Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Prevalence and Influence Factors for Non-Alcoholic Fatty Liver Disease in Long-Term Hospitalized Patients with Schizophrenia: A Cross-Sectional Retrospective Study
Authors Li X , Gao Y, Wang Y, Wang Y, Wu Q
Received 19 November 2022
Accepted for publication 27 January 2023
Published 18 February 2023 Volume 2023:19 Pages 379—389
DOI https://doi.org/10.2147/NDT.S398385
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yuping Ning
Xuelong Li,1– 3 Yakun Gao,4 Yongmei Wang,2,3,5 Ying Wang,2,3,5,* Qing Wu1– 3,5,*
1School of Mental Health and Psychological Sciences, Anhui Medical University, Hefei, People’s Republic of China; 2Department of Psychiatry, Affiliated Psychological Hospital of Anhui Medical University, Hefei, People’s Republic of China; 3Anhui Mental Health Center, Hefei, People’s Republic of China; 4Affiliated Hospital of Weifang Medical College, Weifang, People’s Republic of China; 5Hefei Fourth People’s Hospital, Hefei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qing Wu, Department of Psychiatry, Affiliated Psychological Hospital of Anhui Medical University, 316 Huangshan Road, Hefei, 230000, People’s Republic of China, Tel +86-13856919530, Email [email protected] Ying Wang, Department of Psychiatry, Affiliated Psychological Hospital of Anhui Medical University, 316 Huangshan Road, Hefei, 230000, People’s Republic of China, Tel +86-13866136686, Email [email protected]
Purpose: Long-term hospitalized patients with schizophrenia (SCZ) are vulnerable to physical illness, leading to impaired life expectancy and treatment outcomes. There are few studies on the influence of non-alcoholic fatty liver disease (NAFLD) in long-term hospitalized patients. This study aimed to investigate the prevalence of and influence factors for NAFLD in hospitalized patients with SCZ.
Patients and Methods: This cross-sectional retrospective study included 310 patients who had experienced long-term hospitalization for SCZ. NAFLD was diagnosed based on the results of abdominal ultrasonography. The T-test, Mann–Whitney U-test, correlation analysis, and logistic regression analysis were used to determine the influence factors for NAFLD.
Results: Among the 310 patients who had experienced long-term hospitalization for SCZ, the prevalence of NAFLD was 54.84%. Antipsychotic polypharmacy (APP), body mass index (BMI), hypertension, diabetes, total cholesterol (TC), apolipoprotein B (ApoB), aspartate aminotransferase (AST), alanine aminotransferase (ALT), triglycerides (TG), uric acid, blood glucose, gamma-glutamyl transpeptidase (GGT), high-density lipoprotein, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio significantly differed between the NAFLD and non-NAFLD groups (all P< 0.05). Hypertension, diabetes, APP, BMI, TG, TC, AST, ApoB, ALT, and GGT were positively correlated with NAFLD (all P< 0.05). The results of the logistic regression analysis indicated that APP, diabetes, BMI, ALT, and ApoB were the influence factors for NAFLD in patients with SCZ.
Conclusion: Our results suggest a high prevalence of NAFLD among patients hospitalized long-term due to severe SCZ symptoms. Moreover, a history of diabetes, APP, overweight/obese status, and increased levels of ALT and ApoB were identified as negative factors for NAFLD in these patients. These findings may provide a theoretical basis for the prevention and treatment of NAFLD in patients with SCZ and contribute to the development of novel targeted treatments.
Keywords: schizophrenia, non-alcoholic fatty liver disease, prevalence, influence factors, long-term hospitalization
Introduction
The mechanism underlying schizophrenia (SCZ) development is multimodal, involving neurodevelopmental, genetic expression, and environmental factors.1,2 The lifetime prevalence of SCZ is approximately 1%, with typical onset in early adulthood, severely impairing the social and occupational functioning of patients and creating a serious burden on families and society.3 SCZ is also associated with reduced life expectancy, with patients having an average life expectancy 15 years shorter than that of the general population, and a 2% lifetime risk of suicide.4 A recent study has shown that there is high comorbidity between SCZ and non-alcoholic fatty liver disease (NAFLD).5 Additionally, physical diseases exert a negative impact on the recurrence of SCZ.6 As the most prevalent type of liver disease, NAFLD refers to steatosis in >5% of the liver parenchyma after excluding alcohol abuse and long-term use of steatogenic drugs.7 NAFLD prevalence has been increasing worldwide, with the average exceeding 30% in 2019.8 A recent national epidemiological survey found that the prevalence of NAFLD among Chinese adults was approximately 29.2%, with an annual incidence of approximately 5%.9 NAFLD increases the risk of chronic kidney disease, type 2 diabetes mellitus, and cardiovascular disease, but cardiovascular disease is a common and life-threatening complication of NAFLD,10 as well as the leading cause of death in patients with SCZ.11
A large study in Brazil including 749,720 patients with severe mental illness (61.71% SCZ patients) found that cardiovascular disease caused significantly more deaths in hospitalized than non-hospitalized patients.12 Patients with SCZ who are hospitalized long-term are especially at risk of cardiovascular disease. The highest percentage of somatic diseases associated with death in patients with SCZ are circulatory and digestive diseases, resulting in a mortality rate almost 3.6 times higher than that of the general population.13,14 Patients with SCZ show evidence of adipose dysfunction such as reduced lipocali, which is a major factor in the increased risk of CVD in patients with NAFLD.15,16 Therefore, the prevalence of NAFLD, which has important implications for improving life expectancy and disease burden in long-term hospitalized patients with SCZ, should be explored.
However, previous studies on NAFLD in patients with SCZ have some limitations. A large observational study of 66,273 patients with mental disorders in China reported a higher prevalence of NAFLD in patients with SCZ than in those with other mental disorders, further noting that NAFLD risk was strongly associated with antipsychotic use, diabetes, and other metabolic disorders.17 A recent study also showed the adverse effect of antipsychotics on the risk of NAFLD in patients with SCZ.18 However, the relevance of some important blood indicators is not mentioned. Yan et al19 reported that BMI, drug dose, medication combinations, and TG were significantly associated with the onset of NAFLD in young male patients with SCZ. Nonetheless, their study was limited to young male hospitalized patients with SCZ and did not include women and older adults. Additionally, these studies did not examine the influence factors of NAFLD in long-term hospitalized patients with SCZ, and this gap in the literature should be addressed. Long-term hospitalized patients with SCZ were at high risk of developing NAFLD; the resulting complications such as cardiovascular disease increase the burden of disease and treatment outcomes, reducing the likelihood that patients will return to society. The present study aimed to explore the prevalence of NAFLD and its risk factors in long-term hospitalized patients with SCZ by analyzing general disease data and important blood indicators. We sought to provide a framework upon the development of strategies that can reduce the prevalence of NAFLD in this population, which may in turn help reduce disease burden and improve prognosis.
Materials and Methods
Study Design and Population
This cross-sectional retrospective study was approved by the Institutional Review Board of the Anhui Mental Health Center (Hefei, China). Due to the retrospective nature of the review, the Institutional Review Board of the Anhui Mental Health Center did not request informed consent from the participants and all data was collected anonymously.
We retrospectively reviewed the records of patients with SCZ who were continuously hospitalized for at least 2 years due to severe psychiatric symptoms or severely impaired social functioning at the Anhui Mental Health Center (Hefei, China) between August 2022 and October 2022. SCZ was diagnosed based on the criteria outlined in the 10th revision of the International Classification of Diseases (ICD-10) and was confirmed by two psychiatrists. The inclusion criteria were 1) age ≥18 years; 2) no history of hepatitis or other diseases causing a fatty liver, other than being treated with antipsychotics; 3) no prior treatment with hepatoprotective drugs; 4) no history of long-term alcohol use. The exclusion criteria were 1) one week or more difference between liver ultrasound and blood test; 2) history of neurodevelopmental or neurodegenerative disorders, including intellectual disability and Alzheimer’s disease;
Trained research assistants collected the following sociodemographic and clinical data from the electronic health records of Anhui Mental Health Center: sex, age, years of education, marital status at the time of consultation, weight, height, history of hypertension and diabetes, duration of SCZ, antipsychotic drug number and dose, Brief Psychiatric Rating Scale (BPRS) scores, and laboratory results, including biochemical parameters—total cholesterol (TC), triglycerides (TG), alanine aminotransferase (ALT), alkaline phosphatase, aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), apolipoprotein A1, apolipoprotein B (ApoB), creatinine, high-density lipoprotein cholesterol (HDL), uric acid, and blood glucose—and hematological parameters—platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR), and neutrophil-to-lymphocyte ratio (NLR).
The height and weight of all patients were measured manually by a nurse, and body mass index (BMI) was calculated by dividing weight by the height squared. Antipsychotic polypharmacy (APP) was defined as concurrent use of at least two antipsychotic medications. The antipsychotic drug doses were uniformly converted into dose equivalents of chlorpromazine.20
Definition of NAFLD
The diagnostic criteria for NAFLD were 1) imaging or histological evidence of liver steatosis and 2) no other cause of fat accumulation in the liver, such as heavy drinking, hepatitis C, medication, or genetic diseases. After excluding patients with a history of alcoholic liver disease, hepatolenticular degeneration, viral hepatitis, autoimmune hepatitis, toxic liver disease, and biliary cirrhosis, liver ultrasound examination was conducted by experienced medical staff. Due to the high diagnostic accuracy of hepatic steatosis, ultrasound is widely used for the examination and diagnosis of NAFLD.21
Blood Tests
Patients’ blood samples were collected after an overnight fast and sent to the hospital laboratory for analysis within 1 h of collection. Plasma biochemical parameters were measured with an automatic biochemistry analyzer (AU480, Beckman Coulter, Brea, CA, USA) using commercial kits (Roche, Basel, Switzerland). Routine blood analysis was performed using an automatic hematology analyzer (Mindray BC-2800, Shenzhen, China). MLR, NLR, and PLR were calculated manually based on the hemogram results.
BPRS
BPRS was used to assess the severity of the patients’ symptoms.22 BPRS is divided into five subscales: positive symptoms (hallucinatory behavior, unusual thought content, conceptual disorganization, and grandiosity), negative symptoms (blunted affect, motor retardation, and emotional withdrawal), affinity (anxiety, guilt feelings, depressive mood, and somatic concern), resistance (hostility, uncooperativeness, and suspiciousness), and activation (excitement, tension, and mannerisms-posturing). The total score reflects the severity of the patient’s mental symptoms.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics version 26.0 (IBM Corp., Armonk, NY, USA). The normality of continuous variables was tested using the Shapiro–Wilk test. Categorical data are expressed as numbers with percentages. Continuous variables are expressed as means with standard deviations or medians with interquartile ranges, as appropriate. Normally distributed continuous variables were compared using the independent samples T-test, whereas those with a skewed distribution were compared using the Mann–Whitney U-test. The chi-square test was used to analyze intergroup differences in categorical data. For the representation of correlations between variables and NAFLD, Spearman’s rank correlation coefficients were used for continuous variables, and Cramer’s V correlation coefficients were used for categorical variables. To investigate the influence factors for NAFLD, each variable was analyzed using univariate logistic regression analysis, and then meaningful variables were included in the multiple logistic regression analysis to derive possible influencing factors. All P-values were two-sided. P-values <0.05 were considered statistically significant.
Results
Baseline Characteristics
We identified 337 long-term hospitalized patients with SCZ. After excluding 15 patients with a history of alcohol use and 12 patients with liver diseases, 310 eligible patients were included in this study.
Among the 310 patients (men, n=178, 57.42%; female, n=132, 42.58%), 170 (54.84%) had comorbid NAFLD (Table 1). The average ages were 48.36±11.67 and 47.81±13.3 years in the NAFLD and non-NAFLD groups, respectively. Significant differences in hypertension, diabetes, APP, BMI, TC, AST, ALT, TG, GGT, high-density lipoprotein cholesterol, ApoB, uric acid, blood glucose, NLR, and PLR were observed between the groups (all P<0.05).
 |
Table 1 Comparison of the Baseline Characteristics Between the NAFLD and Non-NAFLD Groups |
Correlations Between Variables and NAFLD
As shown in Table 2, the following variables were positively correlated with NAFLD: hypertension (r=0.159, P=0.005), diabetes (r=0.208, P<0.001), APP (r=0.131, P=0.021), BMI (r=0.596, P<0.001), ALT (r=0.321, P<0.001), AST (r=0.182, P=0.001), GGT (r=0.256, P<0.001), TC (r=0.181, P=0.001), TG (r=0.342, P<0.001), and ApoB (r=0.356, P<0.001). No significant correlations were observed between any other variables and NAFLD (all P>0.05).
 |
Table 2 Correlation Between the Variables and NAFLD in Patients with SCZ |
Influence Factors for NAFLD
As shown in Table 3, variables that differed significantly between the NAFLD and non-NAFLD groups were further analyzed via logistic regression analysis. The following variables were found to be statistically significant in univariate logistic regression analysis: hypertension (odds ratio [OR]=2.374, 95% confidence interval [CI]=1.283−4.393, P=0.006), blood glucose (OR=1.477, 95% CI=1.1.099−1.985, P=0.010), PRL (OR=0.994, 95% CI=0.988−0.999, P=0.019), diabetes (OR=3.910, 95% CI=1.812−8.438, P=0.001), APP (OR=1.718, 95% CI=1.084−2.722, P=0.021), BMI (OR=1.536, 95% CI=1.389−1.98, P<0.001), ALT (OR=1.072, 95% CI=1.045−1.100, P<0.001), AST (OR=1.074, 95% CI=1.034−1.115, P<0.001), GGT (OR=1.027, 95% CI=1.012−1.042, P<0.001), apoB (OR=55.120, 95% CI=14.321−212.148, P<0.001), TC (OR=1.535, 95% CI=1.178−2.000, P=0.001), HDL (OR=0.364, 95% CI=0.158−0.837, P=0.017), and TG (OR=1.639, 95% CI=1.214−2.213, P=0.001). After including the above significant variables in the multiple logistic regression analysis, the following variables were found to be statistically significant: diabetes (OR=4.291, 95% CI=1.380−13.342, P=0.012), APP (OR=2.261, 95% CI=1.110−4.400, P=0.016), BMI (OR=1.456, 95% CI=1.299−1.634, P<0.001), ALT (OR=1.063, 95% CI=1.007−1.122, P=0.027), and apoB (OR=13.134, 95% CI=1.115−154.728, P=0.041).
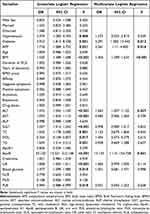 |
Table 3 Influence Factors for NAFLD in Patients with SCZ |
Discussion
We investigated the prevalence of and influence factors for NAFLD in long-term hospitalized patients with SCZ. Our analysis indicated that 54.84% of these patients had comorbid NAFLD, which is a significantly higher rate than that observed in the general Chinese population and in patients with other mental disorders.17 When compared with the non-NAFLD group of the current study, the NAFLD group exhibited trends toward significance in terms of abnormal levels of liver enzymes, lipids, inflammatory markers, and glucose, as well as significant increases in the prevalence of obesity, hypertension, diabetes, and APP. Moreover, diabetes, APP, BMI, ALT, and ApoB may be influence factors for NAFLD in these patients.
In this study, BMI and diabetes showed a strong significant relationship with NAFLD. For most adults, BMI is mainly related to changes in body weight. Weight gain is associated with a significant expansion of adipose tissue, which may lead to adipocyte dysfunction and insulin resistance, as well as an impaired ability of adipocytes to store fat, leading to the release of free fatty acids into the circulation and their absorption and accumulation in ectopic organs, such as the liver, increasing the risk of NAFLD.23 Abnormal weight gain is common in patients with SCZ. Previous studies have identified unhealthy lifestyle and dietary habits and antipsychotic drug use as the main causes of weight gain in patients with SCZ.24 Weight gain may be associated with gray matter atrophy in the brain. In the recent years, several studies have demonstrated significant gray matter atrophy in patients with SCZ, including atrophy of the frontal lobe, temporal pole, occipital lobe and putamen, which begins early in the disease process and gradually worsens as the disease progresses.25–27 The reduced size of the putamen, a component of the striatum, may increase the likelihood of binge eating by inhibiting the sensitivity of dopamine D2 receptors to make food intake less rewarding.28,29 Additionally, reduced gray matter volume in the precentral gyrus of the frontal lobe impairs the patient’s ability to perceive the size and weight of food, increasing the likelihood of binge eating and the risk of obesity.30 Notably, obesity is an important influencing factor in diabetes and, in turn, these two conditions affect each other.31 Unmedicated patients with first-episode SCZ have significant insulin resistance and impaired glucose tolerance that persisted for the subsequent duration of the disease,32 which significantly increases the risk of diabetes. When diabetes is present in patients with SCZ, insulin resistance exacerbates adipocyte dysfunction and perpetuates the production of de novo fat in the liver, with increased free serum fatty acids and adipose tissue inflammation following the course of disease.33 Free fatty acids account for 60% of the liver fat content in patients with NAFLD, which is a significantly higher proportion than that of de novo fat in the liver, and the increase in free fatty acids leads to increased liver fat accumulation and promotes NAFLD development.34
In the current study, APP was identified as another influence factor for NAFLD in patients with SCZ. Antipsychotic medications are widely used in the treatment of SCZ, and their rational use plays an irreplaceable role in reducing the length of hospitalization and improving treatment adherence among patients.35 However, other observational studies have found that APP may not only increase the number of hospitalizations of patients with SCZ, but may also prolong the length of stay, suggesting that APP may have adverse effects on patients who are hospitalized long-term.36,37 The side effects associated with antipsychotic medications, such as dyslipidemia, weight gain, systemic inflammation, and insulin resistance, increase the incidence of NAFLD.38,39 Centorrino et al40 found that when the total dose of antipsychotics was similar, there was a 56% higher risk of adverse effects and a 55% longer hospital stay in patients with SCZ treated with APP compared to SCZ patients treated with monotherapy. A review by Gallego et al found that APP is strongly associated with metabolic abnormalities such as hyperprolactinemia and diabetes mellitus.41 Further studies found that APP increased the occupancy of dopamine receptors, histamine H1 receptors, adrenergic α1 and α2 receptors, and muscarinic M3 receptors, and while this increased clinical efficacy to some extent, it also increased the risk of hyperprolactinemia and abnormal leptin secretion, resulting in significant weight gain.42 Additionally, APP may reduce the responsiveness of pancreatic beta cells to blood glucose and impair glucose metabolism through antagonism of 5-hydroxytryptamine-related receptors and M3 receptors, reducing the ability to transport glucose and increasing the risk of insulin resistance.43,44 The above studies show that APP increases the risk of metabolic abnormalities in patients with SCZ and increases the risk of NAFLD. The World Federation of Societies of Biological Psychiatry guidelines for the treatment of schizophrenia recommend that to reduce the incidence of adverse drug reactions, the use of APP should only be considered in certain individual cases (eg, treatment-resistant patients with SCZ).45 The effect of antipsychotic drugs on NAFLD cannot be ignored; therefore, medical professionals should choose a single medication based on the effective antipsychotic treatment for each patient.
A recent study by Aaroe et al46 found that the serum ALT levels are related to the development of NAFLD in patients with SCZ. Other recent studies have reported that higher ALT levels are positively associated with the risk of hepatic steatosis and metabolic syndrome in patients with SCZ. A large cohort study conducted in China demonstrated that higher activity of ALT over time significantly increased the risk of metabolic syndrome in individuals with SCZ,47 which increases NAFLD risk.48 A previous study has suggested ALT as a marker of liver cell damage, reflecting the response of hepatocytes to oxidative stress in patients with NAFLD.49 However, Sookoian et al50 proposed a different view. They reported that, in the context of NAFLD, the body exists in a state of metabolic overload, with significantly increased expression of liver-related genes and proteins to accommodate the high energy demand, resulting in changes in the amount of amino acids released into the circulation. Therefore, they suggested that ALT and other transaminases reflect the degree of hepatocyte damage and play an active role in determining liver metabolism.
Apolipoprotein is closely related to lipid metabolism. ApoA1 can reduce circulating cholesterol level by combining with high-density lipoproteins.51 Unlike apoA1, apoB, the important scaffold protein for assembling extremely low-density lipoprotein, is significantly positively related to the risk of hyperlipidemia.52 Mabrouk et al53 found that the reduced activity of paraoxonase 1 was associated with lipid disorders in patients with SCZ exhibiting significant increases in ApoB levels. In addition, antipsychotic drugs increase serum ApoB levels in patients with SCZ by affecting cholesterol biosynthesis.54 Therefore, APP may make this effect even more pronounced. Previous studies have identified a positive association between ApoB and NAFLD prevalence, which is consistent with our finding that elevated serum ApoB level is a risk factor for NAFLD.55,56
This study had some limitations. First, due to the retrospective cross-sectional design, we could not determine a temporal-causal relationship between NAFLD and the identified risk factors. Second, because the sample size was small and the study population included only long-term hospitalized patients, there may have been a degree of selection bias, making it difficult to extrapolate the results to all patients with SCZ. Third, different antipsychotic drugs have different pathways of action and different degrees of effect on NAFLD. Herein, we did not classify or analyze antipsychotic medications, which may have affected the accuracy of the results. Lastly, although the correlations of waist circumference, waist-to-height ratio, and waist-to-hip ratio with NAFLD have been verified,57 we were unable to include these parameters in the study due to the limitations of the retrospective design. Therefore, further large-scale prospective studies with larger sample sizes that address these limitations are warranted to complement and validate our findings.
Conclusion
Our findings suggest that long-term hospitalized patients with SCZ have a significantly high risk of NAFLD, with influence factors including above-normal BMI, diabetes, APP, and increased levels of ALT and ApoB. Hence, weight, ALT levels, and ApoB levels should be monitored during SCZ treatment, particularly in those with APP and comorbid diabetes, and Influence factors should be actively addressed to prevent NAFLD. Our findings may provide directions for the prevention and treatment of NAFLD in patients with SCZ and promote the development of more effective targeted therapies for improving prognosis and disease burden in these patients.
Abbreviations
ALP, alkaline phosphatase; ALT, alanine aminotransferase; ApoB, apolipoprotein B; APP, antipsychotic polypharmacy; AST, aspartate aminotransferase; BMI, body mass index; BPRS, Brief Psychiatric Rating Scale; GGT, gamma-glutamyl transpeptidase; MLR, monocyte-to-lymphocyte ratio; NAFLD, non-alcoholic fatty liver disease; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SCZ, schizophrenia; TC, total cholesterol; TG, triglycerides.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The study protocol conformed with the Declaration of Helsinki and was approved by the Institutional Review Board of the Anhui Mental Health Center (permit numbers HSY-IRB-YJ-YYYX-WQ001). Due to the retrospective nature of the review, the Institutional Review Board of the Anhui Mental Health Center did not request informed consent from the participants and all data was collected anonymously. All participants in this study signed a patient privacy confidentiality agreement.
Consent for Publication
As retrospective studies do not require signed informed consent according to local laws and regulations, this requirement was not applicable to the present study.
Acknowledgments
We would like to thank the Anhui Mental Health Center for providing data support for this study. Without the support of the hospital, this work would not have been possible.
Funding
This research was funded by the Hefei Health Applied Medicine Research Project (grant number Hwk2021yb015) and the Hospital Project of Hefei Fourth People’s Hospital (grant numbers HFSY2022YB08 and HFSY2022ZD11). The funders had no role in the study design; in the collection, analysis, or interpretation of the data; in the writing of the manuscript; or in the decision to submit the article for publication.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Singh T, Poterba T, Curtis D, et al. Rare coding variants in ten genes confer substantial risk for schizophrenia. Nature. 2022;604(7906):509–516. doi:10.1038/s41586-022-04556-w
2. Paquin V, Lapierre M, Veru F, King S. Early environmental upheaval and the risk for schizophrenia. Annu Rev Clin Psychol. 2021;17:285–311. doi:10.1146/annurev-clinpsy-081219-103805
3. Cloutier M, Aigbogun MS, Guerin A, et al. The economic burden of schizophrenia in the United States in 2013. J Clin Psychiatry. 2016;77(6):764–771. doi:10.4088/JCP.15m10278
4. McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia-an overview. JAMA Psychiatry. 2020;77(2):201–210. doi:10.1001/jamapsychiatry.2019.3360
5. Zhang R, Sjölander A, Ploner A, Lu D, Bulik CM, Bergen SE. Novel disease associations with schizophrenia genetic risk revealed in ~400,000 UK Biobank participants. Mol Psychiatry. 2022;27(3):1448–1454. doi:10.1038/s41380-021-01387-5
6. Yang C, Zhong X, Zhou H, Wu Z, Zhang M, Ning Y. Physical comorbidities are independently associated with higher rates of psychiatric readmission in a Chinese Han population. Neuropsychiatr Dis Treat. 2020;16:2073–2082. doi:10.2147/NDT.S261223
7. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. doi:10.1002/hep.29367
8. Henry L, Paik J, Younossi ZM. Review article: the epidemiologic burden of non-alcoholic fatty liver disease across the world. Aliment Pharmacol Ther. 2022;56(6):942–956. doi:10.1111/apt.17158
9. Zhou F, Zhou J, Wang W, et al. Unexpected rapid increase in the burden of NAFLD in China from 2008 to 2018: a systematic review and meta-analysis. Hepatology. 2019;70(4):1119–1133. doi:10.1002/hep.30702
10. Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(1 Suppl):S47–64. doi:10.1016/j.jhep.2014.12.012
11. Erlangsen A, Andersen PK, Toender A, Laursen TM, Nordentoft M, Canudas-Romo V. Cause-specific life-years lost in people with mental disorders: a nationwide, register-based cohort study. Lancet Psychiatry. 2017;4(12):937–945. doi:10.1016/S2215-0366(17)30429-7
12. Melo APS, Dippenaar IN, Johnson SC, et al. All-cause and cause-specific mortality among people with severe mental illness in Brazil’s public health system, 2000-2015: a retrospective study. Lancet Psychiatry. 2022;9(10):771–781. doi:10.1016/S2215-0366(22)00237-1
13. Räsänen S, Hakko H, Viilo K, et al. Excess mortality among long-stay psychiatric patients in Northern Finland. Soc Psychiatry Psychiatr Epidemiol. 2003;38(6):297–304. doi:10.1007/s00127-003-0635-2
14. Ringen PA, Faerden A, Antonsen B, et al. Cardiometabolic risk factors, physical activity and psychiatric status in patients in long-term psychiatric inpatient departments. Nord J Psychiatry. 2018;72(4):296–302. doi:10.1080/08039488.2018.1449012
15. Osimo EF, Sweeney M, de Marvao A, et al. Adipose tissue dysfunction, inflammation, and insulin resistance: alternative pathways to cardiac remodelling in schizophrenia. A multimodal, case-control study. Transl Psychiatry. 2021;11(1):614. doi:10.1038/s41398-021-01741-9
16. Targher G, Byrne CD, Tilg H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. 2020;69(9):1691–1705. doi:10.1136/gutjnl-2020-320622
17. Ma Q, Yang F, Ma B, et al. Prevalence of nonalcoholic fatty liver disease in mental disorder inpatients in China: an observational study. Hepatol Int. 2021;15(1):127–136. doi:10.1007/s12072-020-10132-z
18. Koreki A, Mori H, Nozaki S, et al. Risk of Nonalcoholic Fatty Liver Disease in Patients With Schizophrenia Treated With Antipsychotic Drugs: a Cross-sectional Study. J Clin Psychopharmacol. 2021;41(4):474–477. doi:10.1097/JCP.0000000000001421
19. Yan J, Hou C, Liang Y. The prevalence and risk factors of young male schizophrenics with non-alcoholic fatty liver disease. Neuropsychiatr Dis Treat. 2017;13:1493–1498. doi:10.2147/NDT.S137183
20. Leucht S, Samara M, Heres S, Davis JM. Dose equivalents for antipsychotic drugs: the DDD method. Schizophr Bull. 2016;42(suppl 1):S90–S94. doi:10.1093/schbul/sbv167
21. Wong VW, Chan WK, Chitturi S, et al. Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017-Part 1: definition, risk factors and assessment. J Gastroenterol Hepatol. 2018;33(1):70–85. doi:10.1111/jgh.13857
22. Hafkenscheid A. Psychometric evaluation of a standardized and expanded Brief Psychiatric Rating Scale. Acta Psychiatr Scand. 1991;84(3):294–300. doi:10.1111/j.1600-0447.1991.tb03147.x
23. Maher JJ, Leon P, Ryan JC. Beyond insulin resistance: innate immunity in nonalcoholic steatohepatitis. Hepatology. 2008;48(2):670–678. doi:10.1002/hep.22399
24. Adamowicz K, Mazur A, Mak M, Samochowiec J, Kucharska-Mazur J. Metabolic syndrome and cognitive functions in schizophrenia-implementation of dietary intervention. Front Psychiatry. 2020;11:359. doi:10.3389/fpsyt.2020.00359
25. Yang Y, Li X, Cui Y, et al. Reduced Gray Matter Volume in Orbitofrontal Cortex Across Schizophrenia, Major Depressive Disorder, and Bipolar Disorder: a Comparative Imaging Study. Front Neurosci. 2022;16:919272. doi:10.3389/fnins.2022.919272
26. Alemán-Gómez Y, Najdenovska E, Roine T, et al. Partial-volume modeling reveals reduced gray matter in specific thalamic nuclei early in the time course of psychosis and chronic schizophrenia. Hum Brain Mapp. 2020;41(14):4041–4061. doi:10.1002/hbm.25108
27. Rich AM, Cho YT, Tang Y, et al. Amygdala volume is reduced in early course schizophrenia. Psychiatry Res Neuroimaging. 2016;250:50–60. doi:10.1016/j.pscychresns.2016.02.006
28. Stice E, Yokum S, Burger KS, et al. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J Neurosci. 2011;31:4360–4366. doi:10.1523/jneurosci.6604-10.2011
29. Stice E, Yokum S, Blum K, et al. Weight gain is associated with reduced striatal response to palatable food. J Neurosci. 2010;30:13105–13109. doi:10.1523/jneurosci.2105-10.2010
30. Zhang Y, Zhao H, Qiu S, et al. Altered functional brain networks in Prader-Willi syndrome. NMR Biomed. 2013;26:622–629. doi:10.1002/nbm.2900
31. Wang Y, Zeng L, Chen L, et al. The prevalence and clinical characteristics of diabetes mellitus in Chinese inpatients with chronic schizophrenia: a multicenter cross-sectional study. PeerJ. 2021;9:e12553. doi:10.7717/peerj.12553
32. Perry BI, McIntosh G, Weich S, Singh S, Rees K. The association between first-episode psychosis and abnormal glycaemic control: systematic review and meta-analysis. Lancet Psychiatry. 2016;3(11):1049–1058. doi:10.1016/S2215-0366(16)30262-0
33. Lang S, Schnabl B. Microbiota and fatty liver disease-the known, the unknown, and the future. Cell Host Microbe. 2020;28(2):233–244. doi:10.1016/j.chom.2020.07.007
34. Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115(5):1343–1351. doi:10.1172/JCI23621
35. Di Lorenzo R, Ferri P, Cameli M, Rovesti S, Piemonte C. Effectiveness of 1-year treatment with long-acting formulation of aripiprazole, haloperidol, or paliperidone in patients with schizophrenia: retrospective study in a real-world clinical setting. Neuropsychiatr Dis Treat. 2019;15:183–198. doi:10.2147/NDT.S189245
36. Bolstad A, Andreassen OA, Røssberg JI, et al. Previous hospital admissions and disease severity predict the use of antipsychotic combination treatment in patients with schizophrenia. BMC Psychiatry. 2011;11:126. doi:10.1186/1471-244X-11-126
37. Civan Kahve A, Kaya H, Gül Çakıl A, et al. Multiple antipsychotics use in patients with schizophrenia: why do we use it, what are the results from patient follow-ups? Asian J Psychiatr. 2020;52:102063. doi:10.1016/j.ajp.2020.102063
38. May M, Barlow D, Ibrahim R, Houseknecht KL. Mechanisms underlying antipsychotic-induced NAFLD and iron dysregulation: a multi-omic approach. Biomedicines. 2022;10(6):548. doi:10.3390/biomedicines10061225
39. Mouzaki M, Yodoshi T, Arce-Clachar AC, et al. Psychotropic medications are associated with increased liver disease severity in pediatric nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2019;69(3):339–343. doi:10.1097/MPG.0000000000002401
40. Centorrino F, Goren JL, Hennen J, et al. Multiple versus single antipsychotic agents for hospitalized psychiatric patients: case-control study of risks versus benefits. Am J Psychiatry. 2004;161(4):700–706. doi:10.1176/appi.ajp.161.4.700
41. Gallego JA, Nielsen J, de Hert M, et al. Safety and tolerability of antipsychotic polypharmacy. Expert Opin Drug Saf. 2012;11:527–542. doi:10.1517/14740338.2012.683523
42. Jeon SW, Kim YK. Unresolved Issues for Utilization of Atypical Antipsychotics in Schizophrenia: antipsychotic Polypharmacy and Metabolic Syndrome. Int J Mol Sci. 2017;18(10):2174. doi:10.3390/ijms18102174
43. Kelly AC, Sheitman BB, Hamer RM, et al. A naturalistic comparison of the long-term metabolic adverse effects of clozapine versus other antipsychotics for patients with psychotic illnesses. J Clin Psychopharmacol. 2014;34:441–445. doi:10.1097/JCP.0000000000000159
44. Silvestre JS, Prous J. Research on adverse drug events. I. Muscarinic M3 receptor binding affinity could predict the risk of antipsychotics to induce type 2 diabetes. Methods Find Exp Clin Pharmacol. 2005;27:289–304. doi:10.1358/mf.2005.27.5.908643
45. Hasan A, Falkai P, Wobrock T, et al. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318–378. doi:10.3109/15622975.2012.696143
46. Aarøe ASK, Odgaard Maeng K, Leifsdottir Jacobsen R, et al. Hepatic steatosis in patients with schizophrenia: a clinical cross-sectional study. Nord J Psychiatry. 2022;76(2):114–119. doi:10.1080/08039488.2021.1939779
47. Sun H, Liu Q, Wang X, et al. The longitudinal increments of serum alanine aminotransferase increased the incidence risk of metabolic syndrome: a large cohort population in China. Clin Chim Acta. 2019;488:242–247. doi:10.1016/j.cca.2018.10.033
48. Wang M, Wang M, Zhang R, et al. A combined association of serum uric acid, alanine aminotransferase and waist circumference with non-alcoholic fatty liver disease: a community-based study. PeerJ. 2022;10:e13022. doi:10.7717/peerj.13022
49. Sunny NE, Parks EJ, Browning JD, Burgess SC. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 2011;14(6):804–810. doi:10.1016/j.cmet.2011.11.004.
50. Sookoian S, Castaño GO, Scian R, et al. Serum aminotransferases in nonalcoholic fatty liver disease are a signature of liver metabolic perturbations at the amino acid and Krebs cycle level. Am J Clin Nutr. 2016;103(2):422–434. doi:10.3945/ajcn.115.118695
51. van der Vorst EPC. High-density lipoproteins and apolipoprotein A1. Subcell Biochem. 2020;94:399–420. doi:10.1007/978-3-030-41769-7_16
52. Hussain MM, Shi J, Dreizen P. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J Lipid Res. 2003;44(1):22–32. doi:10.1194/jlr.r200014-jlr200
53. Mabrouk H, Mechria H, Mechri A, et al. Paraoxonase 1 activity and lipid profile in schizophrenic patients. Asian J Psychiatr. 2014;9:36–40. doi:10.1016/j.ajp.2013.12.019
54. Canfrán-Duque A, Casado ME, Pastor O, et al. Atypical antipsychotics alter cholesterol and fatty acid metabolism in vitro. J Lipid Res. 2013;54(2):310–324. doi:10.1194/jlr.M026948
55. Nass KJ, van den Berg EH, Faber KN, Schreuder TCMA, Blokzijl H, Dullaart RPF. High prevalence of apolipoprotein B dyslipoproteinemias in non-alcoholic fatty liver disease: the lifelines cohort study. Metabolism. 2017;72:37–46. doi:10.1016/j.metabol.2017.04.004
56. Wang J, Zhu W, Huang S, et al. Serum apoB levels independently predict the development of non-alcoholic fatty liver disease: a 7-year prospective study. Liver Int. 2017;37(8):1202–1208. doi:10.1111/liv.13363
57. Li M, Shu W, Zunong J, et al. Predictors of non-alcoholic fatty liver disease in children. Pediatr Res. 2022;92:322–330. doi:10.1038/s41390-021-01754-6
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
