Back to Journals » International Journal of General Medicine » Volume 14
Prevalence and Multiplicity of Thrombophilia Genetic Polymorphisms of FV, MTHFR, FII, and PAI-I: A Cross-Sectional Study on a Healthy Jordanian Population
Authors Al-Zoubi N , Alrabadi N, Kheirallah K , Alqudah A
Received 11 June 2021
Accepted for publication 29 July 2021
Published 7 September 2021 Volume 2021:14 Pages 5323—5332
DOI https://doi.org/10.2147/IJGM.S324340
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Nabil Al-Zoubi,1 Nasr Alrabadi,2 Khalid Kheirallah,3 Ahmad Alqudah4
1Department of General Surgery/Vascular Surgery, Jordan University of Science and Technology, Irbid, 22110, Jordan; 2Department of Pharmacology, Faculty of Medicine, Jordan University of Science and Technology, Irbid, 22110, Jordan; 3Department of Public Health and Community Medicine, Jordan University of Science and Technology, Irbid, Jordan; 4Department of Laboratories/Jordan University of Science and Technology, Irbid, 22110, Jordan
Correspondence: Nabil Al-Zoubi
Associate Professor of Vascular Surgery and Endovascular Therapy, Department of Surgery, Faculty of Medicine, Jordan University of Science and Technology, PO Box 3030, Irbid 22110, Jordan
Tel + 962 79-5577-4637
Email [email protected]
Background: FV, MTHFR, II, and PAI-I are the most common genes associated with thrombophilia genetic variants, which vary among different populations and ethnic groups. Little is known about the prevalence and multiplicity of these variants in Jordan. The aim of this study was to estimate the prevalence and multiplicity of the FV G1691A, FV H1299R, MTHFR 1298A>C, MTHFR 677C>T, II 20210G>A, and PAI-I 675 4G/5G variants among healthy Jordanians.
Methods: This cross-sectional study was conducted on randomly selected healthy Jordanian participants. Non-Jordanians and those with a history of arterial/venous thrombosis, atherosclerosis, or a history of recurrent abortions were excluded from the study. PCR was used to detect variants in DNA extracted from participants’ blood samples.
Results: A total of 300 subjects were screened: 170 (56.7%) females with an average age of 27.78± 9.32 years and 130 (43.3%) males with an average age of 29.88± 8.55 years. Genetic variants (at least one) were found in 75% of the subjects (81.2% among females and 66.9% among men), while 64.7%, 52%, and 12% were found to have at least two, three, and four variants, respectively. Overall, 21%, 29%, 54.3%, 27.3%, 7.7%, and 66% of participants were found to have FV G1691A, FV H1299R, MTHFR 1298A>C, MTHFR 677C>T, II 20210G>A, and PAI-I 675 4G/5G gene variants, respectively.
Conclusion: Three-quarters of our population had at least one of the thrombophilia genetic variants, and most had more than one variant. The most common variants detected were associated with MTHFR, followed by PAI-I, FV, and then II. We observed that females had higher prevalence estimates than males. However, multiplicity among males was significantly higher than females. Our findings indicated noticeable differences in prevalence estimates compared with other populations.
Keywords: thrombophilia, gene mutations, factor V Leiden, FV, MTHFR, factorII, PAI-I
Introduction
Thrombosis is one of the most common reasons for death among young people.1 It is a multifactorial disease, with major medical impacts occurring in arterial or venous circulation resulting from dynamic interactions among many genetic and acquired risk factors.1 Several known factors are associated with development of the disease, including genetic variants that predispose individuals to a hypercoagulable state known as thrombophilia.2 The variant spectrum of hereditary thrombophilia and subsequent clinical manifestations vary among ethnic groups.3,4 For instance, the FV Leiden and FII 20210G>A prothrombin gene variants both lead to enhanced blood coagulation.5 Elevated levels of homocysteine can result from several variants in the MTHFR gene, and have been identified as risk factors of thrombosis.5 Impaired fibrinolytic function induced by elevated PAI-I expression is commonly observed in patients with the thrombotic disease.6
In Jordan, there is a lack of information regarding the prevalence and multiplicity (a combination of more than one genetic variant) of these genetic variants. Therefore, the present study aimed to determine the prevalence and multiplicity of the most common thrombophilia variants in thye FV, MTHFR, FII, and PAI-I genes among Jordanians. This will help in providing basic knowledge for future studies to aid in the proper diagnosis and treatment of thrombotic diseases.
Methods
This cross-sectional study was approved by the institutional review board (16/123/2019) of Jordan University of Science and Technology and King Abdullah University Hospital and was conducted in accordance with the Declaration of Helsinki. It was performed on healthy Jordanian participants between October 2019 and March 2020. Study participants were randomly selected from blood donors who were geographically distributed in the north, middle, and south of Jordan. Written and informed consent was provided. Those excluded from the study were non-Jordanians, relatives (at least from the paternal side) those with a history of radiation exposure, arterial/venous thrombosis, atherosclerosis (peripheral arterial, cardiovascular, and cerebrovascular disease), or recurrent abortions.
Genetic Analysis
Venous blood samples were collected from participants in vacutainers containing 2 mL EDTA to run molecular analysis for FV, MTHFR, II, and PAI-I genetic variants with PCR and DNA hybridization of amplified target sequences with a Nuclear Laser Medicine screening test for thrombophilic disease 7 mutations. GenxTract was used for DNA extraction with cell lysing and resin, then the DNA material was prepared for PCR. DNA extraction was followed by amplification, where oligonucleotides complementary to sequences within the FV, MTHFR, PAI-I, and II genes were synthesized. PCR products were loaded into specific well plates. Allele-specific capture probes were complementary to nucleotides and able to bind with the gene sequences of either the wild or mutated types. There were three types of probes — normal, mutant, and control — specified and listed within the kit, which had all needed materials according to the manufacturer’s recommendations. The procedure started with incubation in a water bath for 30 minutes at 45°C, followed by washing to remove excess excessive probes and any other unwanted materials. After binding of the conjugate to the probe–target complex, multiple washes were done to remove unbound conjugates, followed by procedural steps for the detection of chromogens. Development of a colored chromogen on the region of the gene on the strip with only one line on the normal site meant that the sample contained the wild type, while two lines meant a heterozygous variant. On the other hand, one line at the mutant site meant that we had the homozygous variant. Strip tests were performed for two polymorphisms/gene variants in FV Leiden (HR2 and R506Q), two polymorphisms in the MTHFR gene (677C>T and 1298A>C), and one polymorphism in the II gene (20210G>A).
Statistical Analysis
Data were analyzed using SPSS 26.0. Frequencies with percentages and means ± SD are reported as appropriate, 95% CIs were used for prevalence estimates, ?2 to compare categorical variables, and t-tests and ANOVA to compare means. P=0.05 was considered statistically significant.
Results
We collected data on 300 healthy Jordanian individuals. This sample consisted of 130 (43.3%) males (average age 29.88±8.55 years) and 170 (56.7%) females (average age 27.78±9.32 years). For the genes we studied, 215 (71.7%) participants had polymorphisms in MTHFR, while 198 (66%), 144 (48%), and 23 (7.7%) had polymorphisms in PAI-I, FV, and II, respectively. Females were significantly older than males (mean difference 2.1 years, P=0.04). Sample characteristics are given in Table 1.
 |
Table 1 Sample characteristics and distribution of polymorphisms with respect to sex |
MTHFR
Most occurrences of polymorphisms were in the MTHFR gene (71.7%). Polymorphisms in this sample were either 1298A>C (54.3%) or 677C>T (27.3%). Females have a significantly higher rates of polymorphisms in this gene than males (77.1% vs 64.6%, P=0.025), but this difference was not present when we tested each on its own. Females had a slight, though significant tendency to have a heterozygous variant of 677C>T more than males and lower tendency to have a homozygous variant (P=0.006). This difference was not found for 1298A>C (Table 1). Most MTHFR polymorphism (94.0%) carriers possessed PAI-I polymorphisms, followed by FV (64.7%), and II (9.3%; Table 2).
 |
Table 2 Multiplicity of genetic polymorphisms with respect to sex |
PAI-I
PAI-I polymorphisms were detected in 198 (66%) subjects. Females had higher rates of polymorphisms in PAI-I than males (72.4% vs 57.7%, P=0.01). This significant difference remained even when considering heterozygous/homozygous against wild-type variants between males and females (P=0.02, Table 1). Most PAI-I polymorphism carriers had polymorphism in MTHFR (94.0%), and this was the most prevalent co-occurrence in the genes we studied, especially for MTHFR-1298A>C (82.3%). FV polymorphisms were present in 72.2% of individuals who possessed a PAI-I polymorphism: 43.7% in FV-1299H>R and 31.8% in FV-1691G>A. Only 11.6% of PAI-I-polymorphism carriers had II polymorphisms (Table 2).
FV
FV polymorphisms were detected in 144 (48.0%) subjects. The 1691G>A polymorphism was significantly more common in females (30% vs 9.2%, P<0.001) and 1299H>R more common in males (38.5% vs 21.8%, P=0.003; Table 1). Because these two results opposed each other, testing FV polymorphisms in general resulted in a nonsignificant difference (P=0.169). Interestingly, there were co-occurrences of II polymorphisms in FV-1691G>A carriers (33.3%), with a higher prevalence in males (66.7%) than females (25.5%). Of male FV-1299H>R carriers, 12% possessed FV-1691G>A, while none of the female FV-1299H>R carriers possessed that polymorphism (Table 2).
II
II polymorphisms were the least prevalent (n=23 subjects, 7.7%), with only nine males and 14 females showing. There was no difference between males and females (P=0.838). II polymorphisms were most commonly associated with FV polymorphisms (14.6%), followed by PAI-I and MTFHR. Figures for co-occurrence for heterozygous and homozygous variants for all genes are given in Table 2.
Multiplicity of Genetic Polymorphisms
We also inspected occurrences in more than just two polymorphisms at the same time. Three-quarters of participants had at least one polymorphism in one the of genes we studied, with females more prevalent in this category than males (81.2% vs 66.9%, P=0.005), while 64.7% of participants had at least two polymorphisms, with higher prevalence in females as well (P=0.015). Participants with at least three, four, or five polymorphisms constituted 52%, 12%, and 1.7% of the sample, respectively, with no differences with respect to sex. None of the participants had six polymorphisms at the same time (Table 3). Figure 1 presents a comparison matrix showing numbers of subjects who had two different variant (hetero, homo, and wild type) associations.
 |
Table 3 Co-occurrence of polymorphisms with respect to sex |
 |
Figure 1 Comparison matrix showing co-occurrence of two polymorphisms for all four genes. The diagonal line represents the reference. |
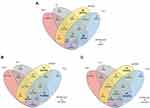
Some scenarios of multiplicity were more prevalent than others: 120 (40%) participants possessed polymorphisms in MTHFR, FV, and PAI-I simultaneously (Figure 2A), while 48 (16%) had polymorphisms in FV and II. The third–most common scenario was having a polymorphism in MTHFR only and wild-type variants in all of PAI-I, FV, and II, which occurred in 26 (8.67%) participants. We also ran this analysis for males and females separately, which resulted in similar distributions (Figure 2B and C). Figure 2 presents the most common associations between more than two genes, with Venn diagrams demonstrating >16 different scenarios for gene multiplicity and comparison between males and females.
Discussion
Thrombophilia has received growing attention since the mutations responsible for more prevalent thrombophilia, such as FV and II 20210G>A (prothrombin) mutations, were discovered in the 1990s.7 Mutations in genes encoding proteins that activate coagulation pathways or inactivate anticoagulation mechanisms play an important role in predisposition and increase the risk of venous thrombosis.8,9 Heritable thrombophilic defects are much more prevalent than was anticipated originally, and it is not at all unusual to find individuals or families with more than one defect.10 Carriers of a genetic risk factor are at increased risk of a first venous thrombosis, particularly when exposed to environmental triggers.2 Such abnormalities can be recognized in 50% of people who have thrombotic episodes.9 However, routine thrombophilia testing is controversial, and research efforts have been focused on selection criteria that may be used to increase the chance of discovering a genetic risk factor.2 In the current investigation, the most common genes with thrombophilic variants (FV, MTHFR, FII, and PAI-I) were screened in our Jordanian sample. About eight in every ten subjects screened seemed to have at least one of these six mutations. The most prevalent variants were for MTHER (71.7%) and PAI-I (66%) while the least prevalent were for II (7.7%). Sex also seems to be an important factor in these genes. Endogamy among Jordanian society could explain the high prevalence of these mutations. The clinical significance of these results is yet to be investigated.
FV
In 1994, Bertina et al first described a defect in the FV gene.11 The most frequent is the 1691G>A mutation known as FV Leiden, which is also the most frequent prothrombotic genetic abnormality leading to thrombophilia.12 This mutation brings the phenotype known as activated protein C resistance, leading to a hypercoagulable state, which increases the risk of thrombosis.13 Clotting has a dominant inheritance, ie, heterozygosity for FV increases the risk of thrombosis as much as five- to tenfold and homozygosity (when both alleles are mutated) 50- to 100-fold.13 In Caucasians, heterozygosity for the FV mutation is the most common heritable thrombophilic defect. It is found in 2%–15% of the general population, and is more prevalent in individuals of northern European extraction than those from southern Europe.10 While this mutation is common in Caucasians, it is almost absent among blacks and Asians.13 The country with the highest frequency reported in the eastern Mediterranean region is Lebanon (14.4%).12
Our study showed that the prevalence of FV variants in the Jordanian general population is 48% (21% for Leiden and 29% for 1299H>R), which is the highest in reported literature, even when compared to neighboring Lebanon. Although more common in females than males, this difference was statistically insignificant (P=0.084). As mentioned, the most frequent mutation is 1691G>A. On the contrary, we found that 1299H>R was more common than 1691G>A. We observed that only males had both H1299R and 1691G>A variants. Further studies should be conducted to discover whether this observation has clinical significance (Table 1).
II (Prothrombin)
In 1996, Poort et al described single amino-acid genetic variation in the 3ʹ untranslated region of the gene that codes for prothrombin.11 Prothrombin has procoagulant, anticoagulant, and antifibrinolytic activities, and thus a disorder involving prothrombin results in multiple imbalances in hemostasis.11 The prevalence of this defect in northern Europe is 2% in general, but up to 6.5% has been reported in southern Europe, where the prothrombin 20210G>A mutation might be the most prevalent heritable thrombophilic defect.11 Our data estimated that the prevalence of 20210G>A was 7.7%, which is slightly higher than what has been reported in other populations. Althoughmore common in females than males, this difference was statistically insignificant (P=0.422). We also we found that heterogeneous variants were more common than homogeneous variants (Table 1).
MTHFR
In 1969, McCully made a clinical observation linking elevated plasma-homocysteine concentrations and vascular diseases (premature atherosclerosis and arterial thrombosis).11,13 Elevations in plasma-homocysteine concentration can occur due to genetic defects in the enzymes involved in homocysteine metabolism.11 This is coded by MTHFR on chromosome 1, p36.3 in humans, and there are DNA-sequence variants (genetic polymorphisms) associated with this gene, although the two most common are 677C>T and 1298A>C. The most common form of genetic hyperhomocysteinaemia results from production of a thermolabile variant of methylene tetrahydrofolate reductase, with reduced enzymatic activity (T mutation). The gene encoding for this variant contains an alanine-to-valine substitution at amino acid 677 (677C>T).11 The 677C>T polymorphism shows wide regional and ethnic variation, and prevalence is 2%–21%.15 On the other hand,the 1298A>C mutation does not show as much population variance: its prevalence is more uniform within currently studied groups.15
In our study, MTHFR had the highest prevalence among the studied genetic variants. It was estimated to be mutated in 71.7% of screened participants. The 1298A>C variant was the most common (44%), followed by 677C>T (17.7%). This is different from what has been reported in the literature, where 677C>T is more common than 1298A>C. Also, our results showed that 10% of participants had both 1298A>C and 677C>T. Females had significant higher prevalence of MTHFR variant than males (P=0.013, Table 1).
PAI-I 675 4g/5g
The PAI-I 675 4G/5G polymorphism is related to differential binding of nuclear proteins that affect the rate of transcription of fibrinolytic inhibitors.16 It is the primary inhibitor of tissue- and urokinase-type plasminogen activators and considered a critical regulator of the fibrinolytic system.17 Impaired fibrinolytic function induced by elevated PAI-I expression is commonly observed in patients with thrombotic disease.6 Most studies, however, have reported higher PAI-I plasma levels in individuals with the 4G/5G genetic mutation.18 In the present study, the prevalence of PAI-I 4G/5G was 66%. It showed female predominance (P=0.006, Table 1).
Multiplicity of Variants
A homozygous abnormality or combination of two or more abnormal heterozygous factors can lead to clinically apparent thrombotic disorders at an early age.11 For example, combinations of FV and the II 20210G>A polymorphism are found frequently.10 Therefore, in this study we tried to determine the multiplicity of these genetic variants. We found that three-quarters of participants (75%) had at least one variant. A majority had two, three, and four genetic variants: 64.7%, 52%, and 12%, respectively (Table 2 and Table 3).
PAI-I variants were most commonly associated with MTHFR variants (94.9%), followed by FV (72.2%) and II (11.6%) variants. FV variants were most commonly associated with PAI-I variants (99.3%), followed by MTHFR (96.5%) and II (14.6%) variants. MTHFR variants were most commonly associated with PAI1 variants (87.4%), followed by FV (64.7%) and II (9.30%) variants. II variants were most commonly associated with PAI-I variants (100%), followed by FV (91.3%) and MTHFR (87.0%) variants. All these associations were statistically significant (P=0) except for associations between II and MTHFR variants (P=0.670, Table 3, Figures 1 and 2).
Comparison with Other Similar Studies in Jordan
Eid and Rihani screened 200 healthy Jordanians (40% female) and reported a 15% prevalence of FV Leiden (87% heterozygous, 13% homozygous), 2% prothrombin 20210G>A (100% heterozygous), and 24% MTHFR 677C>T (67% heterozygous, 33% homozygous).19 They concluded that the prevalence of FV Leiden and MTHFR 677C>T was elevated in the Jordanian population; however, the incidence of the 20210G>A variant was relatively low.19 Considering the larger number of subjects (300 vs 200) and different proportion of females to males (56.8% vs 40%) in our study, both results are comparable, as shown in Table 1. Anther study from Jordan estimated that the frequency of FV Leiden, prothrombin 20210G>A, and MTHFR 677C>T mutations in Jordanian thrombotic patients was 25.7%, 6%, and 31.7%, respectively.20 Unfortunately, we could not compare our results to this study, due to differences in the study populations (healthy subjects vs patients). We were unable to find any studies on PAI-I genetic polymorphisms among the Jordanian population.
Sex Variations
Males and females are thought to significantly differ at the cellular and molecular levels, with sex differences reported in platelet function and coagulation-factor activities.21 In this study, we observed that females had higher prevalence of thrombophilia genetic polymorphisms than males (Table 1). Hormones, such as progesterone and testosterone, may also play a part in mediating sex differences in thrombosis, although the evidence for this is extremely limited.21
Limitations
Self-reported history was the only source of data to exclude participants from the study. Some venous thrombosis and atherosclerotic diseases could have been asymptomatic and discovered incidentally. Participants who were relatives on the paternal side were excluded from the study; however, they were not verified on the maternal side. As we screened young healthy subjects, thrombophilia-related diseases may still develop, as these are much more prevalent in later ages.
Perspectives
Our results indicate factors that need further investigation, eg, the effect of the high prevalence found for the studied thrombophilia genetic variants in Jordans compared to other populations regarding thrombophilia-related disorders. Very high prevalence of the MTHFR variant, which is strongly related to cardiovascular and peripheral arterial diseases, could explain the observed high prevalence of these diseases among Jordanians, especially at young ages. We observed variations in the age distributions of these genetic variants, which could be related to environmental factors that can cause genetic variants.
Conclusion
Three-quarters of this healthy Jordanian population had at least one of the thrombophilia genetic variants, and most had more than one. The most common variant detected was associated withMTHFR, followed by PAI-I, II, and then FV. Females had higher prevalence estimates than males. However, the multiplicity among males was significantly higher than females. Our findings indicated noticeable differences in prevalence estimates compared to other populations.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval to the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mandala E, Lafaras C, Tsioni C, et al. Prevalence of thrombophilic mutations in patients with unprovoked thromboembolic disease. A comparative analysis regarding arterial and venous disease. Hippokratia. 2012;16:250–255.
2. Vagdatli E, Serafimidou O, Pantziarela E, Tsikopoulou F, Mitsopoulou K, Papoutsi A. Prevalence of thrombophilia in asymptomatic individuals with a family history of thrombosis. Hippokratia. 2013;17:359–362.
3. Lee SY, Kim EK, Kim MS, et al. The prevalence and clinical manifestation of hereditary thrombophilia in Korean patients with unprovoked venous thromboembolisms. PLoS One. 2017;12(10):e0185785. doi:10.1371/journal.pone.0185785
4. Ardestani MT, Nodushan HH, Aflatoonian A, Ghasemi N, Sheikhha MH. Case control study of the factor V Leiden and factorII G20210A mutation frequency in women with recurrent pregnancy loss. Iran J Reprod Med. 2013;11:61–64.
5. Yenicesu GI, Cetin M, Ozdemir O, et al. A prospective case–control study analyzes 12 thrombophilic gene mutations in Turkish couples with recurrent pregnancy loss. Am J Reprod Immunol. 2010;63:126–136.
6. Tang J, Zhu W, Mei X, Zhang Z. Plasminogen activator inhibitor-1: a risk factor for deep vein thrombosis after total hip arthroplasty. J Orthop Surg Res. 2018;13:8. doi:10.1186/s13018-018-0716-2
7. Weingarz L, Schwonberg J, Schindewolf M. Prevalence of thrombophilia according to age at the first manifestation of venous thromboembolism: results from the MAISTHRO registry. Br J Haematol. 2013;163:655–665. doi:10.1111/bjh.12575
8. Angelopoulou K, Nicolaides A, Deltas CC. Prevalence of Genetic Mutations That Predispose to Thrombophilia in a Greek Cypriot Population. Clin Appl Thrombosis. 2000;6(2):104–107. doi:10.1177/107602960000600211
9. Keify F, Azimi-Nezhad M, Zhiyan-abed N, Nasseri M, Abbaszadegan MR. Inherited genetic markers for thrombophilia in northeastern iran (a clinical-based report). Rep Biochem Mol Biol. 2014;2(2):77–81.
10. Walker ID. Thrombophilia in pregnancy. J Clin Pathol. 2000;53:573–580. doi:10.1136/jcp.53.8.573
11. Khan S, Dickerman JD. Hereditary thrombophilia. Thromb J. 2006;4:15. doi:10.1186/1477-9560-4-15
12. Kreidy R. Factor V-leiden mutation: a common risk factor for venous thrombosis among lebanese patients. Thrombosis. 2012;2012:1–4. doi:10.1155/2012/380681
13. Michel CA, Rocha JB, Costa DC, Lima CA, Batschauer APB. Prevalence of factor V Leiden in patients with venous thrombosis. J Bras Patol Med Lab. 2016;52(4):227–232. doi:10.5935/1676-2444.20160038
14. Spark JI, Laws P, Fitridge R. The incidence of hyperhomocysteinaemia in vascular patients. Eur J Vasc Endovasc Surg. 2003;26:558–561. doi:10.1016/S1078-5884(03)00381-2
15. Morales-Borges RH. Prevalence of MTHFR C677T and A1298C mutations and thrombophilia in puerto rico. J Blood Disorders Transfusion. 2014;5:213.
16. Margaglione M, Cappucci G, d’Addedda M. PAI-1 plasma levels in a general population without clinical evidence of atherosclerosis relation to environmental and genetic determinants. Arterioscler Thromb Vasc Biol. 1998;18:562–567. doi:10.1161/01.ATV.18.4.562
17. Fay WP, Parker AC, Condrey LR, Shapiro AD. Human plasminogen activator inhibitor-1 (PAI-1) deficiency: characterization of a large kindred with a null mutation in the PAI-1 gene. Blood. 1997;90:204–208. doi:10.1182/blood.V90.1.204.204_204_208
18. Francis CW. Plasminogen activator inhibitor-1 levels and polymorphisms. Arch Pathol Lab Med. 2002;126:1401–1404. doi:10.5858/2002-126-1401-PAILAP
19. Eid SS, Rihani G. Prevalence of factor V Leiden, prothrombin G20210A, and MTHFR C677T mutations in 200 healthy. Clin Lab Sci. 2004;17(4):200–202.
20. Eid SS, Shubeilat T. Prevalence of factor V Leiden, prothrombin G20210A, and MTHFR G677A among 594 thrombotic Jordanian patients Jordanians. Blood Coagul Fibrinolysis. 2005;16(6):417–421. doi:10.1097/01.mbc.0000175478.46831.52
21. Nordstrom SM, Weiss EJ. Weiss. Sex differences in thrombosis. Expert Rev Hematol. 2008;1(1):3–8. doi:10.1586/17474086.1.1.3
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.