Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Prevalence and Factors Associated with Subclinical Hypothyroidism in Major Depressive Disorder Patients with Dyslipidemia
Authors Zhan L , Yin H, Gao Y, Li Y, Ma J
Received 8 September 2023
Accepted for publication 24 October 2023
Published 27 October 2023 Volume 2023:19 Pages 2309—2318
DOI https://doi.org/10.2147/NDT.S435138
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Taro Kishi
Luyu Zhan,1,2,* Huimin Yin,1,2,* Yujun Gao,3,* Yi Li,1,2 Jun Ma2,3
1Wuhan Mental Health Center, School of Mental Health and Psychological Sciences, Anhui Medical University, Wuhan, People’s Republic of China; 2Department of Psychiatry, Wuhan Mental Health Center, Wuhan, People’s Republic of China; 3Department of Psychiatry, Renmin Hospital of Wuhan University, Wuhan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jun Ma; Yi Li, Wuhan Mental Health Center, No. 89, Gongnongbing Road, Wuhan, Hubei Province, People’s Republic of China, Email [email protected]; [email protected]
Background: Major depressive disorder (MDD) is a common psychiatric disorder with a high prevalence of comorbidity with subclinical hypothyroidism. The aim of this study was to investigate the prevalence and factors influencing the comorbidity of subclinical hypothyroidism in patients with dyslipidemic MDD who were hospitalized for the first time in a Chinese population.
Methods: The study incorporated 708 first-time hospitalized MDD patients, all with dyslipidemia. Data collection encompassed socio-demographic information, blood pressure, fasting blood glucose (FBG), lipid, and thyroid hormone levels. Participants were evaluated using the Hamilton Depression Scale (HAMD), Hamilton Anxiety Scale (HAMA), and Positive Symptom Subscale (PSS).
Results: The prevalence of subclinical hypothyroidism in dyslipidemic MDD patients with the first hospitalization was 39.97%. The course of the disease, age at onset, HAMA score, and low-density lipoprotein cholesterol (LDL-C) were risk factors for subclinical hypothyroidism in dyslipidemic MDD patients. The course of disease, age at onset, HAMA score, HAMD score, FBG, and systolic blood pressure (SBP) levels were observed to influence serum Thyroid Stimulating Hormone (TSH) levels.
Conclusion: MDD patients with dyslipidemia have a high prevalence of subclinical hypothyroidism, and the outcome is associated with anxiety, fasting glucose, and lipids. This study provides a potential biomarker for the identification of co-morbid subclinical hypothyroidism in MDD patients with dyslipidaemia.
Keywords: major depressive disorder, prevalence, dyslipidemia, subclinical hypothyroidism
Introduction
Major depressive disorder (MDD) is a very common psychiatric disorder that is part of the affective disorder spectrum and shares a common biological basis with other affective disorder spectrum disorders.1 MDD is characterised by depressed mood, diminished interest and loss of pleasure, accompanied by somatic symptoms such as loss of appetite and insomnia, and which remains one of the leading causes of the global burden of disease,2 with an estimated annual economic cost of approximately $12 billion.3 The lifetime prevalence of MDD in China is 3.4% and the 12-month prevalence is 2.1%.4 Although there have been many studies that have made very significant progress in better understanding the pathophysiology of MDD, the pathogenesis of MDD remains unclear.5 MDD has been shown to be associated with increased morbidity and mortality from vascular disease.6,7 American Heart Association identifies MDD as a tier II risk factor for early cardiovascular disease.8 Disorders of lipid metabolism are often observed in patients with MDD in clinical practice.9 And dyslipidemia is an important risk factor for cardiovascular disease.10 Many studies have demonstrated a relationship between MDD and dyslipidemia,11–13 which not only affects the prognosis of MDD patients but also increases the risk of premature death.14
The pathophysiological mechanism of MDD is also closely related to the somatic endocrine metabolic state, and the thyroid gland is one of the glands responsible for metabolism in the body, which secretes thyroid hormones that can affect the body’s energy metabolism, cardiovascular function, cerebral neurotransmission, and other physiological processes.15 It has been shown that the prevalence of MDD is higher in patients with subclinical hypothyroidism,16,17 and several studies on subjects of different ethnic and cultural backgrounds have shown that the incidence of depression increases immediately after hypothyroidism or thyroidectomy.18,19 Thyroid function is not only closely related to MDD but also affects lipid metabolism in the body.20 Hypothyroidism, which leads to the development of hypercholesterolaemia, is mainly associated with reduced activity of low-density lipoprotein (LDL) receptors. In vivo control of free triiodothyronine (FT3) over sterol regulatory element binding protein 2 (SREBP-2), which regulates cholesterol biosynthesis by limiting the activity of the degradative enzyme 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA), is reduced.21 At the same time, hypercholesterolaemia may have toxic effects on the pituitary-thyroid axis. Thyroid follicular cells from mice fed a hypercholesterolemic diet showed significant accumulation of lipid droplets, cytoplasmic loss and mitochondrial degeneration under electron microscopy.22 It has also been shown that mice fed a high cholesterol diet have elevated serum cholesterol levels and TSH levels, significantly elevated pituitary cholesterol levels, and structural changes in TSH cells in the anterior pituitary compared to mice fed a normal diet.23 The above studies have shown that MDD, dyslipidemia and thyroid function are closely related to each other.
Therefore, we conducted the present study with an aim to find out the prevalence of subclinical hypothyroidism in patients with dyslipidaemic MDD at the time of first hospitalisation and the factors influencing it. Aims of the study: 1. To assess the prevalence of subclinical hypothyroidism in patients with dyslipidemic MDD. 2. To analyse the factors influencing subclinical hypothyroidism. 3. And to explore the relationship between demographic data, clinical parameters and serum TSH levels.
Materials and Methods
Subjects
A total of 708 patients with MDD with dyslipidemia were admitted to the Wuhan Mental Health Center between July 2017 and August 2022.
To be eligible for the study, patients needed to meet the following criteria: meet the 10th revision diagnostic standards for MDD according to the International Classification of Diseases (ICD-10); meet the dyslipidemia criteria outlined in the guidelines for the prevention and treatment of dyslipidemia in Chinese adults24 (total cholesterol (TC) ≥ 5.2 mmol/l or triglycerides (TG) ≥ 1.7 mmol/l or low-density lipoprotein cholesterol (LDL-C) ≥ 3.4 mmol/l or high-density lipoprotein cholesterol (HDL-C) < 1.0 mmol/l);25 have no prior history of hospitalization before the day of admission; be between 18 and 60 years old; and have a score of ≥24 on the 17-item Hamilton Depression Scale (HAMD-17).
The following formula was used to determine the sample size: 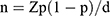 . n, sample size; Z, 95% confidence interval, equal to 1.96; d, marginal error, equal to 0.05; and p, expected prevalence, equal to 0.35. The estimated sample size was 350 cases. A total of 739 inpatients were screened, of whom 31 were excluded for the following reasons: During pregnancy or lactation (n =3); Having a history of psychoactive substance dependence (n =7); Suffering from a serious physical illness or personality disorders (n =3); Those with a clear history of diabetes before the onset of MDD and treated with glucose - lowering medications (n =18). (shown in Figure 1). Finally, a total of 708 individuals were included in this study. Therefore, the sample size in this study was able to provide a sufficient level of certainty for statistical analyses.
. n, sample size; Z, 95% confidence interval, equal to 1.96; d, marginal error, equal to 0.05; and p, expected prevalence, equal to 0.35. The estimated sample size was 350 cases. A total of 739 inpatients were screened, of whom 31 were excluded for the following reasons: During pregnancy or lactation (n =3); Having a history of psychoactive substance dependence (n =7); Suffering from a serious physical illness or personality disorders (n =3); Those with a clear history of diabetes before the onset of MDD and treated with glucose - lowering medications (n =18). (shown in Figure 1). Finally, a total of 708 individuals were included in this study. Therefore, the sample size in this study was able to provide a sufficient level of certainty for statistical analyses.
 |
Figure 1 Flow chart. Abbreviations: SCH, subclinical hypothyroidism; NTF, normal thyroid function. |
The study was approved by the Ethics Committee of Wuhan Mental Health Centre under approval number KY20170201.01. All participants provided written informed consent signed by the patients themselves or their families.
Research Design
In this study, a cross-sectional design was used to examine the prevalence of subclinical hypothyroidism in patients with dyslipidaemic MDD who were hospitalized for the first time and to analyse the factors affecting thyroid function. We defined patients with thyroid stimulating hormone (TSH) >4.2uIU/mL and free tetraiodothyronine (FT4) as in the normal range as the subclinical hypothyroidism (SCH) subgroup and other patients as the normal thyroid function (NTF),26,27 and compared the two clinical subgroups concerning demographic and general clinical data. Risk factors for subclinical hypothyroidism in dyslipidemic MDD patients were analyzed, and the relation between TSH levels and socio-demographic and clinical parameters was examined through multiple linear regression analysis.
We collected general clinical data on the day of admission for patients with MDD who met the inclusion criteria. These data included patients’ age, gender, age of onset, course of disease, marital status, history of outpatient treatment. History of suicide was obtained by asking for specific information in the medical history. The severity of depressive symptoms was assessed using the HAMD-17, anxiety symptoms using the 14-item Hamilton Anxiety Scale (HAMA-14), psychotic symptoms using the Positive Symptom Subscale (PSS) comprising items P1-P7 from the Positive and Negative Symptom Scale (PANSS), and illness severity using the Clinical Global Impression Inventory (CGI). In addition, the following indicators were extracted from the medical record system, including the patient’s blood lipid profile (specifically: TC, TG, LDL-C, HDL-C), fasting blood glucose (FBG) level, body mass index (BMI), blood pressure level (specifically: systolic blood pressure, SBP; diastolic blood pressure, DBP), and thyroid function (specifically: TSH: thyroid stimulating hormone; FT3: free triiodothyronine; FT4: free tetraiodothyronine).
The relevant psychometric measures were assessed by two psychiatrists with primary care levels who had received uniform training at the medical centers where the sample was obtained.
Data Analysis
Numerical variables that conformed to a normal distribution were expressed as means and standard deviations, whereas categorical variables were expressed as counts. Independent samples t-tests were used to compare continuous variables across subgroups, and chi-square tests were used to compare ratios. To examine variables affecting subclinical hypothyroidism, variables that differed in univariate analysis were included as independent variables in a binary logistic regression model with subclinical hypothyroidism as the outcome variable. Finally, using multiple linear regression analysis to determine the influencing factors of thyroid function in the target population. A variance inflation factor (VIF) was used to assess multicollinearity between independent variables, with VIF > 5 indicating the presence of multicollinearity. The threshold of significance was set at less than 0.05 and all p-values were two-tailed. Statistical analyses were performed with SPSS 26 (SPSS, Inc., Chicago, IL).
Results
Differences Between the Two Groups in MDD with or without Subclinical Hypothyroidism
Among the 708 patients with dyslipidemic MDD (There were 416 cases of one type of dyslipidaemia, 222 cases of two types of dyslipidaemia, 68 cases of three types of dyslipidaemia and 2 cases of four types of dyslipidaemia), 283 had subclinical hypothyroidism, representing 39.97% of the total sample. This report showed differences in some clinical parameters between the two subgroups of patients with SCH and NTF. HAMD, HAMA and PSS scores and some metabolic parameters (FBG, TC, LDL-C, BMI, SBP, DBP) were significantly higher in the SCH subgroup than in the NTF subgroup. However, patients in the SCH subgroup had a shorter duration of disease (Table 1).
 |
Table 1 The Demographic and General Clinical Data in Different Clinical Subgroups |
Determinants of MDD Patients with Subclinical Hypothyroidism: Based on a Binary Logistic Regression Model
A binary logistic regression model (backward: Wald) was constructed with subclinical hypothyroidism as the outcome variable and parameters that differed in the univariate analysis as independent variables, showing that course of disease (B = 0.07, p = 0.003, OR = 1.07), onset age (B = 0.07, p < 0.001, OR = 1.07), HAMA score (B = 0.19, p <. 001, OR = 1.21) and LDL-C (B = 0.29, p = 0.049, OR = 1.33) were risk factors for dyslipidemia MDD with subclinical hypothyroidism, while HAMD score (B = −0.26, p < 0.001, OR = 0.77), FBG level (B = −0.96, p < 0.001, OR = 0.39), SBP level (B = −0.15, p < 0.001, OR = 0.86) were protective factors, and these results are summarized in Table 2.
 |
Table 2 Binary Logistic Regression Analyses of Determinants of Thyroid Dysfunction in Dyslipidemic MDD Patients |
onstruction of Multiple Linear Regression Analysis with TSH Level as the Outcome Variable
Multiple linear regression equations were constructed using TSH level as the outcome variable and general clinical data and metabolic parameters as independent variables. The results showed that SBP (B = 0.11, t = 7.96, p < 0.001), Onset age (B = −0.04, t = −3.27, p = 0 0.001), FBG (B = 0.80, t = 5.29, p < 0.001), HAMD (B = −0.13, t = −2.69, p = 0 0.008), HAMA (B = 0.37, t = 9.55, p < 0.001) and LDL-C (B = 0.27, t = 2.03, p = 0 0.043) were significantly affected thyroid function (Table 3).
 |
Table 3 Factors Affecting TSH Levels: a Model Based on Multiple Linear Regression Analysis |
Discussion
This study investigated the prevalence of subclinical hypothyroidism and associated factors influencing it in a large sample of first-time hospitalized patients with dyslipidaemic MDD. The main findings were as follows: (1) The prevalence of subclinical hypothyroidism among dyslipidemic MDD patients was 39.97%. The HAMD, HAMA and PSS scores and some metabolic parameters (FBG, TC, LDL-C, BMI, SBP, DBP) were significantly higher in the SCH subgroup than in the NTF subgroup. (2) Risk factors for subclinical hypothyroidism in the target population included course of disease, age at onset, HAMA score, and LDL-C levels. (3) Course of disease, age at onset, HAMA score, HAMD score, FBG, and SBP levels significantly influenced serum TSH levels.
Our study showed that the prevalence of subclinical hypothyroidism in dyslipidemic MDD patients was 39.97%. There are many reports on the prevalence of subclinical hypothyroidism diagnosed by MDD, but there is a great deal of heterogeneity in the reported prevalence rates, and there are several potential factors contributing to this heterogeneity. In a previous study of first-onset, unmedicated MDD patients in a Chinese population, the prevalence of subclinical hypothyroidism was 60.7%,28 which is much higher compared with the prevalence of subclinical hypothyroidism in our present study (39.97%). In contrast, in a study on the relationship between depression and comorbid thyroid disease in a European population, the point prevalence of MDD combined with hypothyroidism was found to be only 13.2%29, which is significantly lower than the prevalence found in our study. There may be several reasons for these differences: first, MDD patients have reduced activity and exercise due to the effects of the disease, and some of them have overeating habits, leading to an increase in the proportion of obese and overweight patients.30 These poor lifestyles will eventually lead to abnormal lipid levels. Antidepressants or antipsychotics can affect metabolism, leading to adverse effects such as weight gain and dyslipidaemia.31 Secondly, depression and abnormal lipid metabolism may share common acute systemic responses, such as the hypothalamic-pituitary-adrenal axis, which also predispose patients with MDD to lipid metabolism disorders.32 Lipids also adversely affect thyroid function, and the thyroid gland may be one of the target organs for lipotoxicity, with abnormal lipid metabolism being a potential risk factor for subclinical hypothyroidism.33 In addition, excessive accumulation of cholesterol may cause thyroid dysfunction.34,35 In our study, the diagnostic criteria for subclinical hypothyroidism were broader and therefore the prevalence was higher than that of hypothyroidism.
Another important result of this study was the finding that the course of disease, onset age, HAMA scores, and LDL-C levels were risk factors for comorbid subclinical hypothyroidism in patients with dyslipidemic MDD. A study on the effect of thyroid function on the risk of readmission after hospital discharge in patients with MDD showed that the longer the duration of disease in MDD patients, the more likely they were to develop subclinical hypothyroidism.36 One study showed that patients with MDD at a later age of onset had higher TSH levels,37 and anxious MDD patients had higher TSH levels than those without anxiety,37 which suggests that a later age of onset and a high HAMA score are more likely to be associated with subclinical hypothyroidism. The above findings are important support for our findings, in addition to the fact that lipid levels are also an important influence on thyroid function. Lipid metabolism plays an important role in thyroid function maintained by thyroid stimulating hormones, and subclinical hypothyroidism is a common endocrine disorder associated with dyslipidemia.38 A study conducted in an Indian population on response to levothyroxine treatment in patients with subclinical hypothyroidism showed higher LDL-C levels in the subclinical hypothyroidism group compared to the control group,39 which is consistent with our study.
We also found that onset age, LDL-C levels, HAMA score, HAMD score, FBG, and SBP significantly affects serum TSH levels. We found that HAMA scores significantly affect serum TSH levels, which is in agreement with several other studies.28,40–42 The results of these studies suggest that the severity of depressive symptoms and the severity of anxiety symptoms in MDD significantly affect serum TSH levels. This is due to the fact that TSH is involved in mood regulation in two ways, either by acting on the α and β subtypes of thyroid hormone receptors in the limbic system to regulate mood43,44 or by modulating the 5-hydroxytryptaminergic and γ-aminobutyric acidergic systems in the brain.45,46 In terms of metabolism, we found that FBG and LDL-C significantly affect serum TSH levels, which is consistent with the view of Zhu et al.47 A study has shown that abnormal thyroid function can increase the risk of developing diabetes and diabetic complications.48 Previous studies have suggested a possible common etiology of MDD, thyroid disease, and diabetes, namely an imbalance in the neuroendocrine network.49 In addition, previous studies validated our view that LDL-C significantly affects TSH levels.50 In terms of blood pressure, it has been demonstrated that subclinical hypothyroidism is associated with hypertension,51 and our study found that SBP was independently and correlated with subclinical hypothyroidism in MDD patients with dyslipidemia, which may be due to subclinical hypothyroidism causing abnormalities in lipid metabolism and subsequently atherosclerosis, which ultimately leads to changes in blood pressure.52
According to our findings, in clinical work, it is important to pay attention to the patient’s age, duration of the disease, anxiety symptoms and to detect the patient’s LDL-C serum level, which can help us to monitor the patient’s thyroid function in a timely manner, to control the progression of the disease, and to avoid any adverse effects on the patient’s prognosis.
There are several limitations of this study that need to be addressed. First, the cross-sectional design of this study did not allow for the determination of causal or reverse causal relationships between thyroid function and clinical variables in patients with MDD. In order to reveal the causal relationship between these effects and subclinical hypothyroidism, future studies should use longitudinal studies. Second, our study population consisted of both first-episode unmedicated patients and patients with a history of outpatient treatment, which may have confounded medication effects. Third, this study was conducted in a Chinese population and should not be generalised to other populations.
Conclusion
In conclusion, this study found a high prevalence of subclinical hypothyroidism in patients with dyslipidaemic MDD. In the target population, thyroid function is affected by a variety of clinical parameters, which provide potential biomarkers for early identification of subclinical hypothyroidism.
Abbreviations
MDD, Major Depressive Disorder; HAMD, Hamilton Depression Scale score; HAMA, Hamilton Anxiety Scale score; SCH, subclinical hypothyroidism; NTF, normal thyroid function; PSS, Positive symptom subscale; CGI-SI, Clinical Global Impression Scale - Severity of Illness; FT3, Free triiodothyronine; FT4, Free tetraiodothyronine; TSH, thyroid stimulating hormone; FBG, fasting blood glucose; TC, total cholesterol; HDL-C, high density lipoprotein cholesterol; TG, triglycerides; LDL-C, low density lipoprotein cholesterol; BMI, Body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; WC, waist circumference.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The ethics committees of the Wuhan mental health center reviewed and approved this study. All subject guardians knew about this study and signed informed consent. All procedures carried out in studies conformed to the 1964 Helsinki Declaration and its subsequent amendments or similar ethical standards.
Acknowledgments
We are grateful to all the medical staff and patients in our study and to those who contributed to the diagnosis and clinical evaluation of the subjects.
Author Contributions
Jun Ma and Yi Li made significant contributions to the conception and design of the study. Luyu Zhan and Huimin Yin were responsible for data collection, analysis, writing and re-editing of the article. Yujun Gao was responsible for adding and revising the content of the manuscript. Jun Ma finalised the published version. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Ng QX, Lim DY, Chee KT. Reimagining the spectrum of affective disorders. Bipolar Disord. 2020;22(6):638–639. doi:10.1111/bdi.12960
2. Collaborators GBDMD. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9(2):137–150. doi:10.1016/S2215-0366(21)00395-3
3. Tanner JA, Hensel J, Davies PE, Brown LC, Dechairo BM, Mulsant BH. Economic burden of depression and associated resource use in Manitoba, Canada. Can J Psychiatry. 2020;65(5):338–346. doi:10.1177/0706743719895342
4. Huang Y, Wang Y, Wang H, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. 2019;6(3):211–224. doi:10.1016/S2215-0366(18)30511-X
5. Malhi GS, Mann JJ. Depression. Lancet. 2018;392(10161):2299–2312. doi:10.1016/S0140-6736(18)31948-2
6. Melo APS, Dippenaar IN, Johnson SC, et al. All-cause and cause-specific mortality among people with severe mental illness in Brazil’s public health system, 2000–15: a retrospective study. Lancet Psychiatry. 2022;9(10):771–781. doi:10.1016/S2215-0366(22)00237-1
7. Geraets AFJ, Schram MT, Jansen JFA, et al. The cardiometabolic depression subtype and its association with clinical characteristics: the Maastricht Study. J Affect Disord. 2022;313:110–117. doi:10.1016/j.jad.2022.06.045
8. Goldstein BI, Carnethon MR, Matthews KA, et al. Major depressive disorder and bipolar disorder predispose youth to accelerated atherosclerosis and early cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132(10):965–986. doi:10.1161/CIR.0000000000000229
9. Lemche AV, Chaban OS, Lemche E. Depression contributing to dyslipidemic cardiovascular risk in the metabolic syndrome. J Endocrinol Invest. 2017;40(5):539–546. doi:10.1007/s40618-016-0601-y
10. Kopin L, Lowenstein C. Dyslipidemia. Ann Intern Med. 2017;167(11):ITC81–ITC96. doi:10.7326/AITC201712050
11. Parekh A, Smeeth D, Milner Y, Thure S. The role of lipid biomarkers in major depression. Healthcare. 2017;5(1). doi:10.3390/healthcare5010005
12. Persons JE, Fiedorowicz JG. Depression and serum low-density lipoprotein: a systematic review and meta-analysis. J Affect Disord. 2016;206:55–67. doi:10.1016/j.jad.2016.07.033
13. Chien IC, Lin CH, Chou YJ, Chou P. Increased risk of hyperlipidemia in patients with major depressive disorder: a population-based study. J Psychosom Res. 2013;75(3):270–274. doi:10.1016/j.jpsychores.2013.06.003
14. Laursen TM, Nordentoft M, Mortensen PB. Excess early mortality in schizophrenia. Annu Rev Clin Psychol. 2014;10(1):425–448. doi:10.1146/annurev-clinpsy-032813-153657
15. Burch HB, Ingelfinger JR. Drug effects on the thyroid. N Engl J Med. 2019;381(8):749–761. doi:10.1056/NEJMra1901214
16. Yang R, Du X, Li Z, et al. Association of subclinical hypothyroidism with anxiety symptom in young first-episode and drug-naive patients with major depressive disorder. Front Psychiatry. 2022;13:920723. doi:10.3389/fpsyt.2022.920723
17. Gorkhali B, Sharma S, Amatya M, Acharya D, Sharma M. Anxiety and depression among patients with thyroid function disorders. J Nepal Health Res Counc. 2020;18(3):373–378. doi:10.33314/jnhrc.v18i3.2499
18. Qiao D, Liu H, Zhang X, et al. Exploring the potential of thyroid hormones to predict clinical improvements in depressive patients: a machine learning analysis of the real-world based study. J Affect Disord. 2022;299:159–165. doi:10.1016/j.jad.2021.11.055
19. Choi KW, Kim Y, Fava M, et al. Increased morbidity of major depressive disorder after thyroidectomy: a nationwide population-based study in South Korea. Thyroid. 2019;29(12):1713–1722. doi:10.1089/thy.2019.0091
20. Grais IM, Sowers JR. Thyroid and the heart. Am J Med. 2014;127(8):691–698. doi:10.1016/j.amjmed.2014.03.009
21. Duntas LH, Brenta G, Renewed A. Focus on the association between thyroid hormones and lipid metabolism. Front Endocrinol. 2018;9:511. doi:10.3389/fendo.2018.00511
22. Ayuob NN, El-Hawwary AA, Huwait EA, Mubarak WAE, Balgoon MJ. Red grape juice protects the rat thyroid gland against hypercholesterolemic changes. Ultrastructural and biochemical evidences. Rom J Morphol Embryol. 2019;60(3):921–929.
23. Yang J, Zhang X, Liu Z, et al. High-cholesterol diet disrupts the levels of hormones derived from anterior pituitary basophilic cells. J Neuroendocrinol. 2016;28(3):12369. doi:10.1111/jne.12369
24. Joint committee issued Chinese guideline for the management of dyslipidemia in A. [2016 Chinese guideline for the management of dyslipidemia in adults]. Zhonghua Xin Xue Guan Bing Za Zhi. 2016;44(10):833–853. Chinese. doi:10.3760/cma.j.issn.0253-3758.2016.10.005
25. Li H, Zhang X, Sun Q, Zou R, Li Z, Liu S. Association between serum lipid concentrations and attempted suicide in patients with major depressive disorder: a meta-analysis. PLoS One. 2020;15(12):e0243847. doi:10.1371/journal.pone.0243847
26. Li X, Zhen D, Zhao M, et al. Natural history of mild subclinical hypothyroidism in a middle-aged and elderly Chinese population: a prospective study. Endocr J. 2017;64(4):437–447. doi:10.1507/endocrj.EJ16-0549
27. Zhao M, Yang T, Chen L, et al. Subclinical hypothyroidism might worsen the effects of aging on serum lipid profiles: a population-based case-control study. Thyroid. 2015;25(5):485–493. doi:10.1089/thy.2014.0219
28. Lang X, Hou X, Shangguan F, Zhang XY. Prevalence and clinical correlates of subclinical hypothyroidism in first-episode drug-naive patients with major depressive disorder in a large sample of Chinese. J Affect Disord. 2020;263:507–515. doi:10.1016/j.jad.2019.11.004
29. Fugger G, Dold M, Bartova L, et al. Comorbid thyroid disease in patients with major depressive disorder - results from the European Group for the Study of Resistant Depression (GSRD). Eur Neuropsychopharmacol. 2018;28(6):752–760. doi:10.1016/j.euroneuro.2018.03.011
30. Mills JG, Thomas SJ, Larkin TA, Deng C. Overeating and food addiction in major depressive disorder: links to peripheral dopamine. Appetite. 2020;148:104586. doi:10.1016/j.appet.2020.104586
31. Franch Pato CM, Molina Rodriguez V, Franch Valverde JI. Sindrome metabolico y antipsicoticos atipicos. Posibilidad de prediccion y control [Metabolic syndrome and atypical antipsychotics: possibility of prediction and control]. Rev Psiquiatr Salud Ment. 2017;10(1):38–44. Spanish. doi:10.1016/j.rpsm.2016.09.003
32. Marazziti D, Rutigliano G, Baroni S, Landi P, Dell’Osso L. Metabolic syndrome and major depression. CNS Spectr. 2014;19(4):293–304. doi:10.1017/S1092852913000667
33. Zhao M, Tang X, Yang T, et al. Lipotoxicity, a potential risk factor for the increasing prevalence of subclinical hypothyroidism? J Clin Endocrinol Metab. 2015;100(5):1887–1894. doi:10.1210/jc.2014-3987
34. Song Y, Liu J, Zhao K, Gao L, Zhao J. Cholesterol-induced toxicity: an integrated view of the role of cholesterol in multiple diseases. Cell Metab. 2021;33(10):1911–1925. doi:10.1016/j.cmet.2021.09.001
35. Shao SS, Zhao YF, Song YF, et al. Dietary high-fat lard intake induces thyroid dysfunction and abnormal morphology in rats. Acta Pharmacol Sin. 2014;35(11):1411–1420. doi:10.1038/aps.2014.82
36. Yang L, Yang X, Yang T, et al. The effect of thyroid function on the risk of psychiatric readmission after hospitalization for major depressive disorder. Psychiatry Res. 2021;305:114205. doi:10.1016/j.psychres.2021.114205
37. Wang X, Wang Q, Ren H, et al. The prevalence and clinical correlates of anxiety in Chinese patients with first-episode and drug-naive major depressive disorder at different ages of onset. J Affect Disord. 2023;325:306–312. doi:10.1016/j.jad.2023.01.032
38. Khazan M, Amouzegar A, Gharibzadeh S, Mehran L, Tohidi M, Azizi F. Prevalence of hypothyroidism in patients with dyslipidemia: Tehran Thyroid Study (TTS). Horm Metab Res. 2014;46(13):980–984. doi:10.1055/s-0034-1389997
39. Vishnoi G, Chakraborty B, Garda H, Gowda SH, Goswami B. Low mood and response to Levothyroxine treatment in Indian patients with subclinical hypothyroidism. Asian J Psychiatr. 2014;8:89–93. doi:10.1016/j.ajp.2013.12.004
40. Wang Q, Xu X, Ren H, Tan L, Zhang XY. Prevalence and risk factors of thyroid dysfunction in outpatients with overweight or obese first-episode and drug-naive major depressive disorder. J Affect Disord. 2023;328:135–140. doi:10.1016/j.jad.2023.02.068
41. Luo G, Li Y, Yao C, Li M, Li J, Zhang X. Prevalence of overweight and obesity in patients with major depressive disorder with anxiety: mediating role of thyroid hormones and metabolic parameters. J Affect Disord. 2023;335:298–304. doi:10.1016/j.jad.2023.05.008
42. Dai W, Liu J, Xie H, et al. Association between subclinical hypothyroidism and psychotic features in Chinese young adults with first-episode and untreated major depressive disorder. J Affect Disord. 2023;333:209–215. doi:10.1016/j.jad.2023.04.067
43. Naicker M, Abbai N, Naidoo S. Bipolar limbic expression of auto-immune thyroid targets: thyroglobulin and thyroid-stimulating hormone receptor. Metab Brain Dis. 2019;34(5):1281–1298. doi:10.1007/s11011-019-00437-w
44. Fliers E, Unmehopa UA, Alkemade A. Functional neuroanatomy of thyroid hormone feedback in the human hypothalamus and pituitary gland. Mol Cell Endocrinol. 2006;251(1–2):1–8. doi:10.1016/j.mce.2006.03.042
45. Nuguru SP, Rachakonda S, Sripathi S, Khan MI, Patel N, Meda RT. Hypothyroidism and depression: a narrative review. Cureus. 2022;14(8):e28201. doi:10.7759/cureus.28201
46. Karakatsoulis GN, Tsapakis EM, Mitkani C, Fountoulakis KN. Subclinical thyroid dysfunction and major depressive disorder. Hormones. 2021;20(4):613–621. doi:10.1007/s42000-021-00312-3
47. Zhu Q, Jiang G, Lang X, et al. Prevalence and clinical correlates of abnormal lipid metabolism in first-episode and drug-naive patients with major depressive disorder with abnormal glucose metabolism. Sci Rep. 2023;13(1):8078. doi:10.1038/s41598-023-35290-6
48. Kalra S, Aggarwal S, Khandelwal D, Grzmil P. Thyroid dysfunction and dysmetabolic syndrome: the need for enhanced thyrovigilance strategies. Int J Endocrinol. 2021;2021:9641846. doi:10.1155/2021/9641846
49. Ramirez LA, Perez-Padilla EA, Garcia-Oscos F, Salgado H, Atzori M, Pineda JC. A new theory of depression based on the serotonin/kynurenine relationship and the hypothalamic pituitary- adrenal axis. Biomedica. 2018;38(3):437–450. doi:10.7705/biomedica.v38i3.3688
50. Lei Y, Yang J, Li H, Zhong H, Wan Q. Changes in glucose-lipid metabolism, insulin resistance, and inflammatory factors in patients with autoimmune thyroid disease. J Clin Lab Anal. 2019;33(7):e22929. doi:10.1002/jcla.22929
51. Gaitonde DY, Rowley KD, Sweeney LB. Hypothyroidism: an update. Am Fam Physician. 2012;86(3):244–251.
52. Berta E, Lengyel I, Halmi S, et al. Hypertension in thyroid disorders. Front Endocrinol. 2019;10:482. doi:10.3389/fendo.2019.00482
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
