Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Pretreatment Neutrophil-to-Lymphocyte Ratio as Prognostic Biomarkers in Patients with Unresectable Hepatocellular Carcinoma Treated with Hepatic Arterial Infusion Chemotherapy Combined with Lenvatinib and Camrelizumab
Authors Xiao Y , Zhu G, Xie J, Luo L , Deng W , Lin L, Tao J, Hu Z, Shan R
Received 24 July 2023
Accepted for publication 2 November 2023
Published 9 November 2023 Volume 2023:10 Pages 2049—2058
DOI https://doi.org/10.2147/JHC.S432134
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr David Gerber
Yongqiang Xiao,* Guoqing Zhu,* Jin Xie,* Laihui Luo, Wei Deng, Liucong Lin, Jiahao Tao, Zhigao Hu, Renfeng Shan
Department of General Surgery, the First Affiliated Hospital of Nanchang University, Nanchang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhigao Hu; Renfeng Shan, Department of General Surgery, the First Affiliated Hospital of Nanchang University, No. 17, Yongwaizheng Street, Nanchang, Jiangxi, 330006, People’s Republic of China, Tel/Fax +86-791-88692522, Email [email protected]; [email protected]
Purpose: This study aimed to assess the prognostic significance of the neutrophil-lymphocyte ratio (NLR) in patients with unresectable hepatocellular carcinoma (u-HCC) treated with hepatic artery infusion chemotherapy (HAIC) combined with lenvatinib and camrelizumab.
Patients and Methods: We conducted a retrospective cohort study involving patients diagnosed with u-HCC who underwent HAIC combined with lenvatinib and camrelizumab. Patients were stratified into two cohorts using the median NLR as the cutoff point. We then assessed treatment response, overall survival (OS), progression-free survival (PFS), and adverse events in these patient groups.
Results: Between October 2020 and April 2022, a total of 88 patients were enrolled in the study. The overall cohort exhibited a median PFS of 7.9 months, while the median OS was not reached, and a median NLR of 3.46. Notably, the group with NLR< 3.46 demonstrated significantly superior OS (not reached vs 9.6 months, p = 0.017) and PFS (18.3 vs 5.3 months, p = 0.0015) compared to the NLR≥ 3.46 group. Furthermore, multivariate analysis revealed that an alpha-fetoprotein (AFP) ≥ 400 ng/mL [hazard ratio (HR), 2.133; 95% confidence interval (CI), 1.102– 4.126; p = 0.024], Barcelona Clinical Hepatocellular Carcinoma (BCLC) stage C (HR, 2.319; 95% CI, 1.128– 4.764; p = 0.022), and NLR ≥ 3.46 (HR, 2.35; 95% CI, 1.239– 4.494; p = 0.009) were identified as independent risk factors for OS. Additionally, multivariate analysis demonstrated that AFP ≥ 400 ng/mL, BCLC stage C, and NLR ≥ 3.46 were independent negative factors of PFS.
Conclusion: NLR can be associated with outcomes in patients with u-HCC treated with HAIC combined with lenvatinib and camrelizumab.
Keywords: unresectable hepatocellular carcinoma, neutrophil-to-lymphocyte ratio, hepatic arterial infusion chemotherapy, lenvatinib, camrelizumab
Introduction
Hepatocellular carcinoma (HCC) is the most prevalent primary liver cancer and the third major cause of cancer-related deaths worldwide; the World Health Organization estimates that the number of deaths caused by liver cancer will reach 1.3 million by 2040.1,2 While surgical resection remains an effective treatment for HCC, approximately 50% of patients with HCC are ineligible for radical treatment options, including surgical resection, once diagnosed due to late onset of symptoms, which is referred to as unresectable HCC (u-HCC), and these patients will eventually be treated with systemic therapy.3,4 Owing to the encouraging results of IMbrave 150, the combination strategy of atezolizumab plus bevacizumab, two agents with different mechanisms of action, was approved as the standard of care for patients with u-HCC.5 Lenvatinib or camrelizumab has been shown to improve the survival of patients with u-HCC.6,7 Furthermore, hepatic arterial infusion chemotherapy (HAIC) demonstrated a high rate of response and improved survival in advanced HCC,8,9 and the combination of HAIC and sorafenib improved survival benefits compared with sorafenib alone.10,11 Therefore, HAIC combined with lenvatinib and camrelizumab may be an effective option for u-HCC.12–14
The neutrophil-to-lymphocyte ratio (NLR), a marker of systemic inflammation, is related to the prognosis of several cancers.15–21 HCC is often driven by persistent liver inflammation, and raised NLR suggests the existence of a pro-inflammatory immunological milieu.22 The relationship between NLR and treatment outcomes has been established in studies utilizing surgical resection, sorafenib, lenvatinib, HAIC, or transarterial chemoembolization (TACE) plus camrelizumab as the therapy for HCC.23–28 However, the connection between NLR and the outcomes associated with u-HCC patients treated with HAIC combined with lenvatinib and camrelizumab has yet to be explored.
Herein, we explored the efficacy and safety of in u-HCC patients treated with HAIC combined with lenvatinib and camrelizumab, as well as the association between NLR on progression-free survival (PFS), overall survival (OS), and adverse events (AEs).
Materials and Methods
Patients
Patients with u-HCC who were treated with HAIC combined with lenvatinib and camrelizumab at the First Affiliated Hospital of Nanchang University from October 2020 to April 2022 were retrospectively analyzed. The inclusion criteria were as follows: (1) patients diagnosed with HCC by dynamic computed tomography (CT)/magnetic resonance imaging (MRI) or pathology according to the European Association for the Study of the Liver Clinical Practice Guidelines: Management of Hepatocellular Carcinoma;29 (2) patients has at least one follow-up information; (3) patients who had not received any HCC-related treatment; (4) patients were not diagnosed with malignancy other than HCC.
The research received approval from the Hospital Ethics Committee and adhered to the ethical principles of the Declaration of Helsinki.
HAIC
The HAIC procedure was performed as previously described.30 Based on the Seldinger technique, a senior interventionalist inserted a catheter through the right femoral artery under the guidance of digital subtraction angiography to confirm the location of the tumor and blood supply vessels. Then, a 2.7F catheter was placed at the tumor’s main blood supply artery. Therapeutic schemes included FOXFOL, oxaliplatin 85 mg/m2, via continuous infusion for 2 hours, leucovorin 400 mg/m2, via continuous infusion for 2 hours, and 5-fluorouracil 2400 mg/ m2, via continuous infusion for 46 or 23 hours depending on patient tolerance, or RALOX, oxaliplatin 100 mg/ m2, via continuous infusion for 2 hours and raltitrexed 3 mg/ m2, via continuous infusion for 2 hours. In particular, the drug doses were modified based on the patient’s liver function reserve and tolerance to chemotherapy. The appropriate catheter was removed after completing HAIC and reinserted for the subsequent HAIC cycle. HAIC was conducted once every 3 weeks and up to 6 times.
Lenvatinib and Camrelizumab
Patients with u-HCC received lenvatinib and camrelizumab either before or three days after receiving HAIC treatment. Patients weighing <60 kg were prescribed an oral lenvatinib dose of 8 mg/day, and those weighing ≥60 kg were prescribed 12 mg/day. Camrelizumab 200 mg was given intravenously to patients every three weeks. The drug dose was reduced or the drug discontinued in case of disease progression or intolerable toxicity.
Evaluation and Data Collection
3 weeks after each HAIC therapy, or every 6–8 weeks after HAIC treatment was completed, all patients were evaluated by two senior imaging specialists using dynamic CT or MRI to assess efficacy according to Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) and the Modified Response Evaluation Criteria in Solid Tumors (mRECIST). OS was defined as the period from the start of treatment to the date of all-cause death, while PFS was the time between the start of therapy and the occurrence of radiographic progression or death. The last visit date for patients who were still living and the date of death for those who died during follow-up were used to determine when follow-up ended. The disease control rate (DCR) was derived by adding complete response (CR), partial response (PR), and stable disease (SD) rates. The objective response rate (ORR) was estimated by summing the rates for CR and PR. The National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0) were used to identify all adverse events (AEs) related to the therapy.
All variables were collected prior to starting treatment. NLR was defined as the ratio of neutrophil count to lymphocyte count in peripheral blood, and was categorized into low and high NLR groups using mean or median as the cutoff value after testing for normality. Based on previous studies, we employed the following cut-off values for continuous clinical features: age, 60 years,31 and alpha-fetoprotein (AFP), 400 ng/mL.29 The modified albumin-bilirubin (mALBI) score was used to assess the patient’s liver function.32 Furthermore, we used the Liver Cancer Study Group of Japan criteria to establish portal vein tumor thrombosis (PVTT) and hepatic vein tumor thrombus (HVTT) staging based on tumor thrombus location.33 The Barcelona Clinic Liver Cancer (BCLC) categorization system was used to determine the stage of HCC.34
Statistical Analysis
Continuous variables that followed a normal distribution were presented as mean ± standard deviation, while those not adhering to a normal distribution were expressed as median and interquartile range (IQR). The χ2 test and Fisher’s exact test were used to evaluate categorical variables. Median survival time and 95% confidence interval (CI) were determined using Kaplan–Meier survival analysis, and survival curve comparisons were conducted with the Log-rank test. A Cox proportional hazard model was used for multivariate survival analysis. Factors with p-value < 0.1 in univariate analysis were used as covariates in the multivariate COX proportional hazard model for OS, and factors significantly associated with OS were included in the multivariate analysis for PFS. Statistical significance was set at p < 0.05. Statistical analysis was performed using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, NY, USA) and R, version 4.2.1 (http://www.r-project.org/).
Results
A total of 98 patients were treated with HAIC combined with lenvatinib and camrelizumab between October 2020 and April 2022, of which 10 patients were excluded (10 patients had previously received treatment). Finally, 88 patients were enrolled in the study, and their baseline characteristics are shown in Table 1. All continuous variables are non-normally distributed. Among the enrolled patients, there were 13 females and 75 males, with a median age of 53.0 (46.3–62.0) years. There were 18, 23, 43, and 4 mALBI grade 1, 2a, 2b, and 3, respectively. Two patients had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 1. Additionally, AFP ≥ 400 ng/mL was found in 49 patients, 23 patients had a solitary lesion, and notably, the majority (81/88) had hepatitis B. There were 22 patients with Vp3, 15 patients with Vp4, 2 patients with Vv2, and 2 patients with Vv3. It is noteworthy that 2 patients had both HVTT and PVTT. The BCLC stage A, B, and C had 6, 25, and 57 patients, respectively. The median NLR was 3.46 (1.96–4.40).
 |
Table 1 Demographic Characteristics of the Patients |
For NLR with some baseline characteristics shown in Table S1, except for the maximum tumor diameter [8.5 (6.4–10.6) vs 11.1 (6.8–13.5) cm, p = 0.015], no statistical difference in the baseline characteristics was found between the low NLR group (< 3.46) and high NLR group (≥ 3.46).
Therapeutic Response
In accordance with the RECIST v1.1 criteria, the CR, PR, SD, and progressive disease (PD) were 0 (0), 22 (25.0%), 54 (61.4%), and 12 (13.6%), respectively, while the ORR and DCR were 25% and 86.4%, respectively (Table 2). Meanwhile, according to mRECIST criteria, the CR, PR, SD, and PD were 5 (5.7%), 42 (47.7%), 29 (33.0%), and 12 (13.6%), respectively, with ORR and DCR of 53.4% and 86.4%, respectively (Table 2). There was no significant difference between the low NLR group (< 3.46) and the high NLR group (≥ 3.46).
 |
Table 2 Therapeutic Response to HAIC Combined with Lenvatinib and Camrelizumab |
Overall Survival and Progression-Free Survival
The median PFS for all patients was 7.9 months (95% CI, 6.3–9.5 months), and the median OS did not reach (95% CI, not available) (Figure 1a and b). When stratified into low and high NLR groups based on NLR, the median PFS was 18.3 months (95% CI 6.9–29.7 months) in the low NLR group and 5.3 months (95% CI 3.4–7.3 months, p = 0.0015) in the high NLR group (Figure 1c). The median OS was not reached (95% CI not achieved) in the low NLR group and 9.6 months (95% CI 4.3–14.9 months, p = 0.017) in the high NLR group (Figure 1d).
Factors Affecting Overall Survival and Progression-Free Survival
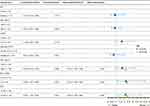
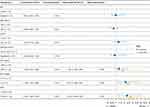
Univariate analysis of baseline clinical characteristics indicated that AFP (p = 0.090), BCLC stage (p = 0.032), and NLR (p = 0.020) were significantly associated with OS (Figure 2). Multivariable analysis with these factors revealed that AFP ≥400 ng/mL [hazard ratio (HR), 2.133; 95% CI, 1.102–4.126; p = 0.024], BCLC stage C (HR, 2.319; 95% CI, 1.128–4.736; p = 0.022), and the NLR ≥ 3.46 (HR, 2.359; 95% CI, 1.239–4.494; p = 0.009) were significant independent predictors of OS (Figure 2).
Factors associated with PFS in the univariate analysis are listed in Figure 3. Risk factors that exhibited significant effects on OS were incorporated into a multivariate analysis to evaluate their influence on PFS. The results indicated that AFP ≥400 ng/mL (HR, 1.790; 95% CI, 1.039–3.086; p = 0.036), BCLC stage C (HR, 2.437; 95% CI, 1.348–4.407; p = 0.003), and the NLR ≥ 3.46 (HR, 2.742; 95% CI, 1.584–4.746; p < 0.001) were independently associated with PFS.
Adverse Events
Table S2 shows the AEs observed after treatment with HAIC combined with lenvatinib and camrelizumab. The most common adverse events were elevated transaminase (76.1%), fatigue (55.7%), and nausea and vomiting (48.9%). The majority of AEs were mild, and there were no unexpected AEs. Moreover, no patients died from AEs. Notably, no significant difference was observed in the overall or grade 3/4 AEs between the low group (NLR< 3.46) and the high NLR group (NLR ≥ 3.46).
Follow-Up Duration and Sequential Therapy After Progression
The median follow-up duration was 11.4 (7.0–15.7) months for all patients with u-HCC treatment with HAIC combined with lenvatinib and camrelizumab (Table 1). When split into high and low NLR groups, the low NLR group had a longer follow-up than the high NLR group (12.7 (8.6–17.6) vs 9.8 (5.3–14.7) months; Table S1).
There are 45 patients with u-HCC who received HAIC combined with lenvatinib and camrelizumab observed imaging progression. Six patients were treated with regorafenib, 13 patients underwent vascular intervention, 3 received local ablation, 3 had radiation therapy, and 11 patients were given with best supportive care because they could not tolerate or refused sequential therapy. Notably, nine patients could not be evaluated for subsequent strategies due to a lack of accurate follow-up information (Table 3).
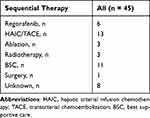 |
Table 3 Sequential Therapy After Progression |
Discussion
Our retrospective study, for the first time, explored the efficacy and safety of employing HAIC combined with lenvatinib and camrelizumab in u-HCC patients, while also assessing the prognostic significance of the NLR. According to our research, patients with a high NLR group, NLR≥ 3.46, had a worse prognosis than those with a low NLR group, NLR < 3.46. Multivariate analysis revealed that high NLR was an independent risk factor for OS and PFS. Furthermore, the multivariate analysis demonstrated that AFP level and BCLC stage were also independently associated with OS and PFS.
While previous reports have explored the use of HAIC in conjunction with molecularly targeted drugs and immune checkpoint inhibitors for u-HCC treatment, these studies often exhibited varying degrees of heterogeneity.35 In contrast, our study sought to mitigate this heterogeneity. Furthermore, we meticulously documented the adverse events experienced by the patients and the subsequent treatment strategies following disease progression in a comprehensive manner.
NLR, as a marker of inflammation, plays a significant role in the progression of various malignancies, including melanoma, breast, prostate, non-small cell lung, ovarian, and colorectal cancers, and serves as a prognostic predictor of these malignancies.15–21 Furthermore, the relationship between NLR and treatment outcomes in u-HCC has been reported in treatment regimens such as lenvatinib, HAIC, or TACE in combination with camrelizumab.25–28 Elevated NLR may be associated with more aggressive tumors.22 Neutrophils contribute to immunosuppression by inhibiting T lymphocyte proliferation and natural killer cell cytotoxicity through the production of nitric oxide, reactive oxygen species, and arginase 1. Neutrophils can also promote the expansion of T regulatory cells and hinder T cell proliferation and cytotoxicity via the cell surface expression of CD274.36 Additionally, neutrophils induce CD4+ T cells to release immunosuppressive cytokines such as interleukin 10 and transforming growth factor β. They enhance the recruitment of immunosuppressive T regulatory cells and M2 phenotype macrophages by expressing chemokine ligands 2 and 17.37 Furthermore, neutrophils produce matrix metalloproteinase 9 and stimulate the proliferation and survival of stem-like cancer cells.36 These factors collectively impair antitumor immune responses, promote tumor cell proliferation and invasiveness, and stimulate extracellular matrix remodeling and angiogenesis. Lymphocytes are recognized as the pivotal effector of antitumor immunity. A reduction in lymphocytes demonstrates that anti-tumor immunity is compromised. In summary, a high NLR may signify a disrupted immune microenvironment within the tumor, where the pro-tumor effect outweighs the anti-tumor effect.
Patients in the high NLR group had larger tumors in our study, potentially contributing to their less favorable prognosis compared to the low NLR group. Furthermore, our findings showed that more advanced tumor stages and higher AFP levels were indicative of a poorer prognosis, aligning with prior research. Given the predominance of hepatitis B virus exposure among HCC patients in China, the majority of individuals in our study were afflicted with hepatitis B-related HCC (81/88). Since distinct etiologies result in diverse tumor immune microenvironments, it is imperative to include a more comprehensive representation of HCC patients with various etiologies.38 Although previous studies have revealed that mALBI is associated with OS and PFS in u-HCC patients,39 our study did not yield the same findings, possibly due to limitations in our sample size.
Moreover, AEs were evaluated in all patients, and notably, no fatal AEs were recorded, which showed that HAIC combined with lenvatinib and camrelizumab for u-HCC was well tolerated. Tada et al40 reported that patients with high NLR (NLR≥3) were more likely to suffer AEs. However, this was not observed in our analysis, which could be attributed to the utilization of different NLR cutoff values. The follow-up period was shorter in the high NLR group than in the low NLR group, which was due to a higher number of endpoint events in the high NLR group that contributed to the termination of follow-up. Furthermore, second-line therapies that have been authorized have only been assessed in patients who have previously received sorafenib therapy,3 and there is still a lack of high-quality evidence regarding subsequent treatment after progression on combination therapy, contributing to the substantial heterogeneity in sequential treatment regimens in our study.
This study has some limitations. It is a single-center, retrospective cohort study, which may introduce inherent information and selection bias. Additionally, it is a single-arm study without a control group and has a relatively small sample size. Therefore, further validation is required through multicenter, prospective, randomized, controlled clinical trials. Furthermore, due to the limited follow-up time, the median OS could not be observed, and a more extended duration is necessary to accumulate a sufficient number of endpoint events. Finally, this study has revealed the potential prognostic value of NLR in HAIC combined with lenvatinib and camrelizumab for the treatment of patients with u-HCC.
Conclusion
In summary, our study demonstrates that NLR, a readily accessible and cost-effective marker, is correlated with the prognosis of patients with u-HCC undergoing treatment with HAIC combined with lenvatinib and camrelizumab. However, larger prospective studies may be necessary to validate and establish reliable cutoff values.
Abbreviations
AEs, adverse events; AFP, alpha-fetoprotein; BCLC, Barcelona clinic liver cancer; CR, complete response; CT, computed tomography; CI, confidence interval; DCR, disease control rate; HR, Hazard ratio; HAIC, hepatic arterial infusion chemotherapy; HVTT, hepatic vein tumor thrombus; HCC, hepatocellular carcinoma; MRI, magnetic resonance imaging; mALBI, modified albumin-bilirubin; mRECIST, Modified Response Evaluation Criteria in Solid Tumors; NLR, neutrophil-to-lymphocyte ratio; ORR, objective response rate; OS, overall survival; PR, partial response; PVTT, portal vein tumor thrombosis; PFS, progression-free survival; PD, progressive disease; RECIST v1.1, Response Evaluation Criteria in Solid Tumors version 1.1; SD, stable disease; TACE, transarterial chemoembolization; u-HCC, unresectable hepatocellular carcinoma.
Data Sharing Statement
The data that support the results of this study are obtainable upon request from the corresponding author.
Ethics Statement
The approval (NO. (2022) CDYFYYLK (06-009)) for conducting this study was provided by the First Affiliated Hospital of Nanchang University’s ethical committee. In view of the fact that this was a retrospective study of the patient’s medical data and that no identifying information was used, the waiver of informed consent was approved.
Patient Consent for Publication
In view of the fact that this was a retrospective study of the patient’s medical data and that no identifying information was used, the waiver of informed consent was approved.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Rumgay H, Arnold M, Ferlay J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77(6):1598–1606. doi:10.1016/j.jhep.2022.08.021
3. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. doi:10.1038/s41572-020-00240-3
4. Yang C, Zhang H, Zhang L, et al. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2022;20(4):203–222. doi:10.1038/s41575-022-00704-9
5. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
6. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
7. Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, Phase 2 trial. Lancet Oncol. 2020;21(4):571–580. doi:10.1016/S1470-2045(20)30011-5
8. He M-K, Le Y, Li Q-J, et al. Hepatic artery infusion chemotherapy using mFOLFOX versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: a prospective non-randomized study. Chin J Cancer. 2017;36(1):83. doi:10.1186/s40880-017-0251-2
9. Lyu N, Lin Y, Kong Y, et al. FOXAI: a Phase II trial evaluating the efficacy and safety of hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin for advanced hepatocellular carcinoma. Gut. 2018;67(2):395–396. doi:10.1136/gutjnl-2017-314138
10. Ikeda M, Shimizu S, Sato T, et al. Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin versus sorafenib for advanced hepatocellular carcinoma: randomized phase II trial. Ann Oncol. 2016;27(11):2090–2096. doi:10.1093/annonc/mdw323
11. He M, Li Q, Zou R, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5(7):953–960. doi:10.1001/jamaoncol.2019.0250
12. Luo L, Xiao Y, Zhu G, et al. Hepatic arterial infusion chemotherapy combined with PD-1 inhibitors and tyrosine kinase inhibitors for unresectable hepatocellular carcinoma: a tertiary medical center experience. Front Oncol. 2022;12:1004652. doi:10.3389/fonc.2022.1004652
13. He MK, Liang RB, Zhao Y, et al. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv Med Oncol. 2021;13:17588359211002720. doi:10.1177/17588359211002720
14. Mei J, Tang YH, Wei W, et al. Hepatic arterial infusion chemotherapy combined with PD-1 inhibitors plus lenvatinib versus PD-1 inhibitors plus lenvatinib for advanced hepatocellular carcinoma. Front Oncol. 2021;11:618206. doi:10.3389/fonc.2021.618206
15. Zaragoza J, Caille A, Beneton N, et al. High neutrophil to lymphocyte ratio measured before starting ipilimumab treatment is associated with reduced overall survival in patients with melanoma. Br J Dermatol. 2016;174(1):146–151. doi:10.1111/bjd.14155
16. Yalon M, Toren A, Jabarin D, Fadida E, Constantini S, Mehrian-Shai R. Elevated NLR may be a feature of pediatric brain cancer patients. Front Oncol. 2019;9:327. doi:10.3389/fonc.2019.00327
17. Peng B, Wang YH, Liu YM, Ma LX. Prognostic significance of the neutrophil to lymphocyte ratio in patients with non-small cell lung cancer: a systemic review and meta-analysis. Int J Clin Exp Med. 2015;8(3):3098–3106.
18. Gu X, Gao X, Li X, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in prostate cancer: evidence from 16,266 patients. Sci Rep. 2016;6(1):22089. doi:10.1038/srep22089
19. Iimori N, Kashiwagi S, Asano Y, et al. Clinical significance of the neutrophil-to-lymphocyte ratio in endocrine therapy for stage IV breast cancer. In Vivo. 2018;32(3):669–675. doi:10.21873/invivo.11292
20. Cho H, Hur HW, Kim SW, et al. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother. 2009;58(1):15–23. doi:10.1007/s00262-008-0516-3
21. Grenader T, Nash S, Adams R, et al. Derived neutrophil lymphocyte ratio is predictive of survival from intermittent therapy in advanced colorectal cancer: a post hoc analysis of the MRC COIN study. Br J Cancer. 2016;114(6):612–615. doi:10.1038/bjc.2016.23
22. Motomura T, Shirabe K, Mano Y, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol. 2013;58(1):58–64. doi:10.1016/j.jhep.2012.08.017
23. Okamura Y, Ashida R, Ito T, Sugiura T, Mori K, Uesaka K. Preoperative neutrophil to lymphocyte ratio and prognostic nutritional index predict overall survival after hepatectomy for hepatocellular carcinoma. World J Surg. 2015;39(6):1501–1509. doi:10.1007/s00268-015-2982-z
24. Mano Y, Shirabe K, Yamashita Y, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg. 2013;258(2):301–305. doi:10.1097/SLA.0b013e318297ad6b
25. Terashima T, Yamashita T, Iida N, et al. Blood neutrophil to lymphocyte ratio as a predictor in patients with advanced hepatocellular carcinoma treated with hepatic arterial infusion chemotherapy. Hepatol Res. 2015;45(9):949–959. doi:10.1111/hepr.12436
26. Tada T, Kumada T, Hiraoka A, et al. Neutrophil-to-lymphocyte ratio is associated with survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Liver Int Apr. 2020;40(4):968–976. doi:10.1111/liv.14405
27. Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two Phase III studies. J Hepatol. 2017;67(5):999–1008. doi:10.1016/j.jhep.2017.06.026
28. Guo Y, Ren Y, Chen L, et al. Transarterial chemoembolization combined with camrelizumab for recurrent hepatocellular carcinoma. BMC Cancer. 2022;22(1):270. doi:10.1186/s12885-022-09325-6
29. European Association for the Study of the Liver. Electronic address EEE, European Association for the Study of the L. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
30. Miyaki D, Aikata H, Honda Y, et al. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma according to Child-Pugh classification. J Gastroenterol Hepatol. 2012;27(12):1850–1857. doi:10.1111/j.1440-1746.2012.07276.x
31. Wen CP, Lin J, Yang YC, et al. Hepatocellular carcinoma risk prediction model for the general population: the predictive power of transaminases. J Natl Cancer Inst. 2012;104(20):1599–1611. doi:10.1093/jnci/djs372
32. Hiraoka A, Michitaka K, Kumada T, et al. Validation and potential of albumin-bilirubin grade and prognostication in a nationwide survey of 46,681 hepatocellular carcinoma patients in Japan: the need for a more detailed evaluation of hepatic function. Liver Cancer. 2017;6(4):325–336. doi:10.1159/000479984
33. Makuuchi M. Surveillance algorithm and diagnostic algorithm for hepatocellular carcinoma. Hepatol Res. 2010;40:6–7.
34. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
35. Tan Z, Zhang J, Xu L, et al. Triple combination of HAIC-FO plus tyrosine kinase inhibitors and immune checkpoint inhibitors for advanced hepatocellular carcinoma: a systematic review and meta-analysis. PLoS One. 2023;18(10):e0290644. doi:10.1371/journal.pone.0290644
36. Geh D, Leslie J, Rumney R, Reeves HL, Bird TG, Mann DA. Neutrophils as potential therapeutic targets in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2022;19(4):257–273. doi:10.1038/s41575-021-00568-5
37. Zhou S-L, Zhou Z-J, Hu Z-Q, et al. Tumor-associated neutrophils recruit macrophages and T-regulatory cells to promote progression of hepatocellular carcinoma and resistance to sorafenib. Gastroenterology. 2016;150(7):1646–1658 e17. doi:10.1053/j.gastro.2016.02.040
38. Llovet JM, Castet F, Heikenwalder M, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19(3):151–172. doi:10.1038/s41571-021-00573-2
39. Tomonari T, Tani J, Sato Y, et al. Initial therapeutic results of atezolizumab plus bevacizumab for unresectable advanced hepatocellular carcinoma and the importance of hepatic functional reserve. Cancer Med. 2022;12(3):2646–2657. doi:10.1002/cam4.5145
40. Tada T, Kumada T, Hiraoka A, et al. Neutrophil–lymphocyte ratio predicts early outcomes in patients with unresectable hepatocellular carcinoma treated with atezolizumab plus bevacizumab: a multicenter analysis. Eur J Gastroenterol Hepatol. 2022;34(6):698–706. doi:10.1097/MEG.0000000000002356
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.