach for COVID-19. Life Sci. 2021;273:119117. doi:10.1016/j.lfs.2021.119117
4. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan HJG. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833. e1833. doi:10.1053/j.gastro.2020.02.055
5. Mahmood Z, Alrefai H, Hetta HF, et al. Investigating virological, immunological, and pathological avenues to identify potential targets for developing covid-19 treatment and prevention strategies. Vaccines. 2020;8(3):443. doi:10.3390/vaccines8030443
6. Moubarak M, Kasozi KI, Hetta HF, et al. The rise of SARS-CoV-2 variants and the role of convalescent plasma therapy for management of infections. Life. 2021;11(8):734. doi:10.3390/life11080734
7. Almaghaslah D, Kandasamy G, Almanasef M, Vasudevan R, Chandramohan S. Review on the coronavirus disease (COVID-19) pandemic: its outbreak and current status. Int J Clin Pract. 2020;74(11):e13637. doi:10.1111/ijcp.13637
8. Abdellatif AA, Tawfeek HM, Abdelfattah A, Batiha GE-S, Hetta HF. Recent updates in COVID-19 with emphasis on inhalation therapeutics: nanostructured and targeting systems. J Drug Deliv Sci Technol. 2021;63:102435. doi:10.1016/j.jddst.2021.102435
9. Dai W, Chen X, Xu X, et al. Clinical characteristics of asymptomatic patients with SARS-CoV-2 in Zhejiang: an imperceptible source of infection. Can Respir J. 2020;2020:2045341. doi:10.1155/2020/2045341
10. Magdy Beshbishy A, Hetta HF, Hussein DE, et al. Factors associated with increased morbidity and mortality of obese and overweight COVID-19 patients. Biology. 2020;9(9):280. doi:10.3390/biology9090280
11. Beshbishy AM, Oti VB, Hussein DE, et al. Factors behind the higher COVID-19 risk in diabetes: a critical review. Front Public Health. 2021;9:591982.
12. Koneru G, Batiha GE-S, Algammal AM, et al. BCG vaccine-induced trained immunity and COVID-19: protective or bystander? Infect Drug Resist. 2021;14:1169. doi:10.2147/IDR.S300162
13. Iltaf S
14. Laszkowska M, Faye AS, Kim J, et al. Disease course and outcomes of COVID-19 among hospitalized patients with gastrointestinal manifestations. Clin Gastroenterol Hepatol. 2020;19(7):1402–1409.
15. Alsharif W, Qurashi A. Effectiveness of COVID-19 diagnosis and management tools: a review. Radiography. 2021;27(2):682–687. doi:10.1016/j.radi.2020.09.010
16. Islam MF, Cotler J, Jason LA. Post-viral fatigue and COVID-19: lessons from past epidemics. Fatigue. 2020;8(2):61–69.
17. World Health Organization. A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October 2021; 2021.
18. Dar HA, Waheed Y, Najmi MH, et al. Multiepitope subunit vaccine design against COVID-19 based on the spike protein of SARS-CoV-2: an in silico analysis. J Immunol Res. 2020;2020:8893483. doi:10.1155/2020/8893483
19. Collaborative G, Collaborative C. SARS-CoV-2 vaccination modelling for safe surgery to save lives: data from an international prospective cohort study. Br J Surg. 2021;108(9):1056–1063.
20. Tenforde MW, Kim SS, Lindsell CJ, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network—United States, March–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(30):993. doi:10.15585/mmwr.mm6930e1
21. Carfì A, Bernabei R, Landi F; Group ftGAC-P-ACS. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi:10.1001/jama.2020.12603
22. Goërtz YMJ, Van Herck M, Delbressine JM, et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020;6(4):00542–02020. doi:10.1183/23120541.00542-2020
23. Garner P. Covid-19 and fatigue—a game of snakes and ladders; 2020.
24. Callard F, Perego E. How and why patients made Long Covid. Soc Sci Med. 2021;268:113426. doi:10.1016/j.socscimed.2020.113426
25. Davido B, Seang S, Tubiana R, de Truchis P. Post-COVID-19 chronic symptoms: a postinfectious entity? Clin Microbiol Infect. 2020;26(11):1448–1449. doi:10.1016/j.cmi.2020.07.028
26. Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2021;93(2):1013–1022.
27. Romero-Sánchez CM, Díaz-Maroto I, Fernández-Díaz E, et al. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020;95(8):e1060–e1070. doi:10.1212/WNL.0000000000009937
28. Papadopoulos AS, Cleare A. Hypothalamic–pituitary–adrenal axis dysfunction in chronic fatigue syndrome. Nature Rev Endocrinol. 2012;8(1):22–32.
29. Sandler CX, Wyller VB, Moss-Morris R, et al. Long COVID and post-infective fatigue syndrome: a review.
30. Wang X, Xu H, Jiang H, et al. Clinical features and outcomes of discharged coronavirus disease 2019 patients: a prospective cohort study. QJM. 2020;113(9):657–665. doi:10.1093/qjmed/hcaa178
31. Hotchin NA, Read R, Smith DG, Crawford DH. Active Epstein-Barr virus infection in post-viral fatigue syndrome. J Infect. 1989;18(2):143–150.
32. Tansey CM, Louie M, Loeb M, et al. One-year outcomes and health care utilization in survivors of severe acute respiratory syndrome. Arch Intern Med. 2007;167(12):1312–1320. doi:10.1001/archinte.167.12.1312
33. Petrie JG, Cheng C, Malosh RE, et al. Illness severity and work productivity loss among working adults with medically attended acute respiratory illnesses: US influenza vaccine effectiveness network 2012–2013. Clin Infect Dis. 2016;62(4):448–455. doi:10.1093/cid/civ952
34. Wootton DG, Dickinson L, Pertinez H, et al. A longitudinal modelling study estimates acute symptoms of community acquired pneumonia recover to baseline by 10 days. Eur Respir J. 2017;49(6):1602170. doi:10.1183/13993003.02170-2016
35. Wyrwich KW, Yu H, Sato R, Powers JH. Observational longitudinal study of symptom burden and time for recovery from community-acquired pneumonia reported by older adults surveyed nationwide using the CAP Burden of Illness Questionnaire. Patient Relat Outcome Meas. 2015;6:215–223. doi:10.2147/PROM.S85779
36. Garrigues E, Janvier P, Kherabi Y, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81(6):e4–e6. doi:10.1016/j.jinf.2020.08.029
37. Srivastava A, Bala R, Devi TP, Anal L. Psychological trauma and depression in recovered COVID-19 patients: a telecommunication based observational study. Trends Psychiatry Psychother. 2021. doi:10.47626/2237-6089-2021-0381
38. Whittaker A, Anson M, Harky A. Neurological manifestations of COVID‐19: a systematic review and current update. Acta Neurol Scand. 2020;142(1):14–22. doi:10.1111/ane.13266
39. Hernández-Fernández F, Valencia HS, Barbella-Aponte RA, et al. Cerebrovascular disease in patients with COVID-19: neuroimaging, histological and clinical description. Brain. 2020;143(10):3089–3103. doi:10.1093/brain/awaa239
40. Craparo G, La Rosa VL, Marino G, et al. Risk of post-traumatic stress symptoms in hospitalized and non-hospitalized COVID-19 recovered patients. A cross-sectional study. Psychiatry Res. 2022;308:114353. doi:10.1016/j.psychres.2021.114353
41. Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi:10.1136/bmj.m2094
42. DHL Ng, CY Choy, YH Chan, et al. Fever patterns, cytokine profiles, and outcomes in COVID-19. Open Forum Infect Dis. 2020;7(9):ofaa375.
43. Chen D, Xu W, Lei Z, et al. Recurrence of positive SARS-CoV-2 RNA in COVID-19: a case report. Int J Infect Dis. 2020;93:297–299. doi:10.1016/j.ijid.2020.03.003
44. Cheung KS, Hung IFN, Chan PPY, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic Review and meta-analysis. Gastroenterology. 2020;159(1):81–95. doi:10.1053/j.gastro.2020.03.065
45. Parasa S, Desai M, Thoguluva Chandrasekar V, et al. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(6):e2011335. doi:10.1001/jamanetworkopen.2020.11335
46. Kecler-Pietrzyk A, Orsi G, Carthy J, Torreggiani WC. Enteritis and severe abdominal pain as the first presentation of Covid-19. Ir Med J. 2020;113(6):102.
47. Al-Aly Z, Xie Y, Bowe BJN. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594(7862):259–264. doi:10.1038/s41586-021-03553-9
48. Ayoubkhani D, Khunti K, Nafilyan V, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021:372. doi:10.1136/bmj.n693
49. Lam MH, Wing YK, Yu MW, et al. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med. 2009;169(22):2142–2147. doi:10.1001/archinternmed.2009.384
50. Donnelly JP, Wang XQ, Iwashyna TJ, Prescott H. Readmission and death after initial hospital discharge among patients with COVID-19 in a large multihospital system. JAMA. 2021;325(3):304–306. doi:10.1001/jama.2020.21465
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
