Back to Journals » Therapeutics and Clinical Risk Management » Volume 17
Pretreatment Systemic Immune-Inflammation Index Predict Needs for Teeth Extractions for Locally Advanced Head and Neck Cancer Patients Undergoing Concurrent Chemoradiotherapy
Authors Yilmaz B , Somay E , Selek U , Topkan E
Received 16 August 2021
Accepted for publication 10 October 2021
Published 18 October 2021 Volume 2021:17 Pages 1113—1121
DOI https://doi.org/10.2147/TCRM.S334556
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Busra Yilmaz,1 Efsun Somay,2 Ugur Selek,3,4 Erkan Topkan5
1Department of Dentomaxillofacial Radiology, Faculty of Dentistry, Baskent University, Ankara, Turkey; 2Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Baskent University, Ankara, Turkey; 3Department of Radiation Oncology, School of Medicine, Koc University, Istanbul, Turkey; 4Department of Radiation Oncology, MD Anderson Cancer Center, The University of Texas, Houston, TX, USA; 5Department of Radiation Oncology, Faculty of Medicine, Baskent University, Adana, Turkey
Correspondence: Erkan Topkan
Department of Radiation Oncology, Baskent University Medical Faculty, Adana, 01120, Turkey
Tel +90-533-7381069
Fax +90-322-3444452
Email [email protected]
Background: To evaluate the utility of pretreatment systemic immune-inflammation index (SII) in predicting the teeth caries and need for tooth extraction after concurrent chemoradiotherapy (C-CRT) for locally advanced squamous-cell head and neck cancer (LA-SCHNC) patients.
Methods: The records of LA-SCHNC patients who underwent formal dental evaluations at pre- and post-C-CRT periods were retrospectively analyzed. The pretreatment SII values were calculated using the platelet, neutrophil, and lymphocyte measures acquired on the first day of C-CRT: SII=Platelets×neutrophils/lymphocytes. Receiver operating characteristic (ROC) curve analysis was employed to identify the ideal pre-C-CRT SII cutoff that may predict the teeth caries and the need for tooth extraction after the C-CRT. The primary endpoint was the link between the pre-C-CRT SII and the need for tooth extraction during the follow-up period.
Results: A sum of 126 patients were included. Median follow-up was 4.9 years (range: 2.7– 7.8). Nasopharyngeal and laryngeal cancers comprised the majority (75.4%) study cohort. Post-treatment teeth extractions were reported in 62.7% patients. The optimal cutoff was 558 [Area under the curve (AUC): %76.8 sensitivity: 72.3%; and specificity: 70.9%] that grouped the patients into two subgroups with significantly different post-C-CRT tooth extraction rates: Group 1: SII≤ 558 (n = 70) and SII> 558 (n = 56), respectively. Correlation analysis revealed a significant relationship between the pretreatment SII and the tooth extraction rates after the C-CRT (rs:0.89: P = 0.001). The comparative analysis displayed that the teeth extractions rates were significantly higher in the SII> 558 group (77.1% versus 51.4% for SII≤ 558; Hazard ratio: 1.68; P = 0.001). Further analyses showed that the pre-C-CRT SII> 558 was the unique factor associated with meaningfully higher necessities for post-C-CRT teeth extractions.
Conclusion: The present outcomes intimated that high pretreatment SII levels were linked to significantly increased post-treatment teeth extractions in LA-SCHNC patients undergoing definitive C-CRT.
Keywords: concurrent chemoradiotherapy, head and neck cancers, systemic immune-inflammation index, tooth extraction
Introduction
In comparison to radiation (RT) alone or sequential chemo-RT (CRT) regimens, concurrent CRT (C-CRT) significantly improved locoregional disease control and survival outcomes in patients with locally advanced squamous cell head and neck cancers (LA-SCHNC).1,2 Unfortunately, these benefits came at the price of increased acute complications such as mucositis and taste abnormalities, as well as late complications like trismus and radiation-associated dysphagia, and the resulting decreases in personal quality of life (QOL) measures.3,4 Teeth losses during and after C-CRT is one such grave complication, which is critical given the teeth’s requisite roles in swallowing and chewing functions.5,6 Moreover, tooth loss can result in life-threatening osteoradionecrosis of the bruised jaws.1 Hence, to avoid such complications, it is recommended that all LA-SCHNC patients have complete oral examinations before and after the C-CRT for the timely management of a particular patient’s overall oral and dental hygiene, if needed.5,7
Despite the efficient provision of dental care before RT or C-CRT, tooth loss is nevertheless reported to be a common health concern in LA-SCHNC patients treated with RT or C-CRT.8 Our knowledge of the actual prevalence of tooth loss following definitive C-CRT is surprisingly limited, although available evidence shows that dental caries may occur in up to 11–80% of patients in post-RT 6-months to 9-years of follow-up.9–18 Post-RT dental caries and subsequent teeth losses are perpetually linked to the attending local and systemic inflammatory processes like diminished saliva secretion and buffering functions, development of acid-producing cariogenic infections caused by the streptococcus and lactobacillus species, apical periodontitis, and alterations in the chemical and micro-morphological composition of the enamel and dentin.5,6 However, to the best of our knowledge, only a few studies have questioned the value of the immune and/or inflammation biomarkers in predicting teeth losses following C-CRT. Recently, Sirin et al revealed that the patients with higher-grade apical periodontitis per Periapical Index (PAI) scoring system had higher high-sensitivity C-reactive protein (hsCRP) and neutrophil-to-lymphocyte ratio (NLR) than either the control or lower PAI score groups.19
Systemic immune-inflammation index (SII), a blend of the platelet (PLT), neutrophil, and lymphocyte counts, is another biomarker that reflects the harmony between the patient’s inflammatory and immune status irrespective of the underlying cause.20 In past investigations, a significant relationship between the SII and prognosis of many clinical situations, including the malignant tumors, coronary artery diseases, ulcerative diseases, and some chronic diseases like psoriasis.20–24 Nevertheless, despite the availability of strong basic proofs, SII has never been researched for its value in predicting the teeth losses of LA-SCHNC patients after the C-CRT. Consequently, we intended to retrospectively evaluate the significance of pretreatment SII measures for predicting the post-C-CRT teeth losses in LA-SCHNC patients who received pretreatment oral care at our institution.
Patients and Methods
Study Population
We reviewed the institutional data charts of LA-SCHNC patients who had oral and dental evaluations before C-CRT at Baskent University Adana Research and Treatment Center Dentistry Clinics between January 2010 and December 2018. To be qualified, the following criteria were required to be fulfilled: Aged >18, treated for head and neck tumor primaries [nasopharynx, larynx, parotid gland, maxillary sinus, hypopharynx, tonsil, lip, oropharynx, and oral cavity tumors] histopathologic proof of SCC histology, proven locally advanced disease per American Joint Cancer Committee (AJCC) 8th ed, no history of other cancers or systemic chemotherapy or RT to the head and neck region before the dental evaluations, planned to undergo definitive C-CRT, and available pre-C-CRT and follow-up records of panoramic radiography, periapical digital radiography, and pre-C-CRT complete blood count tests. History of steroid usage at the past 30 days before the C-CRT onset, complete edentulism, and traumatic or other unnatural teeth losses during the follow-up period were determined as the exclusion criteria for this present analysis (Figure 1).
 |
Figure 1 A flowchart summarizing the patient selection, evaluation, treatment, and follow up details. |
Ethics, Consent and Permission
The present retrospective study design was approved by the institutional review board of Baskent University Medical Faculty before the compilation of any data. Eligible patients provided signed informed consent before the initiation of oral and dental assessments and C-CRT either themselves or legitimately charged caregivers for the acquisition and interpretation of blood samples, sociodemographic and medical records, dental X-rays, and academic presentations.
Baseline Clinical Oral Examination
All pre-C-CRT oral and dental health-related conditions, including facial swelling, a toothache, gingival edema, abscesses, dental caries, and pain on dental percussion were acquired from the medical records. An experienced oral and maxillofacial surgeon and a dentomaxillofacial radiologist handled all of the evaluations. All patients had standard oral and dental exams before therapy, even if they were asymptomatic, as recommended by the American Dental Association (ADA) and the US Food and Drug Administration (FDA).25 The total number of teeth, presence, the number of decayed teeth (crown cavities and/or root cavities), residual roots, periodontal treatments, canal treatments, number of filled and/or extracted teeth were identified from the pre-C-CRT dentistry records.19,25 Radiographic dental examinations were performed using panoramic and periapical radiographs for each patient according to our institutional standards for such patients. All digital panoramic radiographs were acquired with the same X-ray device (J Morita, Veraviewepocs 2D, Kyoto, Japan) utilizing the following parameters: 70 kVp, 10 mA, and 9 s. Periapical radiographs were acquired using the parallelism technique using an intraoral X-ray unit (New Life Radiology, BEST-X-AC, Torino, Italy) set at 70 kVp, 8 mA, 0.4–0.8 s and a digital sensor with its accompanying software (Handy Medical Equipment Co, Ltd, Shanghai, China). Patients were positioned for the imaging procedures as recommended by the manufacturer.
Systemic Immune-Inflammation Index (SII) Assessment
We used Hu’s original equation to calculate pretreatment SII values: SII = [(Platelets) × (neutrophils/lymphocytes)], by utilizing the platelet, neutrophil, and lymphocyte measures procured from the standard complete blood count analysis performed on the first day of the C-CRT.26
Chemoradiotherapy Protocol
The RT technique was 3D-conformal RT between January 2007 and June 2011 and simultaneous integrated boost intensity-modulated RT (SIB-IMRT) thereafter. All target volumes were defined by using the pretreatment co-registered computed tomography (CT), 18-FDG-PET–CT, and/or magnetic resonance imaging scans of the involved primary site and the whole neck. Although there were minor variations in target volume definitions depending on the index tumor primary, the target volumes and related treatment doses were generally defined as depicted in Table 1. The prescribed doses for the high-, intermediate-, and low-risk planned target volumes (PTVs) were 66–70 Gy, 59.4 Gy, and 45–54 Gy, respectively, according to institutional practice. RT was delivered on a daily fractionation basis regardless of the technique used: 5 days per week for 33 days. Chemotherapy consisted of seven weekly doses of cisplatin (40 mg/m2) administered concurrently with RT.
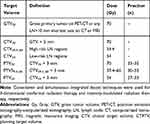 |
Table 1 Details of Radiotherapy Protocol |
Follow-Up Oral and Dental Examination
All follow-up oral and dental examinations were accomplished using the same methodology described in the “Baseline clinical oral examination” section. The treatment commitments for each patient were determined and reported using the principles prespecified before.
Statistical Analysis
The primary endpoint was the relationship between pretreatment SII values and the need for tooth extractions during the post-C-CRT follow-up. Medians and ranges were calculated to describe continuous variables, while categorical variables were expressed with percentage frequency distributions. To compare the patient groups, the Chi-square test, Student’s t-test, or Spearman correlation analyses were used, as indicated. Receiver operating characteristic (ROC) curve analyses were utilized to estimate the pre-C-CRT cutoff(s) that, if present, might divide the entire research cohort into two SII groups with differing results. A two-sided P-value <0.05 was considered significant. Bonferroni correction and related P-values were utilized for comparing three or more groups to depreciate the chance factors.
Results
The current retrospective database search identified a total of 246 LA-SCHNC patients who were referred to a dental clinic for oral and dental evaluations before starting the recommended C-CRT. However, as shown in Figure 1, 126 patients were eligible for the current study because the remaining 120 cases were excluded from the analysis due to proven active infection (n = 58), history of steroid or immune suppressant use at past 30 days before the C-CRT commencement (n = 34), complete edentulism (n = 19), and traumatic or other unnatural tooth loss during the follow-up period (n = 9). The median age was 56 years (range: 18–76) with a male gender predominance (72.2%), as shown in Table 2. Nasopharyngeal (42.1%) and laryngeal (33.3%) cancers accounted for the majority (75.4%) of the study cohort. Of the 126 eligible patients, mirroring the poor oral and dental care, teeth losses were inscribed in 79.4% of cases, and 54.8% of patients had >4 teeth losses. Tooth caries requiring interventions were diagnosed in 72.8% cases, with 31.7% cases presenting with >4 decayed teeth (Table 2). As depicted in Table 3, 120 patients (95.2%) experienced tooth/teeth decay, with 42 (33.3%) having >4 decayed teeth. In addition, 79 (62.7%) patients underwent teeth extractions after the C-CRT at a follow-up period of 4.9 years (range: 2.7–7.8).
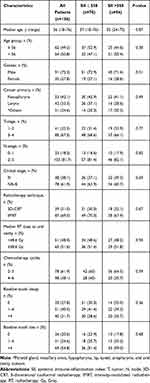 |
Table 2 Baseline and Treatment Characteristics for the Entire Study Cohort and per SII Status |
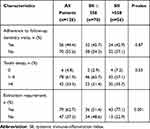 |
Table 3 Outcomes After the Concurrent Chemoradiotherapy |
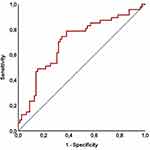
The ROC curve analyses were used to uncover the possible connections between pretreatment SII levels and post-treatment tooth decay and extraction rates. We were unable to find a cutoff that demonstrated a significant relationship between pretreatment SII values and post-treatment tooth decay prevalence [Area under the curve (AUC): 51.2%; sensitivity: 50.4%; and specificity: 48.1%], which could be related to the high (95.2%) tooth decay prevalence in the entire study cohort. The ROC curve analysis results, on the other hand, revealed the optimal cutoff value at 558 (AUC: %76.8 sensitivity: 72.3%; and specificity: 70.9%) (Figure 2), which divided the entire patient population into two subgroups with significantly different post-C-CRT tooth extraction rates: Group 1: SII ≤ 558 (n = 70) and SII > 558 (N = 56), respectively. Although pretreatment features were evenly distributed between the two cohorts, Spearman correlation analysis indicated a robust and significant connection between pretreatment SII values and tooth extraction rates following C-CRT (rs: 0.89: P = 0.001) (Table 3). As demonstrated in Table 3, our comparative analyses displayed that the rates of tooth extractions were significantly higher in the SII > 558 group than its SII ≤ 558 counterpart (77.1% versus 51.4%; P = 0.001). Further analysis showed that the pre-C-CRT SII > 558 was the only predictor associated with significantly increased post-C-CRT tooth extraction requirements (P = 0.001): age group (P = 0.85), gender (P = 0.53), cancer type (P = 0.49), T-stage (P = 0.39), N-stage (P = 0.32), clinical stage (P = 0.19), radiotherapy technique (P = 0.28), median radiotherapy dose to oral cavity (P = 0.14), cycles of chemotherapy (P = 0.35), presence of pretreatment tooth decay (P = 0.46), and presence of pretreatment tooth loss (P = 0.29).
Discussion
The present study investigated the value of pretreatment SII values in predicting severe teeth problems after C-CRT in radically treated LA-SCHNC patients. High pre-C-CRT SII levels were associated with significantly increased tooth loss due to a prevalence of dental caries and a greater need for extractions, according to our findings. Hence, our results suggested that the baseline high SII levels may serve useful in identifying the LA-SCHNC patients’ group with considerably elevated risks for post-C-CRT tooth loss.
The quantity of blood-borne inflammatory cells in the systemic circulation and the microenvironment of various tissue pathologies, including the oral and dental tissues, is significantly raised or lowered depending on the concerned cell type.19,27 The significant link between systemic inflammation and malignant-, metabolic-, cardiovascular-, and dental disorders has prompted the use of individual inflammatory cells or their unique combinations in the prognostication of these disease states.19,28–33 Leukocytes, monocytes, specialized macrophages, neutrophils, lymphocytes, and PLTs account for the vast majority of the inflammatory cell aggregation in diseased oral and dental tissues, much as they do in other injured tissues.34–37 PLT aggregation, vascular occlusion, tissue ischemia, lymphocyte-mediated immunological response, generation of abundant proinflammatory cytokines/chemokines, and synchronization of the innate and adaptive immunities are all tasks these cells perform during inflammatory and immune responses.38 As a result, various authors have investigated the cellular markers of systemic inflammatory response in patients with dental problems, either as single-cell measures or in their two-cell combinations: particularly the NLR and platelet-to-lymphocyte ratio (PLR), with most studies reporting provocative positive results.33,38–40 However, to the best of our knowledge, the novel SII, which is calculated by simultaneously using the components of the NLR and PLR, has never been examined in LA-SCHNC patients for its practical value for accurate prognostication of dental problems after definitive C-CRT.
The novel finding of the present study was the show of an independent and robust relationship between the high pretreatment SII values and increased risks of post-C-CRT tooth loss in LA-SCHNC patients (77.1% versus 51.4% for SII ≤ 558 group; P = 0.001). Teeth extraction rates observed in both SII groups (77.1% and 51.4% in high- and low-SII groups, respectively) appear to be higher than the 19.8% reported by Frydrych et al in LA-SCHNCs treated with RT resin.12 Although the exact causes of this disparity are strenuous to explain, our findings may have mirrored real-world experiences in countries where general oral and dental care is poorer than those found in selected study populations. In the absence of comparable SII studies in LA-SCHNC patients, it is difficult to draw solid conclusions on the apparent link between pretreatment SII levels and the need for tooth extractions following the C-CRT described here. Nonetheless, our findings appear to be consistent with the results of prior research examining the potential connection between systemic inflammation biomarkers and dental outcomes in such individuals.19,33,36,41–43 Of note, previously, Sirin et al previously demonstrated that the NLR levels of patients with grade 3 apical periodontitis (≥1 tooth with a PAI score of 5) were significantly higher than those of patients with grade 1 (1 tooth with a PAI score of 3 or 4; P < 0.05) and grade 2 (≥1 tooth with PAI scores 3 or 4; P < 0.05) apical periodontitis.19 Likewise, Dogan et al reported that the NLR was higher in the hyperlipidemic patients with periodontitis than in the group without periodontitis (P < 0.05).44 In a more recent Chinese study, Lu et al reported that the NLR values, but not PLR, were positively correlated with the risk and clinical parameters of aggressive periodontitis, with the risk of aggressive periodontitis increasing by 20.6% for each 0.1 increase in NLR below NLR<3 values in their healthy control group, reaching saturation when NLR > 3.45 Torrungruang et al46 and Acharya et al,33 on the other hand, reported that both high NLR and PLR indices were robustly linked to severity of chronic periodontitis. Although these studies are fundamentally different from the one presented here, they all indicate a strong link between severe tooth problems and the systemic immune/inflammation response, regardless of the disease type and therapy used.
Although radiation-induced salivary gland dysfunction, root surface and periodontal injury, and systemic chemotherapy effects seem to be the well-recognized driving causes for teeth caries and losses after RT and/or C-CRT, the explicit mechanisms underlying the causal relationship between high pre-C-CRT SII values and increased teeth losses remain unsolved. Nevertheless, we may deduce some discerning hypotheses by comprehending the pivotal roles of local and systemic inflammation on dental caries and the ensuant teeth losses along with the immune and inflammatory cell constituents of the unique SII formula, namely the PLTs, neutrophils, and lymphocytes. Increased counts of peripheric blood PLTs are recognized as a strong indicator of a continuing exacerbated systemic inflammatory response condition that may prompt occlusion of small blood vessels with sequent bony ischemia in the jawbones, and thus, the teeth roots and other supportive tissues: well-proven causes of teeth losses.41,42,47,48 Neutrophils are among the most abundant immune cells of the oral cavity and periodontal tissues, assuming significant roles in local/systemic immunity and inflammation with their phagocytotic and reactive oxygen species plus cytokine/chemokine manufacturing and secreting functions.19,43,49,50 In contrast to inflammatory neutrophils, lymphocytes are immune cells that migrate to the wounded tooth pulp to attack inflammation-producing sources.51,52 Accordingly, increased lymphocyte counts may indicate an active immune response against dental inflammation. The precise mechanisms might probably be more complicated. However, enlightened with such fundamental proof, we can still rationally propose that the high levels of SII may mirror an exacerbated inflammatory and ischemic condition with an accompanying diminished immunity in LA-SCHNCs undergoing conclusive C-CRT, which may result in increased risk for posttreatment teeth caries and subsequent teeth losses. As previously stated, the current high rates of tooth decay may be linked to our country’s socio-economic situation and societal attitudes about self-care. Another reasonable explanation might be that majority of our treated tumors were locally advanced and located in the nasopharynx, larynx, parotid glands, and maxillary sinuses, all of which are sites where high-dose RT regions invariably involve varying quantities of teeth, their roots, and supporting tissues. Bolstering this remark, previously Sun et al reported an overall teeth decay rate of 80% for nasopharyngeal cancer patients.18
The present research is handicapped with several facts. First, the present results apply to a single institutional retrospective study with limited cohort size. Second, albeit the SII was a dynamic biomarker with striking time-dependent fluctuations, our SII measures addressed a single time-point estimation acquired just before the C-CRT commencement. Therefore, future large-scale studies investigating the relationship between SII values acquired during or after C-CRT spans and clinical endpoints may be beneficial in identifying a better SII cutoff that has a more apparent connection with outcomes, such as the tooth losses examined here. And third, concluding on a cause-and-effect premise is problematic due to the lack of immunological and inflammatory chemokine/cytokine measures, as well as tests for cariogenic bacteria before, during, and after the C-CRT durations. As a result, the current findings should be treated with care and regarded as hypothesis-generating rather than authoritative until the results of large-scale prospective investigations confirm them.
Conclusion
High pre-C-CRT SII levels were associated with considerably higher post-treatment tooth extractions, according to the findings of our present retrospective cohort analysis of 126 LA-SCHNC patients. As a result, if further research confirms these findings, the novel immune, and inflammation marker SII, which is inexpensive, easy to obtain, and calculate, could be used to supervise the formal oral and dental evaluations as well as the required treatments for LA-SCHNC patients treated with conclusive C-CRT.
Abbreviations
SII, systemic immune-inflammation index; C-CRT, concurrent chemoradiotherapy; LA-SCHNC, locally advanced squamous-cell head and neck cancer; ROC, receiver operating characteristic; AUC, area under the curve; RT, radiotherapy; CRT, chemo-RT; QOL, quality of life; PAI, periapical index; hsCRP, high-sensitivity C-reactive protein; NLR, neutrophil-to-lymphocyte ratio; PLT, platelet; PLR, platelet-to-lymphocyte ratio; AJCC, American joint cancer committee; ADA, American dental association; FDA, US food and drug administration; SIB-IMRT, simultaneous integrated boost intensity-modulated RT; PTVs, planning target volumes.
Data Sharing Statement
Data cannot be shared publicly because the data is owned and saved by Baskent University Medical Faculty. Data are available from the Baskent University Radiation Oncology Institutional Data Access/Ethics Committee (contact via Baskent University Ethics Committee) for researchers who meet the criteria for access to confidential data: contact address, [email protected].
Ethics Committee Approval and Patient Consent
This study protocol was approved by the institutional review board of Baskent University Medical Faculty and was in compliance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Pignon JP, le Maître A, Maillard E, et al.; MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi:10.1016/j.radonc.2009.04.014
2. Blanchard P, Baujat B, Holostenco V, et al.; MACH-CH Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): a comprehensive analysis by tumour site. Radiother Oncol. 2011;100:33–40. doi:10.1016/j.radonc.2011.05.036
3. De Felice F, Musio D, Terenzi V, et al. Treatment improvement and better patient care: which is the most important one in oral cavity cancer? Radiat Oncol. 2014;9:263. doi:10.1186/s13014-014-0263-x
4. De Felice F, de Vincentiis M, Luzzi V, et al. Late radiation-associated dysphagia in head and neck cancer patients: evidence, research and management. Oral Oncol. 2018;77:125–130. doi:10.1016/j.oraloncology.2017.12.021
5. Deng J, Jackson L, Epstein JB, et al. Dental demineralization and caries in patients with head and neck cancer. Oral Oncol. 2015;51:824–831. doi:10.1016/j.oraloncology.2015.06.009
6. Jawad H, Hodson NA, Nixon PJ. A review of dental treatment of head and neck cancer patients, before, during and after radiotherapy: part 1. Br Dent J. 2015;218:65–68. doi:10.1038/sj.bdj.2015.28
7. Sohn HO, Park EY, Jung YS, et al. Effects of professional oral hygiene care in patients with head-and-neck cancer during radiotherapy: a randomized clinical trial. Indian J Dent Res. 2018;29:700–704. doi:10.4103/ijdr.IJDR_226_17
8. Güll FD, Deppe H, Kesting M, et al. Periodontal disease-like bone loss after adjuvant radiotheraphy in the head and neck region: a case report and review of the literature. Quintessence Int. 2017;48:451–457.
9. Siala W, Mnejja W, Elloumi F, et al. Late toxicities after conventional radiotherapy for nasopharyngeal carcinoma: incidence and risk factors. J Radiother. 2014;2014:268340.
10. Bonan PR, Lopes MA, Pires FR, et al. Dental management of low socioeconomic level patients before radiotherapy of the head and neck with special emphasis on the prevention of osteoradionecrosis. Braz Dent J. 2006;17:336–342. doi:10.1590/S0103-64402006000400013
11. Duarte VM, Liu YF, Rafizadeh S, et al. Comparison of dental health of patients with head and neck cancer receiving IMRT vs conventional radiation. Otolaryngol Head Neck Surg. 2014;150:81–86. doi:10.1177/0194599813509586
12. Frydrych AM, Slack-Smith LM, Parsons R. Compliance of post-radiation therapy head and neck cancer patients with caries preventive protocols. Aust Dent J. 2017;62:192–199. doi:10.1111/adj.12491
13. Hey J, Seidel J, Schweyen R, et al. The influence of parotid gland sparing on radiation damages of dental hard tissues. Clin Oral Investig. 2013;17:1619–1625. doi:10.1007/s00784-012-0854-6
14. Meng L, Liu J, Peng B, et al. The persistence of Streptococcus mutans in nasopharyngeal carcinoma patients after radiotherapy. Caries Res. 2005;39:484–489. doi:10.1159/000088184
15. Mougeot JC, Stevens CB, Almon KG, et al. Caries-associated oral microbiome in head and neck cancer radiation patients: a longitudinal study. J Oral Microbiol. 2019;11:1586421. doi:10.1080/20002297.2019.1586421
16. Rudat V, Meyer J, Momm F, et al. Protective effect of amifostine on dental health after radiotherapy of the head and neck. Int J Radiat Oncol Biol Phys. 2000;48:1339–1343. doi:10.1016/S0360-3016(00)00768-9
17. Schuurhuis JM, Stokman MA, Witjes MJH, et al. Patients with advanced periodontal disease before intensity-modulated radiation therapy are prone to develop bone healing problems: a 2-year prospective follow-up study. Support Care Cancer. 2018;26:1133–1142. doi:10.1007/s00520-017-3934-y
18. Sun HB, Gao XJ, Deng J, et al. Progress of oral sequelae during head-neck radiotherapy. Chin J Dent Res. 2010;13:51–55.
19. Sirin DA, Ozcelik F, Uzun C, et al. Association between C-reactive protein, neutrophil to lymphocyte ratio and the burden of apical periodontitis: a case-control study. Acta Odontol Scand. 2019;77:142–149. doi:10.1080/00016357.2018.1522447
20. Yilmaz A, Mirili C, Bilici M, et al. A novel predictor in patients with gastrointestinal stromal tumors: systemic immune-inflammation index (SII). J BUON. 2019;24:2127–2135.
21. Geng Y, Shao Y, Zhu D, et al. Systemic immune-inflammation index predicts prognosis of patients with esophageal squamous cell carcinoma: a propensity score-matched analysis. Sci Rep. 2016;6:39482. doi:10.1038/srep39482
22. Yang YL, Wu CH, Hsu PF, et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Invest. 2020;50:e13230. doi:10.1111/eci.13230
23. Xie Y, Zhuang T, Ping Y, et al. Elevated systemic immune inflammation index level is associated with disease activity in ulcerative colitis patients. Clin Chim Acta. 2021;517:122–126. doi:10.1016/j.cca.2021.02.016
24. Dincer Rota D, Tanacan E. The utility of systemic-immune inflammation index for predicting the disease activation in patients with psoriasis. Int J Clin Pract. 2021;75:e14101.
25. White SC, Pharoah MJ. Oral Radiology-E-Book: Principles and Interpretation.
26. Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212–6222. doi:10.1158/1078-0432.CCR-14-0442
27. Colotta F, Allavena P, Sica A, et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi:10.1093/carcin/bgp127
28. Murata M. Inflammation and cancer. Environ Health Prev Med. 2018;23:50. doi:10.1186/s12199-018-0740-1
29. Singh N, Baby D, Rajguru JP, et al. Inflammation and cancer. Ann Afr Med. 2019;18:121–126. doi:10.4103/aam.aam_56_18
30. Esser N, Legrand-Poels S, Piette J, et al. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141–150. doi:10.1016/j.diabres.2014.04.006
31. Carrizales-Sepúlveda EF, Ordaz-Farías A, Vera-Pineda R, et al. Periodontal disease, systemic inflammation and the risk of cardiovascular disease. Heart Lung Circ. 2018;27:1327–1334. doi:10.1016/j.hlc.2018.05.102
32. Afari ME, Bhat T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: an update. Expert Rev Cardiovasc Ther. 2016;14:573–577. doi:10.1586/14779072.2016.1154788
33. Acharya AB, Shetty IP, Jain S, et al. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in chronic periodontitis before and after nonsurgical therapy. J Indian Soc Periodontol. 2019;23:419–423.
34. Van Dyke TE. Role of the neutrophil in oral disease: receptor deficiency in leukocytes from patients with juvenile periodontitis. Rev Infect Dis. 1985;7:419–425. doi:10.1093/clinids/7.3.419
35. Seymour GJ, Powell RN, Davies WI. The immunopathogenesis of progressive chronic inflammatory periodontal disease. J Oral Pathol. 1979;8:249–265. doi:10.1111/j.1600-0714.1979.tb01826.x
36. Houri-Haddad Y, Wilensky A, Shapira L. T-cell phenotype as a risk factor for periodontal disease. Periodontol 2000. 2007;45:67–75. doi:10.1111/j.1600-0757.2007.00227.x
37. Croce K, Libby P. Intertwining of thrombosis and inflammation in atherosclerosis. CurrOpinHematol. 2007;14:55–61.
38. Thomas MR, Storey RF. The role of platelets in inflammation. ThrombHaemost. 2015;114:449–458.
39. Pejcić A, Kesić L, Pesić Z, et al. White blood cell count in different stages of chronic periodontitis. Acta Clin Croat. 2011;50:159–167.
40. López R, Loos BG, Baelum V. Hematological features in adolescents with periodontitis. Clin Oral Investig. 2012;16:1209–1216. doi:10.1007/s00784-011-0628-6
41. Romandini M, Laforí A, Romandini P, et al. Periodontitis and platelet count: a new potential link with cardiovascular and other systemic inflammatory diseases. J Clin Periodontol. 2018;45:1299–1310. doi:10.1111/jcpe.13004
42. Al-Rasheed A. Elevation of white blood cells and platelet counts in patients having chronic periodontitis. Saudi Dent J. 2012;24:17–21. doi:10.1016/j.sdentj.2011.10.006
43. Medara N, Lenzo JC, Walsh KA, et al. Peripheral neutrophil phenotypes during management of periodontitis. J Periodontal Res. 2021;56:58–68. doi:10.1111/jre.12793
44. Doğan B, Fentoğlu Ö, Kırzıoğlu FY, et al. Lipoxin A4 and neutrophil/lymphocyte ratio: a possible indicator in achieved systemic risk factors for periodontitis. Med Sci Monit. 2015;21:2485–2493. doi:10.12659/MSM.895115
45. Lu R, Li W, Wang X, et al. Elevated neutrophil-to-lymphocyte ratio but not platelet-to-lymphocyte ratio is associated with generalized aggressive periodontitis in a Chinese population. J Periodontol. 2021;92:507–513. doi:10.1002/JPER.20-0282
46. Torrungruang K, Ongphiphadhanakul B, Jitpakdeebordin S, et al. Mediation analysis of systemic inflammation on the association between periodontitis and glycaemic status. J Clin Periodontol. 2018;45:548–556. doi:10.1111/jcpe.12884
47. Szabó A, Janovszky Á, Pócs L, et al. The periosteal microcirculation in health and disease: an update on clinical significance. Microvasc Res. 2017;110:5–13. doi:10.1016/j.mvr.2016.11.005
48. Michaud DS, Fu Z, Shi J, et al. Periodontal disease, tooth loss, and cancer risk. Epidemiol Rev. 2017;39:49–58. doi:10.1093/epirev/mxx006
49. Silvestre-Roig C, Fridlender ZG, Glogauer M, et al. Neutrophil diversity in health and disease. Trends Immunol. 2019;40:565–583. doi:10.1016/j.it.2019.04.012
50. Hirschfeld J. Neutrophil subsets in periodontal health and disease: a mini review. Front Immunol. 2020;10:3001. doi:10.3389/fimmu.2019.03001
51. Jontell M, Okiji T, Dahlgren U, et al. Immune defense mechanisms of the dental pulp. Crit Rev Oral Biol Med. 1998;9:179–200. doi:10.1177/10454411980090020301
52. Nakanishi T, Takahashi K, Hosokawa Y, et al. Expression of macrophage inflammatory protein 3alpha in human inflamed dental pulp tissue. J Endod. 2005;31:84–87. doi:10.1097/01.don.0000143414.22112.57
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.