Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Prevalence and Associated Factors of Urinary Tract Infection in Patients with Diabetic Neuropathy: A Hospital-Based Cross-Sectional Study
Authors Wang X, Wang Y, Luo L, Tan L, Cai W, Chen L, Ren W
Received 21 December 2022
Accepted for publication 15 April 2023
Published 3 May 2023 Volume 2023:16 Pages 1261—1270
DOI https://doi.org/10.2147/DMSO.S402156
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Xiufen Wang,1– 3 Ying Wang,1,2 Li Luo,3 Liuting Tan,4 Wenzhi Cai,1 Ling Chen,1 Wei Ren1
1Department of Nursing, Shenzhen Hospital, Southern Medical University, Shenzhen, Guangdong, People’s Republic of China; 2School of Nursing, Southern Medical University, Guangzhou, Guangdong, People’s Republic of China; 3Department of the Third Pulmonary Disease, The Third People’s Hospital of Shenzhen, Shenzhen, Guangdong, People’s Republic of China; 4Department of Endocrine, The Third People’s Hospital of Shenzhen, Shenzhen, Guangdong, People’s Republic of China
Correspondence: Wei Ren; Ling Chen, Department of Nursing, Shenzhen Hospital, Southern Medical University, 1333 Xinhu Road, Baoan District, Shenzhen, Guangdong Province, 518101, People’s Republic of China, Tel +86-755-23360006, Fax +86-755-23323777, Email [email protected]; [email protected]
Introduction: Diabetic neurogenic bladder is one of the common complications in patients with diabetic neuropathy. However, studies reporting the prevalence and associated factors of urinary tract infections (UTIs) in patients with diabetic neuropathy are rare. Therefore, the present study aimed to explore the prevalence and influencing factors of UTI in patients with diabetic neuropathy.
Methods: A hospital-based cross-sectional study that recruited patients with diabetic neuropathy was conducted from January 2019 to December 2021. Collected data included patient demographic information (age, sex, education level, body mass index), clinical data (duration of diabetes, method of administration), and laboratory tests. Multivariable logistic regression models were used to identify the factors associated with UTI risk. The strength of association was expressed as the odds ratio (OR) and 95% confidence interval (95% CI).
Results: A total of 579 patients were recruited (male, 68.2%; overall average age, 57.89 years). Using multivariate analysis with adjustment for confounding factors, female sex (odds ratio [OR]: 4.12; 95% CI: 2.24– 7.60; P < 0.001), hypodermic insulin injection (OR: 2.10; 95% CI: 1.02– 4.35; P = 0.045), chronic kidney disease (OR: 3.12; 95% CI: 1.11– 8.80; P = 0.032), history of UTI (OR = 45.92; 95% CI: 8.62– 244.76; P < 0.001), positive urinary nitrite (OR: 32.87; 95% CI: 7.37– 146.70; P < 0.001), and high residual urine volume (OR: 2.19, 95% CI: 1.17– 4.10; P = 0.014) were independent risk factors for UTI in patients with diabetic neuropathy. Compared with the patients aged < 45 years, UTI prevalence increased 2.91-fold in patients aged 45– 54 years (OR: 3.91; 95% CI: 1.02– 15.03; P = 0.047) and 3.87-fold in those aged ≥ 65 years (OR: 4.87; 95% CI: 1.23– 19.25; P = 0.024).
Conclusion: The main findings of this study showed that older age, female sex, hypodermic insulin injection, CKD, history of UTI, and positive urinary nitrite were independent risk factors for UTI in patients with diabetic neuropathy. To minimize the occurrence and resulting disease burden of UTI, knowledge regarding UTI risk factors in patients with diabetic neuropathy is critical to designate interventions.
Keywords: epidemiology, prevalence, urinary tract infection, diabetic neuropathy, risk factors
A Letter to the Editor has been published for this article.
Introduction
Diabetes mellitus (DM) is a disease of almost epidemic proportions worldwide. As of 2015, more than 415 million adults have DM, and this number is estimated to increase to 642 million by 2040.1 In the United States, an estimated 30.2 million adults or 12.2% had DM in 2015, of which 7.2 million (23.3%) were not aware or did not report having DM.2 In China, the estimated overall prevalence of DM is as high as 11.6%.3
Diabetic neuropathy is a group of clinical syndromes caused by different pathophysiological mechanisms with diverse manifestations and is the most common chronic complication of DM.4,5 The most common types of diabetic neuropathy are distal symmetric polyneuropathy (DSPN) and autonomic neuropathy; DSPN accounts for about 75% of diabetic neuropathy and is commonly referred to as diabetic peripheral neuropathy.6 In Korea7 and in Taiwan, China,8 DSPN accounts for 43% and 21.3% of diabetic neuropathy, respectively.
In addition, diabetic neurogenic bladder (DNB) is a common complication in patients with DM. The primary signs include overactive bladder, detrusor overactivity, poor bladder emptying or urinary overflow incontinence, decreased bladder sensory function, and increased bladder volume. Diabetic neuropathy, including DSPN and autonomic neuropathy, is a common pathogenic factor of DNB. Due to the lack of validated and standardized measures, the incidence of DNB is difficult to estimate;9 urinary dysfunction is reported to be a common complication of diabetes, occurring in more than 80% of DM patients.10 In China, the incidence of DNB in patients with DM lasting more than 10 years is 25%, and it can be as high as 50% in patients >15 years of age.11
Urinary tract infections (UTIs) are the second-most common complication of DNB, and about 130–175 million UTIs occur annually worldwide.12 The prevalence of UTIs assessed in hospital-acquired infections were 12.9% in the United States, 19.6% in Europe, and 24% in developing countries.13 Compared with patients without UTIs, patients with UTIs have a prolonged hospital stay and experience a longer treatment,14 which leads to an increased societal and familial burden.15
The risk factors for DNB-UTI are numerous and complex, and there is a lack of predictive tools. Nonetheless, research concerning UTI in heart failure, DM and nervous system disease has achieved some progress.16–18 However, risk factors for UTI often change due to different disease comorbidities. Therefore, it is necessary for clinicians to more carefully consider the prevalence of UTI and its related factors in diabetic patients with neuropathy.
Methods
Study Design
This cross-sectional study was conducted at the Department of Endocrinology in The Third People’s Hospital of Shenzhen from January 2019 to December 2021. A total of 579 confirmed patients with DM complicated with neuropathy were recruited. These patients were categorized into two groups according to the presence or absence of UTI.
Study size was determined according to the following parameters: true proportion = 24%, null hypothesis = 19%, power (1−β) = 80%, and type I error = 5%.
Participants
Inclusion criteria were as follows: (1) neuropathy in type II DM diagnosed in accordance with the diagnostic criteria of ICD-10 disease code E11 for DM, (2) UTI diagnosed based on the 2018 Urological Association of Asia guidelines, (3) age >18 years, (4) able to communicate independently without mental disorders, and (5) provided informed consent.
Exclusion criteria were as follows: (1) incomplete medical records; (2) did not undergo urine routine, blood routine, liver and kidney function tests, or urinary imaging examinations; (3) urinary system-related diseases, such as urinary obstruction, congenital kidney malformation, polycystic kidney structural abnormalities, and other diseases; (4) use of antibiotics before admission or infection at another site; (5) pregnant women, postpartum women, vaginitis, and other similar conditions; (6) male patients with ureteral stricture, long penile prepuce, calculi, balanoposthitis, recent urologic surgery, prostatic hypertrophy; (7) other confounding factors caused by UTI; and (8) age ≤18 years.
Diagnostic Criteria for UTI
Clinical symptoms of UTI included urgency, frequency, dysuria, low back pain, pain during urination, and urinary retention. Signs included fever (patient axillary temperature ≥37.5°C), costovertebral angle tenderness/pain, and suprapubic tenderness. According to the above clinical manifestations, UTI diagnosis was made when one of the above clinical manifestations was present and one of the following conditions was met: (1) positive urine culture, (2) bacteria detected in half of 30 fields by phase contrast microscopy at 400× magnification after centrifugation, (3) bacterial culture by suprapubic aspiration, or (4) diagnosis of UTI in medical records.1
Data Collection
General demographic and clinical information included age (<45, 45–54, 55–64, and ≥65 years), sex, education level (1–6, 7–9, 10–12, and >12 years), and medical insurance types (employed medical insurance, residence medical insurance, self-paying, and others), body mass index (BMI; normal, BMI <24 kg/m2; overweight, BMI ≥24 kg/m2 and <28 kg/m2; obese, BMI ≥28 kg/m2), duration of DM (<10, 10–19, and ≥20 years), mode of insulin administration for DM (oral medicine, oral and intravenous, without medicine, hypodermic injection), complications, and clinical symptoms and signs. Residual urine volume was categorized as normal (<20 mL in men and <50 mL in women) and high (≥20 mL in men and ≥50 mL in women).19 Methods of urination were defined as self-urination, indwelling catheterization, intermittent catheterization, and cystostomy. Moreover, data were collected regarding the following laboratory indicators: hemoglobin A1c, fasting blood glucose, 2-hour postprandial blood glucose, albumin, blood urea nitrogen, serum creatinine, white blood cell count, urine white blood cell count, and urine nitrite value.
If laboratory and imaging studies were performed more than once during the current admission period, the laboratory and imaging results closest to the first positive urine culture were recorded. If a urine culture was negative or no urine culture was obtained, the first laboratory test and imaging results after the current admission were recorded.
Statistical Analysis
Categorical variables are presented as numbers with percentages, and differences between groups were assessed using the chi-squared test. Continuous variables are presented as the mean and standard deviation (SD), and differences between groups were compared using Student’s t-test. Multivariable logistic regression models were used to identify the factors associated with UTI risk, with UTI risk as the dependent variable and associated factors that were statistically significant in the univariable analysis as the independent variables. UTI risk is presented as the odds ratio (OR) with 95% confidence interval (CI). P-values < 0.05 were considered to be statistically significant. Data analysis was performed using SPSS version 24.0 (IBM Corp., Armonk, NY, USA).
Results
Demographical and Clinical Features of Patients with Diabetic Neuropathy
A total of 579 patients with diabetic neuropathy were recruited; male patients accounted for 68.2%. The overall average age was 57.89 years, and more than 60% patients were aged 45–64 years. Moreover, 55% of patients had an education level <10 years, and 61.8% patients had resident medical insurance. More than half of patients had a normal weight, and more than half had a length of DM of <10 years. The combined use of oral and hypodermic injection medicine accounted for 40.9% (Table 1).
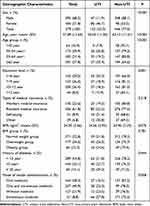 |
Table 1 Association Between Demographic Characteristics and Urinary Tract Infections in Patients with Diabetic Neuropathy |
The most common complications were hypertension (50.1%), vascular disease (40.6%) and retinopathy (38.5%). More than half of patients had a hemoglobin A1c level ≥8.5% and a fasting plasma glucose level ≤7.0 mmol/L, whereas 90.0% had 2-hour postprandial plasma glucose levels ≥10 mmol/L. The positive rate of urine nitrite in this study was 6.1%, and patients with a residual urine volume ≥30 mL accounted for 25.6% (Table 2).
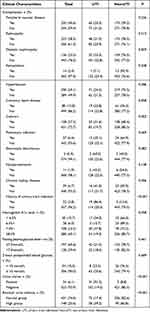 |
Table 2 Association Between Clinical Characteristics and Urinary Tract Infections in Patients with Diabetic Neuropathy |
Univariate Analysis of Associated Factors with UTI in Patients with Diabetic Neuropathy
Table 1 shows an overall 23% prevalence of UTIs in patients with diabetic neuropathy; the prevalence was significantly higher in women than in men (P < 0.001). There was higher risk in the prevalence of UTIs with increased age in patients with diabetic neuropathy (P < 0.001), but a lower risk according to education level (P = 0.001). The mode of insulin administration for DM was related to UTI (P = 0.008).
The prevalence of UTI in patients with diabetic neuropathy was higher in those with cataracts, CKD, history of UTI, and high residual urine volume (P < 0.05; Table 2).
As shown in Table 3, the mean residual urine volume in patients with UTI was significantly higher than that in patients without UTI (P = 0.040).
 |
Table 3 Univariate Analysis of Associated Factors with Urinary Tract Infections in Patients with Diabetic Neuropathy |
Associated Factors of UTI Among Patients with Diabetic Neuropathy in the Multivariate Analysis
Using multivariate analysis after adjustment for the above-mentioned confounding factors, older age, female sex, hypodermic injection mode of treatment administration, CKD, history of UTI, and positive urinary nitrite were associated with UTI in patients with diabetic neuropathy (Table 4 and Figure 1). Compared with patients aged <45 years, UTI risk increased 2.91-fold in those aged 45–54 years and 3.87-fold in those aged ≥65 years (all P < 0.05). UTI risk in women was 4.12-fold higher than in men (P < 0.001). Compared with oral medicine, UTI risk was 2.10-fold higher with hypodermic insulin injection (P = 0.045). UTI prevalence in patients with CKD was 3.12-fold higher than in patients without CKD (P = 0.032). In patients with history of UTI, UTI prevalence was 45.92-fold higher than in patients without history of UTI (P < 0.001). UTI prevalence was 32.87-fold higher in patients with positive urinary nitrite (P < 0.001). Furthermore, UTI prevalence was 2.19-fold higher in patients with residual urine volume ≥30 mL (P = 0.014).
 |
Table 4 Multivariate Analysis of Associated Factors with Urinary Tract Infections in Patients with Diabetic Neuropathy |
Discussion
This is the first report concerning factors associated with UTI in patients with diabetic neuropathy. The main findings are that older age, female sex, hypodermic insulin injection, CKD, history of UTI, and positive urinary nitrite were independent risk factors for UTI in patients with diabetic neuropathy. However, there were no significant associations of other demographic characteristics, other complications, or other laboratory indices with UTI in patients with diabetic neuropathy.
The influencing factors of UTI in patients with diabetic neuropathy have remained unclear. However, studies have reported that the influencing factors of UTI in patients with DM include female sex: UTI was more common in female than male patients with DM and was significantly associated with the presence of neuropathy.20 This is consistent with the present findings, which include that UTI prevalence was 4.12-fold higher in female patients than in male patients with diabetic neuropathy. This may be explained by female urethral anatomy, as the urethra is short and wide and close to both the vagina and anus.
The association of age with UTI in diabetic neuropathy is controversial. Some studies have shown that infection was not related to patient age,20 but some studies indicated that general host factors enhancing UTI risk in patients with DM include age.21 Furthermore, the present study found that UTI prevalence was 4.87-fold higher in patients aged ≥65 years than in patients aged <45 years. One possible explanation is the different study populations, particularly as the age distribution for patients with type I DM and type II DM varies greatly.
Several studies have reported a relationship between CKD and UTI. The possible reason is that patients with UTI use nephrotoxic antibiotics that can cause kidney damage.22 In addition, patients with CKD have an increased risk of UTI, which may be related to immunodeficiency in patients with CKD.23 However, no existing studies have reported the relationship of CKD and UTI in diabetic neuropathy patients. Our studies found that UTI prevalence in patients with CKD was 3.12-fold higher than in those without CKD. This is consistent with the results of previous studies.
History of UTI is a risk factor affecting the occurrence of UTI in patients with DM; moreover, DM is a risk factor for recurrent symptomatic UTI.24 The current study found that UTI prevalence in patients with history of UTI was 45.92-fold higher than in patients without history of UTI. This may be because pathogenic bacteria are not completely eliminated at the time of the first illness and persist in the urinary system for a long time, and the disease subsequently recurs when the patient’s immunity decreases. In addition, studies report that factors enhancing UTI risk in patients with DM include long-term complications, primarily diabetic nephropathy and cystopathy.21 However, no definite relationship between the complication of diabetic nephropathy and UTI in diabetic neuropathy was found in the present study.
Positive urine nitrite often indicates UTI, and this finding is highly specific for UTI.25 The present study found that the incidence of UTI in patients with positive urine nitrite was 32.87-fold higher than in patients with negative urine nitrite. This is consistent with the results of previous studies. This relationship may exist because Gram-negative bacilli such as Escherichia coli can reduce nitrate to nitrite in urine.
Hypodermic insulin injection is generally indicated for patients with diabetic ketoacidosis or hyperosmolar hyperglycemic syndrome.26 However, no studies have reported a relationship between hypodermic insulin injection and UTI risk. The present results demonstrate a 2.1-fold higher UTI prevalence in patients using hypodermic injection than in those using oral administration. This may be related to the unstable control of blood glucose in those using hypodermic insulin injection, as this also increases the urine glucose concentration, thus providing favorable conditions for bacterial reproduction and directly increasing UTI risk in these patients.
Residual urine volume measurement can detect not only urinary retention but also provide early prediction of bladder function. The current study found that UTI prevalence with high residual urine volume was significantly greater than with normal urine volume. This may be because higher residual urine volume indicates bladder dysfunction, which could easily lead to UTI.19 Likewise, our findings suggest that high residual urine volume is associated with UTI in patients with diabetic neuropathy. However, some studies have shown that an increase in residual volume after urination is not related to recurrence of urinary tract infection.27 Therefore, in light of the differences in these studies, further clarification is needed by more prospective cohort studies in the future.
Our study in patients with diabetic neuropathy found that factors associated with UTI in patients with diabetic neuropathy were similar to risk factors for UTI in other populations, such as age, female sex, CKD, and history of previous UTI. These findings help to provide a more comprehensive understanding regarding the risk factors of UTI in patients with diabetic neuropathy, provide clinical patients with ideas for UTI prevention, and avoid the increased disease and economic burden of patients due to UTI as much as possible.
There are some limitations to this study. The first limitation is that this was a single-center study, and data were included only for patients from the same hospital. The results are therefore less representative and likely not generalizable to other patient populations. We will continue to conduct multicenter studies in the future. The second is that this is a cross-sectional study, and the possibility that patients may develop UTIs at a later time cannot be ruled out. Further follow-up studies at different time points will be added in the future. In addition, our study only observed and analyzed the related factors of UTI in patients with diabetes and neuropathy and did not further evaluate the association between diabetic infection and UTI with infections at other common sites. In view of these limitations, we expect to continue conducting representative multicenter prospective cohort studies in the future; such studies will help to further clarify the causal relationship between factors related to UTI in patients with diabetes and neuropathy and to analyze the association between the disease and other common sites of diabetic infection.
Conclusion
In conclusion, this study found that age ≥65 years, female sex, hypodermic insulin injection, CKD, previous history of UTI, positive urinary nitrite, and high residual urine volume are risk factors for UTI in patients with diabetic neuropathy. Understanding the risk factors for UTI in patients with diabetic neuropathy can help clinicians identify high-risk patients early, enabling the earliest possible intervention regarding prevention and treatment strategies. Therefore, we believe that further prospective cohort studies and studies concerning the mechanism of UTI in diabetic patients with neuropathy are necessary to determine whether controlling or reducing risk factors can effectively prevent UTI in such patients.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Ethical Committee of the Third People’s Hospital of Shenzhen, and the study complies with the Declaration of Helsinki. Each participant provided written informed consent.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was partly supported by Science and Innovation Commission of Shenzhen Municipality (JCYJ20190814113003711 and JCYJ20210324142406016) and by Shenzhen Fund for Guangdong Provincial High-level Clinical Key Specialties (SZGSP010).
Disclosure
The authors declare no competing financial interests.
References
1. International Diabetes Federation. IDF diabetes atlas, seventh edition 2015; 2015.
2. American Diabetes Association. 8. pharmacologic approaches to glycemic treatment. Diabetes Care. 2017;40(Suppl1):S64–s74. doi:10.2337/dc17-S011
3. Xu Y, He J, Bi Y, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310(9):948–959. doi:10.1001/jama.2013.168118
4. Williams BC. Textbook of endocrinology - 14th revised edition. Acta Endo. 2019;15:416.
5. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136–154. doi:10.2337/dc16-2042
6. Solomon T, Wu J. Diabetic neuropathy. In: The Diabetic Foot: Medical and Surgical Management. Cham: Springer International Publishing; 2018:
7. Oh J. Clinical spectrum and diagnosis of diabetic neuropathies. Korean J Intern Med. 2020;35(5):1059–1069. doi:10.3904/kjim.2020.202
8. Pai YW, Lin C-H, Lee I-T, et al. Prevalence and biochemical risk factors of diabetic peripheral neuropathy with or without neuropathic pain in Taiwanese adults with type 2 diabetes mellitus. Diabetes Metab Syndr. 2018;12(2):111–116. doi:10.1016/j.dsx.2017.09.013
9. Yuan Z, Tang Z, He C, et al. Diabetic cystopathy: a review. J Diabetes. 2015;7(4):442–447. doi:10.1111/1753-0407.12272
10. Deli G, Bosnyak E, Pusch G, et al. Diabetic neuropathies: diagnosis and management. Neuroendocrinology. 2013;98(4):267–280. doi:10.1159/000358728
11. Peng X, Li S-Y, An Z-M, et al. 美国泌尿协会症状指数评分在女性2型糖尿病神经性膀胱患者中的应用价值研究 [Application Value of American Urological Association symptom index score in female patients with type 2 diabetic neurogenic bladder]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2019;50(4):566–570. Chinese.
12. Nitzan O, Elias M, Chazan B, et al. Urinary tract infections in patients with type 2 diabetes mellitus: review of prevalence, diagnosis, and management. Diabetes Metab Syndr Obes. 2015;8:129–136. doi:10.2147/DMSO.S51792
13. Tandogdu Z, Wagenlehner FM. Global epidemiology of urinary tract infections. Curr Opin Infect Dis. 2016;29(1):73–79. doi:10.1097/QCO.0000000000000228
14. Amos TB, Montejano L, Juneau P, et al. Healthcare costs of urinary tract infections and genital mycotic infections among patients with type 2 diabetes mellitus initiated on canagliflozin: a retrospective cohort study. J Med Econ. 2017;20(3):303–313. doi:10.1080/13696998.2016.1259167
15. Wang W, Xie P, Zhang J, et al. A risk prediction model of urinary tract infections for patients with neurogenic bladder. Int J Neurosci. 2021;131(1):31–39. doi:10.1080/00207454.2020.1732973
16. Zhang L, Zhang F, Xu F, et al. Construction and evaluation of a sepsis risk prediction model for urinary tract infection. Front Med. 2021;8:671184. doi:10.3389/fmed.2021.671184
17. Dubbs SB, Sommerkamp SK. Evaluation and management of urinary tract infection in the emergency department. Emerg Med Clin North Am. 2019;37(4):707–723. doi:10.1016/j.emc.2019.07.007
18. Álvarez-artero E, Campo-Nuñez A, García-García I, et al. Urinary tract infection caused by Enterococcus spp.: risk factors and mortality. An observational study. Rev Clin Esp. 2021;221(7):375–383. doi:10.1016/j.rce.2020.09.005
19. Limin L, Wu J, Ju Y, Li J. Guidelines for urinary system management and clinical rehabilitation in patients with spinal cord injury. Chin J Rehabilit Theor Pract. 2013;19(4):301–307. Chinese.
20. Watts GF, O’Brien SF, Shaw KM. Urinary infection and albumin excretion in insulin-dependent diabetes mellitus: implications for the measurement of microalbuminuria. Diabet Med. 1996;13(6):520–524. doi:10.1002/(SICI)1096-9136(199606)13:6<520::AID-DIA128>3.0.CO;2-D
21. Fünfstück R, Nicolle LE, Hanefeld M, et al. Urinary tract infection in patients with diabetes mellitus. Clin Nephrol. 2012;77(1):40–48. doi:10.5414/CN107216
22. Dicu-Andreescu I, Penescu MN, Căpușă C, et al. Chronic kidney disease, urinary tract infections and antibiotic nephrotoxicity: are there any relationships? Medicina. 2022;59(1):49. doi:10.3390/medicina59010049
23. Shankar M, Narasimhappa S, Urinary Tract SMN. Infection in chronic kidney disease population: a clinical observational study. Cureus. 2021;13(1):e12486. doi:10.7759/cureus.12486
24. Mody L, Juthani-Mehta M. Urinary tract infections in older women: a clinical review. JAMA. 2014;311(8):844–854. doi:10.1001/jama.2014.303
25. Gadalla AAH, Friberg IM, Kift-Morgan A, et al. Identification of clinical and urine biomarkers for uncomplicated urinary tract infection using machine learning algorithms. Sci Rep. 2019;9(1):19694. doi:10.1038/s41598-019-55523-x
26. Karslioglu french E, Donihi AC, Korytkowski MT. Diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome: review of acute decompensated diabetes in adult patients. BMJ. 2019;365:l1114. doi:10.1136/bmj.l1114
27. Dray E, Cameron AP, Clemens JQ, et al. Does post-void residual volume predict worsening urological symptoms in patients with multiple sclerosis? J Urol. 2018;200(4):868–874. doi:10.1016/j.juro.2018.04.068
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

