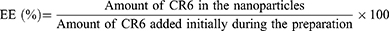Back to Journals » International Journal of Nanomedicine » Volume 17
Preparation of Zein-Based Nanoparticles: Nanoprecipitation versus Microfluidic-Assisted Manufacture, Effects of PEGylation on Nanoparticle Characteristics and Cellular Uptake by Melanoma Cells
Authors Meewan J, Somani S, Almowalad J , Laskar P , Mullin M, MacKenzie G, Khadke S, Perrie Y, Dufès C
Received 18 March 2022
Accepted for publication 15 June 2022
Published 29 June 2022 Volume 2022:17 Pages 2809—2822
DOI https://doi.org/10.2147/IJN.S366138
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Jitkasem Meewan,1 Sukrut Somani,1 Jamal Almowalad,1 Partha Laskar,1 Margaret Mullin,2 Graeme MacKenzie,1 Swapnil Khadke,1 Yvonne Perrie,1 Christine Dufès1
1Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow, G4 0RE, UK; 2Glasgow Imaging Facility, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, G12 8QQ, UK
Correspondence: Christine Dufès, Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow, G4 0RE, UK, Tel +44 1415483796, Fax +44 1415522562, Email [email protected]
Background: The manufacture of nanoparticles using manual methods is hampered by its challenging scale-up and poor reproducibility. To overcome this issue, the production of zein nanoparticles entrapping a lipophilic drug model, coumarin-6, by using a microfluidic system was assessed in this study. The influence of PEG density and chain length on zein nanoparticle characteristics, as well as their uptake efficacy in melanoma cancer cells, was also evaluated.
Methods: Zein nanoparticles were prepared by both manual and microfluidic approaches to allow comparison between the two processes. PEGylated zein nanoparticles with various PEG densities and chain lengths were produced by nanoprecipitation and characterized. Their cellular uptake was evaluated on B16F10 melanoma cancer cells in vitro.
Results: Zein nanoparticles have successfully been produced by both manual and microfluidic approaches. Parameters such as total flow rate and flow rate ratio of the aqueous and organic phases in microfluidic process, as well as the method preparation and aqueous to organic phase volume ratio during nanoprecipitation, have been shown to strongly influence the characteristics of the resulting nanoparticles. Continuous microfluidics led to the production of nanoparticles with low yield and drug entrapment, unlike nanoprecipitation, which resulted in zein nanoparticles with an appropriate size and an optimal drug entrapment efficiency of 64%. The surface modification of the nanoparticles produced by nanoprecipitation, with lower PEG density and shorter PEG chain length made mPEG5K-zein (0.5:1) the most favorable formulation in our study, resulting in enhanced stability and higher coumarin-6 uptake by melanoma cancer cells.
Conclusion: mPEG5K-zein (0.5:1) nanoparticles prepared by nanoprecipitation were the most promising formulation in our study, exhibiting increased stability and enhancing coumarin-6 uptake by melanoma cancer cells.
Keywords: zein nanoparticles, microfluidics, nanoprecipitation, poly(ethylene glycol), cellular uptake
Graphical Abstract:
Introduction
Zein, a prolamin protein found in the endosperm of corn, is approved by the US Food and Drug Administration as generally regarded as safe (GRAS) for use in food, pharmaceutical, and biomedical applications.1–4 Although it is soluble in 50–90% (v/v) aqueous ethanol due to its amphiphilic molecular structure, its large fraction of non-polar amino acids makes it insoluble in water, and its high glutamine content makes it insoluble in absolute alcohol.5 By taking advantage of its different solubilities in ethanol and water, zein was shown to be able to form particles suitable for use as carrier systems for the delivery of essential oils, drugs, and DNA.6–9 Zein nanoparticles can be produced by several preparation methods, the most common one being a simple benchtop process, nanoprecipitation. This method involves mixing a zein ethanolic solution with water to cause the nanoprecipitation of zein and the formation of zein nanoparticles. The payload is trapped within the matrix of the nanoparticles as they form.10–12 The size of these nanoparticles depends on parameters such as mixing process, rate of injection of one phase into another phase, agitation speed, pH of the solution, and the volume ratio of aqueous to organic phase.11,13 In addition, it can vary from batch-to-batch, especially at larger scales, due to the variation of shear force and spatial shear intensity within the solution.10,12 Hence, new methods that can optimize process control and enhance zein nanoparticle quality and consistency are urgently needed.
Over the past decade, microfluidic-based manufacturing systems have been successfully used for obtaining high-quality nanoparticles that can be used for drug delivery. Microfluidic approach allows experimental variables such as temperature, flow rate, and reagent concentration to be modified and controlled in a rapid, reproducible, and precise manner.14 Nanoprecipitation techniques are still widely used to produce nanoparticles, but microfluidics would appear to be a promising alternative to these processes, by allowing a rapid and consistent mixing at the junction where two solvent streams meet using hydrodynamic flow focusing, therefore resulting in a better control of nanoparticle properties such as size, surface characteristics, and drug loading compared with nanoprecipitation.12,14 Despite the advantages of microfluidics, only two research groups have exploited this technology to generate zein nanoparticles.10,12 They demonstrated that rapid and tunable microfluidic mixing can be used to reproducibly synthesize small and homogeneous zein nanoparticles. However, the applicability of this technology for encapsulating drugs or other materials in zein nanoparticles has not been addressed yet.
Because of the protein nature and hydrophobicity of zein, the immunogenicity of zein particles has raised concerns about their potential application as drug and vaccine delivery vehicles.14 To overcome this issue, zein has been conjugated with poly (ethylene glycol) (PEG) in order to create a steric shielding of the delivery system for immune evasion and long circulating half-life in blood.15–17 In our recent work, we demonstrated that PEGylation could confer stealth effects to the zein micelles, since the presence of the protein corona did decrease their uptake by immune cells, but not by melanoma cancer cells in vitro.17 As PEG density and chain length are crucial determinants of shielding efficacy, the modification of zein with PEG of various densities and lengths requires investigation to determine the optimal combination of these two materials to provide stealth efficacy and high cellular uptake for future cancer drug delivery applications.
The aims of this study were therefore 1) to assess the potential of using microfluidics to synthesize zein nanoparticles encapsulating coumarin-6 (CR6) as a lipophilic drug model compared with nanoprecipitation, and 2) to investigate the influence of PEG density and chain length on zein nanoparticle characteristics, as well as their uptake efficacy in melanoma cancer cells in vitro.
Materials and Methods
Cell Lines and Reagents
Yellow zein, coumarin-6 and all other chemicals that are not specifically mentioned below were purchased from Sigma-Aldrich (Poole, UK). Methoxy PEG succinimidyl carboxymethyl ester with a molecular weight (MW) of 5 kDa (mPEG-SCM-5K) and 10 kDa (mPEG-SCM-10K) were obtained from JenKem Technology (Plano, TX). Roswell Park Memorial Institute (RPMI) 1640 cell culture medium, fetal bovine serum (FBS), L-glutamine, penicillin-streptomycin and TrypLE® Express came from Life Technologies (Paisley, UK). Bioware® B16-F10-luc-G5 mouse melanoma cells expressing the firefly luciferase were purchased from Caliper Life Sciences (Hopkinton, MA). Vectashield® containing 4’,6-diamidino-2-phenylindole (DAPI) was from Vector Laboratories (Peterborough, UK). Ultrapure water (with a resistivity of 18.2 MΩ.cm at 25°C) was collected using a Milli-Q® Integral water purification system from Merck (Kenilworth, NJ).
Preparation of Zein Nanoparticles
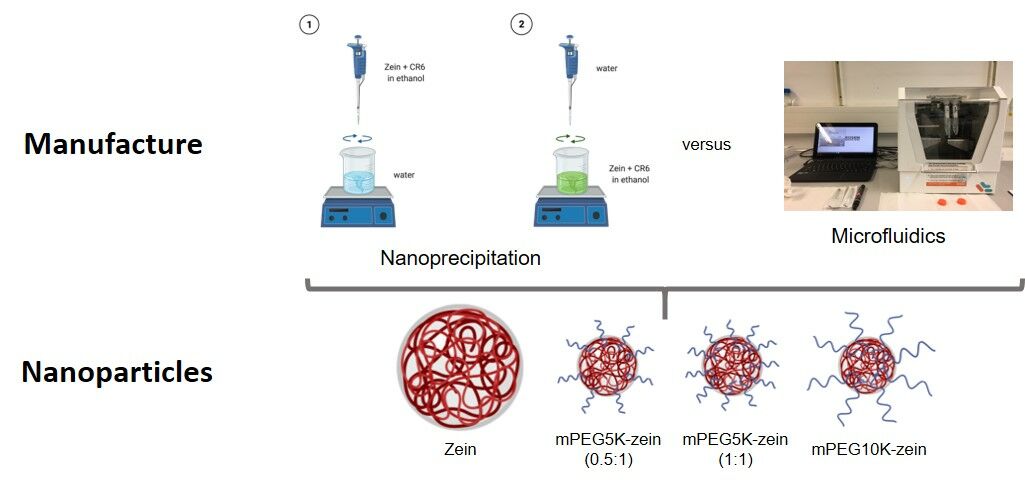
Microfluidics
The preparation of zein nanoparticles using microfluidic mixing on a NanoAssemblrTM Benchtop (Precision NanoSystems Inc., Vancouver, Canada) was adapted from a publication from van Ballegooie et al, with some modifications12 and further optimization of the production parameters (as described in Supplementary Table S1). Briefly, zein (10 mg) was dissolved in 2 mL of 80% (v/v) ethanol (forming 0.5% w/v solution) and stirred at 400 rpm at 25°C overnight. The zein solution was filtered through a 13-mm syringe filter (0.8 µm) to remove any large particulates. Subsequently, 0.01 mg CR6 (5 µL from CR6 stock solution (2 mg/mL in dimethylsulfoxide (DMSO)), corresponding to 0.1% (w/w) of zein) was added to the filtered zein solution, and the mixture was stirred at 200 rpm for 3 h at 37°C. The mixing process took place in a microfluidic cartridge with staggered herringbone structures, which has two inlets: one for the organic phase and the other for the aqueous phase. The CR6-zein solution was loaded into a syringe (1 mL) as the organic phase in the right inlet channel, while the aqueous phase (ultrapure water) was loaded into a 3 mL syringe in the left inlet channel (Figure 1). The flow rate ratio (FRR) between the water and the zein phases was 5:1 and the total flow rate (TFR) was 6 mL/min. The mixing process was carried out at room temperature. The nanoparticles were collected at the microfluidic chip’s outlet channel. Each run was programmed to discard the first 0.35 mL and the last 0.05 mL of the sample to ensure that the variability in fluid dynamics at the start and end of the synthesis run did not affect the final product. The pH of the resulting nanoparticles was increased to 10 by addition of NaOH (1 M) to allow the nanoparticles to be resuspended after centrifugation. The nanoparticles were pelleted following one cycle of centrifugation (10,000 g, for 1 h, at 20°C) to remove the ethanol and the free CR6. Pellets were resuspended in 1 mL ultrapure water and were left to settle at room temperature (20°C) for 1 h before analysis.
 |
Figure 1 Manufacture of zein nanoparticles using a microfluidic cartridge coupled with a NanoAssemblrTM device. |
Nanoprecipitation
In the nanoprecipitation method, 10 mg of zein was dissolved in 2 mL of 80% (v/v) ethanol (forming 0.5% w/v solution) and stirred at 400 rpm at 25°C overnight. The zein solution was filtered through a 13-mm syringe filter (0.8 µm) to remove any large particulates. CR6 (0.01 mg, 5 µL from CR6 stock solution (2 mg/mL in DMSO), corresponding to 0.1% w/w of zein) was added to the filtered zein solution, and the mixture was stirred at 200 rpm for 3 h at 37°C. Zein nanoparticles were then formed either:
- by dropwise addition of the organic phase (zein + CR6 solution) to the aqueous phase (ultrapure water) (Method 1) or
- by dropwise addition of the aqueous phase (ultrapure water) to the organic phase (zein + CR6 solution) (Method 2).
The addition of one phase to the other was carried out under stirring at 700 rpm. The volume ratio of aqueous to organic phase was 5:1, as determined following optimization of production parameters (described in Supplementary Table S2). The resulting nanoparticle suspension was adjusted to pH 10 and purified as described above.
Preparation of mPEG-Zein Nanoparticles
PEGylation of Zein
PEGylated zein was prepared as previously described, with some modifications.17 Briefly, yellow zein (0.1 g) was dissolved in 4 mL of 90% (v/v) ethanol. In order to vary PEG surface density and chain length, various amounts of mPEG-SCM (0.05 g of mPEG-SCM (5 kDa) for mPEG5K-zein (0.5:1 weight ratio); 0.1 g of mPEG-SCM (5 kDa) for mPEG5K-zein (1:1 weight ratio); 0.1 g of mPEG-SCM (10 kDa) for mPEG10K-zein) were dissolved in 1 mL of 90% (v/v) ethanol. PEG and zein solutions were mixed under magnetic stirring (100 rpm, for 3 h, at 25°C). One mL of glycine solution (1 M) was added to stop the reaction and quench the excess PEG ester, followed by the addition of 5 mL citrate buffer (pH 7.4) to precipitate the PEGylated zein. Free PEG and ethanol were removed by dialysis (MW cut-off: 12–14 kDa) against distilled water (2.5 L) under stirring (120 rpm) at 25°C for 48 h. The distilled water was changed 3 times during the dialysis process. The resulting product was then freeze-dried using a Christ Epsilon 2–4 LSC® freeze dryer (Osterode am Harz, Germany). The obtained mPEG-zein conjugates were stored at −20°C for long-term storage. Their composition is summarized in Table 1.
 |
Table 1 Composition of the Three mPEG-Zein Synthesized in the Study |
Preparation of mPEG-Zein Nanoparticles Entrapping CR6
mPEG-zein nanoparticles were prepared from mPEG-zein conjugates by nanoprecipitation Method 2, as described above.
Nanoparticle Characterization
Nanoparticle Morphology
The morphology of the prepared nanoparticles was assessed by transmission electron microscopy (TEM), using a JEOL JEM-1200EX® transmission electron microscope (Jeol, Tokyo, Japan) operating at an accelerating voltage of 80 kV. Each sample was diluted 1:5 in distilled water before being drop cast (3 μL) onto a carbon-coated copper grid (400-mesh size) and was air-dried overnight before imaging.
Determination of Particle Diameter, Size Distribution, and Zeta Potential
The size, polydispersity index (PDI), and zeta potential of zein/mPEG-zein nanoparticles loading CR6 were measured by photon correlation spectroscopy and laser Doppler electrophoresis, using a Malvern Zetasizer Nano-ZS® at 25°C (Malvern Instruments Ltd, Malvern, UK). All samples were diluted with ultrapure water to the desired concentration (1:5 v/v for samples prepared by microfluidics, 1:10 v/v for samples prepared by nanoprecipitation) up to 1 mL before measurement.
CR6 Entrapment Efficiency
Zein/mPEG-zein nanoparticles loading CR6 (50 µL) were dissolved in 950 µL methanol and centrifuged at 9,300 g for 15 min at room temperature using an IEC Micromax® centrifuge (Thermo Scientific, Loughborough, UK). The amount of CR6 entrapped in the nanoparticles was quantified by spectrofluorometry (λexc: 456 nm, λem: 500 nm, slit widths: 5 nm), using a Varian Cary Eclipse® spectrofluorometer (Agilent Technologies, Santa Clara, CA). The entrapment efficiency (EE) was calculated as follows:
Nanoparticle Yield
The amount of zein before and after nanoparticle preparation was determined by Lowry assay, as previously reported.18 Bovine serum albumin (BSA) was used as a standard protein solution and the amount of zein was calculated by correlating the absorbance of each sample with the standard curve of BSA. The particle yield was calculated as follows:
Stability Study
The stability of zein and mPEG-zein formulations was assessed by measuring their particle size with a Malvern Zetasizer Nano-ZS® at specific time points (Days 0, 7, 14, 21, and 28). All samples were kept in glass vials under storage condition at 4°C for 4 weeks (protected from light). Each sample (100 µL) was diluted with ultrapure water up to 1 mL before size measurement at 25°C.
In vitro Analysis
Cell Culture
B16-F10-luc-G5 mouse melanoma cells were grown in RPMI 1640 medium supplemented with 10% (v/v) FBS, 1% (v/v) L-glutamine, and 0.5% (v/v) penicillin-streptomycin. Cultures were maintained at 37°C in a 5% CO2 atmosphere.
Cellular Uptake
Imaging of the cellular uptake of CR6 loaded in zein and mPEG-zein nanoparticles was conducted using confocal microscopy. B16-F10-luc-G5 cells were seeded on coverslips in 6-well plates (1 × 105 cells/well) and were allowed to adhere overnight. They were then treated with CR6 (1 µg per well), either loaded in zein/mPEG-zein nanoparticles or in solution. After 2-h incubation, cells were washed twice with 3 mL phosphate buffer saline (PBS) before being fixed with 2 mL formaldehyde solution (4%) for 10 min. Cells were permeabilized with 2 mL Triton-X 100 solution (0.1% v/v in PBS) for 10 min, before a further incubation with 3 mL BSA (1% w/v in PBS) for 30 min to reduce non-specific binding. Cells were stained with Alexa Fluor® 647 dye (one unit of dye diluted in 200 µL PBS) for 30 min to visualize cell membranes, before a final wash with 3 mL PBS. After staining, they were mounted in Vectashield® containing DAPI for nuclei staining. The cells were examined using a Leica TCS SP8® confocal microscope (Wetzlar, Germany) at x63 magnification (zoom factor of 1.25). DAPI was excited with the 405 nm laser line (emission bandwidth: 415–491 nm), Alexa Fluor® 647 was excited with the 633 nm laser line (emission bandwidth: 645–710 nm), and CR6 was excited with the 488 nm laser line (emission bandwidth: 515–558 nm).
The quantification of the cellular uptake of CR6 loaded in zein/mPEG-zein nanoparticles was carried out by flow cytometry. B16-F10-luc-G5 cells were seeded into 6-well plates at a density of 2 × 105 cells/well and were allowed to grow at 37°C for 24 h, before being treated with CR6 (50 ng per well), either loaded in zein/mPEG-zein nanoparticles or in solution. After 2-h incubation, adherent cells were washed twice with 3 mL PBS and detached (using 200 µL TrypLE® Express and 400 µL complete medium per well). The mean fluorescence intensity (MFI) of CR6 taken up by the cells was analyzed by an Attune NxT® flow cytometer (Thermo Fisher Scientific, Waltham, MA), counting 10,000 cells (gated events) for each sample.
Statistical Analysis
The results were expressed as means ± standard error of the mean (SEM). Statistical analysis was assessed by one-way analysis of variance (ANOVA) followed by Tukey multiple comparison post-test. Unpaired t-test was performed for paired comparisons (Minitab® software, State College, PE). Differences were considered statistically significant for P values lower than 0.05.
Results
Preparation and Characterization of Zein Nanoparticles Entrapping CR6
CR6-loaded zein nanoparticles have been successfully prepared by both microfluidic and manual methods, as demonstrated by TEM imaging, which revealed the presence of spherical-shaped nanoparticles (Figure 2A).
Using microfluidics, an increase in zein concentration led to an increase in the nanoparticle size, while an increase in TFR and FRR (aqueous to organic phase ratio) resulted in a decrease in the nanoparticle size (Supplementary Table S1). The optimal experimental parameters leading to the production of nanoparticles achieving the highest CR6 entrapment by microfluidics were therefore determined to be a zein concentration of 0.5% (w/v), a CR6 loading of 0.1% (w/w), and a TFR of 6 mL/min at a 3:1 FRR (Supplementary Table S1).
Using nanoprecipitation, a zein concentration of 0.5% (w/v) and a CR6 loading of 0.1% (w/w) were maintained constant throughout the experiments to investigate the effects of method preparation and aqueous to organic phase volume ratio on nanoparticle characteristics. The mixing process was found to have a significant impact on the size and EE of the nanoparticles (Supplementary Table S2). Method 1, where zein solution was added to water, yielded nanoparticles with very low EE, unlike nanoparticles that were formed following addition of water to zein solution (Method 2). CR6 entrapment was significantly improved when using this method, despite the large size of the nanoparticles prepared. It decreased when increasing the volume ratio from 4:1 to 5:1. The optimal experimental parameters leading to the production of nanoparticles with the highest CR6 entrapment by nanoprecipitation were therefore determined to be the addition of water to organic phase (Method 2) at 5:1 volume ratio (Supplementary Table S2).
The size of the zein nanoparticles was dependent on the manufacturing approaches, as confirmed by dynamic light scattering (DLS) measurements. It ranged from 98 to 243 nm, with a narrow size distribution (PDI ~0.1) following preparation with all three manufacturing methods. The nanoparticles displayed a negative surface charge (about −26 mV), independently of the preparation method, under the experimental conditions used (Figure 2B). The CR6 EEs of the nanoparticles prepared by microfluidics, nanoprecipitation Method 1, and nanoprecipitation Method 2 were 29.7 ± 0.6%, 24.7 ± 2.6%, and 68.9 ± 1.9%, respectively. Nanoparticles produced by microfluidic manufacturing, compared to those prepared by nanoprecipitation Method 1 (addition of the organic phase to the aqueous phase), displayed no statistical differences in size, PDI, surface charge, or EE. Although the particle size (115.3 ± 6.6 nm for microfluidics and 98.3 ± 2.0 nm for nanoprecipitation Method 1) was suitable for cancer drug delivery, these 2 methods gave low yields and poor EEs. Microfluidic manufacturing, however, seems to be superior over the nanoprecipitation Method 1, as the yield increased from 22.8 ± 2.3% when using nanoprecipitation Method 1 to 29.8 ± 1.6% when using microfluidics. Although the nanoprecipitation Method 2 (addition of water to the organic phase) generated the largest particle size (242.8 ± 2.0 nm), it resulted in a significantly improved EE (68.9 ± 1.9%) and yield (62.2 ± 1.4%) when compared to the other two methods. Among the three manufacturing approaches evaluated in our study, nanoprecipitation Method 2 was therefore found to be the best method for the preparation of zein nanoparticles entrapping CR6 for subsequent experiments.
Characterization of PEGylated Zein Nanoparticles Entrapping CR6
TEM imaging of the PEGylated zein nanoparticles produced by nanoprecipitation Method 2 demonstrated that these nanoparticles were spherical in shape (Figure 3). The size of mPEG5K-zein formulations appeared to be much smaller than that of their 10K counterpart and the control zein.
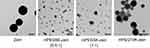 |
Figure 3 Unstained TEM images of zein, mPEG5K-zein (0.5:1), mPEG5K-zein (1:1), and mPEG10K-zein nanoparticles. All nanoparticles were prepared using nanoprecipitation Method 2 (scale bar: 200 nm). |
The hydrodynamic diameter, PDI, zeta potential, and EE of zein and mPEG-zein nanoparticles are summarized in Table 2. PEGylation significantly decreased the size of zein nanoparticles. At constant PEG chain length (5K), the particle size slightly decreased when PEG concentration was doubled. When nanoparticles were prepared with the same PEG to zein molar ratio (mPEG5K-zein (0.5:1) versus mPEG10K-zein), their size increased with increasing chain length. All formulations showed a monodisperse size distribution. Both zein and mPEG-zein nanoparticles displayed a negative surface charge, with a zeta potential of −26 mV for zein and less negative zeta potential values (ranging from −17 to −12 mV) for PEGylated formulations. The EE of CR6 in zein-based nanoparticles was dependent on parameters such as nanoparticle composition and PEG content, with the highest EE obtained from zein nanoparticles. Among PEGylated systems, mPEG10K-zein displayed the highest EE, followed by mPEG5K-zein (0.5:1) and mPEG5K-zein (1:1).
 |
Table 2 Characteristics of Zein and mPEG-Zein Nanoparticles Prepared by Nanoprecipitation Method 2 (n = 6) |
Stability of Zein and mPEG-Zein Nanoparticles
The size of the zein nanoparticles (242.8 ± 2.0 nm at Day 0) was stable for 2 weeks, but significantly increased from Day 15 to reach 929.9 ± 277.9 nm at Day 28 (Figure 4). PEGylation, however, was shown to improve the stability of the zein nanoparticles, as PEGylated formulations were found to be stable for at least 4 weeks when stored at 4°C. Their size remained unchanged (from 138.6 ± 1.8 nm at Day 0 to 137.4 ± 2.0 nm at Day 28 for mPEG5K-zein (0.5:1), and from 133.3 ± 1.7 nm at Day 0 to 131.4 ± 1.2 nm at Day 28 for mPEG5K-zein (1:1)). The size of mPEG10K-zein nanoparticles appeared to increase slightly from 197.3 ± 3.2 nm at Day 0 to 207.9 ± 6.8 nm and 222.8 ± 12.8 nm at Days 14 and 21, respectively, before decreasing to 197.0 ± 2.9 nm at Day 28. However, these changes were not statistically different.
Cellular Uptake
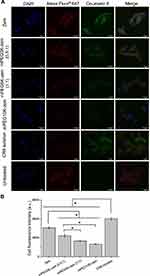
The uptake of CR6 entrapped in zein-based nanoparticles by B16-F10-luc-G5 melanoma cancer cells was qualitatively evaluated using confocal microscopy (Figure 5A). Zein and mPEG-zein nanoparticles were able to deliver CR6 into the cells during 2-h incubation. PEGylation of the nanoparticles led to a lower cellular uptake than that observed with the non-PEGylated formulation. Fluorescent CR6 was predominantly localized in the cytoplasm of the cells after treatment with all the nanoparticle formulations. Its accumulation in the cells was higher after treatment with the CR6 solution than with the nanoparticles.
The cellular uptake was also quantitatively confirmed by flow cytometry (Figure 5B). The highest cellular fluorescence of CR6 was observed following treatment with CR6 solution (MFI 6047 ± 187 arbitrary units (a.u.)), which was 1.3-fold higher than that obtained with zein nanoparticles (MFI 4602 ± 148 a.u.). PEGylation significantly decreased the uptake of the zein nanoparticles. Among the PEGylated formulations, mPEG5K-zein (0.5:1) was more efficacious in delivering CR6 into the cells by 1.3-fold and 1.7-fold in comparison with mPEG5K-zein (1:1) and mPEG10K-zein, respectively. These results indicate that PEGylation with a shorter PEG chain length and lower PEG density led to a higher nanoparticle uptake by the melanoma cells.
Discussion
In this work, zein, a protein-based biopolymer, was chosen as a natural polymer for preparing nanosized particles for drug delivery. Due to the different solubilities of zein in ethanol and water, zein nanoparticles loaded with various active compounds have been produced using nanoprecipitation. The supersaturation of zein occurs when the concentration of the solvent ethanol is reduced by shearing a zein ethanolic solution into water, leading to precipitation and the formation of nanoparticles. However, the manufacture at larger scales is often inconsistent in replicating particle size because nanoprecipitation is a batch process, which results in the variation of shear forces and spatial shear intensity within the solution between batches. Therefore, improved production techniques are required to limit the effects of these factors. Shifting from batch to continuous manufacturing to produce zein nanoparticles is currently being explored to improve particle homogeneity and minimize batch variations.10,12 Microfluidics is one such approach that is being adopted by the industry to produce nanoparticles in a highly reproducible manner and was hence assessed in the present study.
Zein nanoparticles entrapping CR6 were produced by both microfluidic- and manual-based production processes. Using a Y-junction microfluidic system, the modification of zein concentration, total flow rate of the fluidic system, and relative flow rate of the aqueous and organic phases allowed for nanoparticle size and PDI to be controlled. Our parameter optimization results were in good agreement with that published by van Ballegooie et al.12 Using microfluidics, the optimal conditions for achieving the highest CR6 entrapment were found to be a zein concentration of 0.5% w/v, a CR6 loading of 0.1% w/w, and a TFR of 6 mL/min at a 3:1 FRR.
Zein nanoparticles were also manually prepared using nanoprecipitation process. The mixing process was shown to significantly impact the size and EE of the particles. The addition of zein solution to water (Method 1) yielded nanoparticles with very low EE, unlike nanoparticles that were formed when adding water to zein solution (Method 2). This latter process resulted in a significantly enhanced CR6 entrapment. The lower EE observed when increasing aqueous to organic phase volume ratio from 4:1 to 5:1 was found to be similar to the results reported in previous studies.19,20 Our results suggested that nanoprecipitation Method 2 (addition of water to organic phase) at 5:1 volume ratio was suitable for producing zein nanoparticles. Further formulation optimization will be needed to achieve a smaller particle size combined with high CR6 entrapment when using this method.
To compare microfluidic versus manual manufacturing, a volume of water to zein phase ratio of 5:1 was applied based on the results obtained from preliminary studies, meaning that FRR (aqueous to organic phase ratio) of the fluidic system was set at 5:1 and a TFR of 6 mL/min was used. Furthermore, the pH of nanoparticle suspension was increased to 10 once the nanoparticles were formed in order to stabilize them before high-speed centrifugation. Raising the pH of nanoparticle suspension to 10 lowered the surface charge of zein nanoparticles from positive to negative zeta potential values due to the lower isoelectric point of zein at pH 10.7 It prevented irreversible nanoparticle aggregation during handling and facilitated the resuspension of the pellet following centrifugation. This pH adjustment strategy was also successful in improving the EE of zein nanoparticles and significantly decreased the size of the nanoparticles prepared by nanoprecipitation Method 2.
Nanoparticles with similar properties were obtained using microfluidics and nanoprecipitation Method 1. These two approaches produced highly monodisperse zein nanoparticles with the desired size, but with low yield and EE. Nanoprecipitation Method 2, on the other hand, generated zein nanoparticles with the largest size among the three tested manufacturing processes, however remaining below 400 nm, which is the cut-off size allowing extravasation for most tumors.21 Besides, it significantly improved EE and nanoparticle yield. As a result, we decided not to further explore the production of zein-based nanoparticles using the microfluidic setup due to the low EE and yield generated, but instead focused on manual production using nanoprecipitation Method 2 (addition of water to organic phase).
Due to their protein nature and hydrophobicity, zein nanoparticles can cause immunogenicity and may be rapidly cleared by the reticuloendothelial system (RES), which could limit their use for drug delivery.14 To date, one of the most effective strategies to make nanoparticles long-circulating is to graft PEG molecules on their surface, to create a hydrophilic steric stabilization that improves colloidal stability and protects the particles from non-specific protein adsorption, thus reducing opsonization and subsequent phagocytosis.22–24
In this study, PEGylated zein nanoparticles were prepared from mPEG-zein conjugates and the effect of PEG density and chain length on nanoparticle physicochemical properties was investigated. Firstly, to evaluate the effect of the PEG chain length, nanoparticles were produced from mPEG-zein conjugate where the molar ratio of PEG to zein was kept constant and the MW of the PEG was increased from 5 kDa (mPEG5K-zein (0.5:1)) to 10 kDa (mPEG10K-zein). Secondly, to study the influence of PEG density, nanoparticles were prepared from mPEG5K-zein conjugate with doubled PEG content (mPEG5K-zein (0.5:1) versus mPEG5K-zein (1:1)). PEGylation led to a decrease in the size of zein nanoparticles, possibly due to more amphiphilic nature of the mPEG-zein conjugates in comparison with zein, which reduced the interfacial tension between the aqueous and the organic phases.25 When nanoparticles were prepared using the same PEG MW, their size slightly decreased with increasing PEG content. The smaller size with the higher PEG density was also observed in PEG-PLA and PEG-PLGA nanoparticles.25,26 At constant PEG to zein molar ratio, the particle size increased with increasing PEG chain length. This is likely a consequence of the increase in the viscosity of the organic phase during particle preparation and the increase of the extending PEG chain on the final particles.25
PEGylation is known to shield either negative or positive charges on the surface of particles. As expected, incorporating PEG in the formulations decreased zeta potential absolute values of zein nanoparticles. Besides, PEGylation had an impact on the entrapment of CR6 in the nanoparticles. CR6 entrapment appeared to correlate with the particle size, in agreement with a previous study that has shown that smaller size nanoparticles are subject to a more extensive drug loss by diffusion towards the suspending medium due to their larger surface to volume ratio.19 However, when compared between two mPEG5K-zein formulations, a substantial EE reduction was observed with a small decrease in particle size when using high PEG density. This EE decrease could also be due to more limited hydrophobic interactions between CR6 and zein as the PEG content over zein increased.
The long-term stability of zein-based nanoparticles during storage was also examined. In the absence of PEG, zein nanoparticles appeared to form large aggregates, as demonstrated by the size increase observed after 2 weeks. PEG coating effectively prevented the aggregation of the nanoparticles, leading to a better colloidal stability for all three PEGylated formulations. The increase of nanocarrier stability as a result of surface modification with PEG was in accordance with previously published studies.26–28
The size of zein/PEGylated zein nanoparticles is expected to allow the passive accumulation of nanoparticles within solid tumors due to the enhanced permeability and retention (EPR) effect, which is facilitated by the leakiness of tumor vasculature and the lack of lymphatic drainage.29,30 We therefore evaluated the cellular uptake of zein-based nanoparticles in melanoma cancer cells. In vitro studies demonstrated that PEGylation resulted in a significant reduction in cellular uptake efficacy compared to non-PEGylated formulations. This could be explained by the effect of steric shielding of the PEG chains that hinders the interactions between the nanoparticles and the cell surface.31 Among the tested PEGylated formulations, the cellular uptake of CR6 was the highest following incubation with mPEG5K-zein (0.5:1), followed by mPEG5K-zein (1:1) and mPEG10K-zein. Higher cellular uptake of drugs entrapped in nanoparticles with shorter PEG chain length and lower PEG density was consistent with what was previously reported with other nanocarriers.28,32,33 PEGylation was shown to strongly reduce protein binding. In addition, Cruje and Chithrani reported that non-specific protein adsorption may facilitate cancer cell entry.32 Hence, the highest uptake observed in non-PEGylated nanoparticles was most probably due to the absence of PEG molecules able to repel adsorbed proteins. Non-specific protein adsorption was found to increase on nanocarriers with shorter PEG chain lengths and lower grafting densities, resulting in higher nanoparticle internalization by cancer cells.32
Conclusion
Overall, we demonstrated that zein nanoparticles could be produced by both manual and microfluidic approaches. Parameters such as total flow rate and flow rate ratio of the aqueous and organic phases for microfluidics, as well as method preparation and aqueous to organic phase volume ratio for nanoprecipitation, strongly impacted nanoparticle properties. Continuous microfluidic manufacturing led to low nanoparticle yield and poor EE, unlike what was observed with the nanoparticles produced using nanoprecipitation Method 2. Hence, further studies using microfluidics will be required to produce zein nanoparticles with increased yield and drug entrapment. Furthermore, the PEGylation of zein with shorter PEG chain length (with a MW of 5 kDa) and lower PEG density (with a PEG-zein weight ratio of 0.5:1) made mPEG5K-zein (0.5:1) the most favorable formulation in our study, as it improved nanoparticle stability and led to higher drug uptake efficacy by melanoma cancer cells. PEGylated zein nanoparticles, in particular mPEG5K-zein (0.5:1), are therefore highly promising delivery systems that should be further investigated for potential use in cancer drug delivery.
Abbreviations
ANOVA, analysis of variance; a.u., arbitrary units; BSA, bovine serum albumin; CR6, coumarin-6; DAPI, 4’,6-diamidino-2-phenylindole; DLS, dynamic light scattering; DMSO, dimethylsulfoxide; DNA, deoxyribonucleic acid; EE, entrapment efficiency; EPR, enhanced permeability and retention; FBS, fetal bovine serum; FRR, flow rate ratio; GRAS, generally regarded as safe; MFI, mean fluorescence intensity; mPEG-SCM-5K, methoxy PEG succinimidyl carboxymethyl ester with a molecular weight of 5 kDa; mPEG-SCM-10K, methoxy PEG succinimidyl carboxymethyl ester with a molecular weight of 10 kDa; mPEG5K-zein (0.5:1), PEG-zein nanoparticles prepared with mPEG-SCM-5K at a PEG:zein weight ratio of 0.5:1; mPEG5K-zein (1:1), PEG-zein nanoparticles prepared with mPEG-SCM-5K at a PEG:zein weight ratio of 1:1; mPEG10K-zein, PEG-zein nanoparticles prepared with mPEG-SCM-10K at a PEG:zein weight ratio of 1:1; MW, molecular weight; NaOH, sodium hydroxide; PBS, phosphate buffer saline; PDI, polydispersity index; PEG, poly(ethylene glycol); RES, reticuloendothelial system; RPMI, Roswell Park Memorial Institute; SEM, standard error of the mean; TFR, total flow rate; TEM, transmission electron microscopy.
Acknowledgments
The authors would like to acknowledge the CMAC National Facility, housed within the University of Strathclyde’s Technology and Innovation Centre, for providing training and accessing their NanoAssemblrTM instrument. We also would like to thank Dr. Stuart Woods for providing training for using the flow cytometer.
Disclosure
The authors report no conflicts of interest in this work. Jamal Almowalad’s current affiliation is at the Department of Pharmaceutics, College of Pharmacy, Umm Al-Qura University, Al-Abidiyah, Makkah 21955, Kingdom of Saudi Arabia. Partha Laskar’s current affiliation is at the Department of Chemistry -Ångström, Uppsala University, 751 21 Uppsala, Sweden. The graphical abstract was created with BioRender.com.
References
1. Shukla R, Cheryan M. Zein: the industrial protein from corn. Ind Crop Prod. 2001;13:171–192. doi:10.1016/S0926-6690(00)00064-9
2. Lawton JW. Zein: a history of processing and use. Cereal Chem. 2002;79:1–18. doi:10.1094/CCHEM.2002.79.1.1
3. Lin T, Lu C, Zhu L, et al. The biodegradation of zein in vitro and in vivo and its application in implants. AAPS PharmSciTech. 2011;12:172–176. doi:10.1208/s12249-010-9565-y
4. Paliwal R, Palakurthi S. Zein in controlled drug delivery and tissue engineering. J Control Release. 2014;189:108–122. doi:10.1016/j.jconrel.2014.06.036
5. Gianazza E, Viglienghi V, Righetti PG, et al. Amino acid composition of zein molecular components. Phytochemistry. 1977;16:315–317. doi:10.1016/0031-9422(77)80054-X
6. Parris N, Cooke PH, Hicks KB. Encapsulation of essential oils in zein nanospherical particles. J Agric Food Chem. 2005;53:4788–4792. doi:10.1021/jf040492p
7. Regier MC, Taylor JD, Borcyk T, et al. Fabrication and characterization of DNA-loaded zein nanospheres. J Nanobiotechnol. 2012;10:44. doi:10.1186/1477-3155-10-44
8. Dong F, Dong X, Zhou L, et al. Doxorubicin-loaded biodegradable self-assembly zein nanoparticle and its anti-cancer effect: preparation, in vitro evaluation, and cellular uptake. Colloids Surf B Biointerfaces. 2016;140:324–331. doi:10.1016/j.colsurfb.2015.12.048
9. Thapa RK, Nguyen HT, Jeong JH, et al. Synergistic anticancer activity of combined histone deacytylase and proteasomal inhibitor-loaded zein nanoparticles in metastatic prostate cancers. Nanomedicine. 2017;13:885–896. doi:10.1016/j.nano.2016.12.010
10. Olenskyj AG, Feng Y, Lee Y. Continuous microfluidic production of zein nanoparticles and correlation of particle size with physical parameters determined using CFD simulation. J Food Eng. 2017;211:50–59. doi:10.1016/j.jfoodeng.2017.04.019
11. Tarhini M, Benlyamani I, Hamdani S, et al. Protein-based nanoparticle preparation via nanoprecipitation method. Materials. 2018;11:394. doi:10.3390/ma11030394
12. van Ballegooie C, Man A, Andreu I, et al. Using a microfluidics system to reproducibly synthesize protein nanoparticles: factors contributing to size, homogeneity, and stability. Processes. 2019;7:290. doi:10.3390/pr7050290
13. Pascoli M, De lima R, Fraceto LF. Zein nanoparticles and strategies to improve colloidal stability: a mini-review. Front Chem. 2018;6:1–5. doi:10.3389/fchem.2018.00006
14. Hurtado-Lopez P, Murdan S. An investigation into the adjuvanticity and immunogenicity of zein microspheres being researched as drug and vaccine carriers. J Pharm Pharmacol. 2006;58:769–774. doi:10.1211/jpp.58.6.0007
15. Podaralla S, Averineni R, Alqahtani M, et al. Synthesis of novel biodegradable methoxy poly(ethylene glycol)–zein micelles for effective delivery of curcumin. Mol Pharm. 2012;9:2778–2786. doi:10.1021/mp2006455
16. Song R, Zhou Y, Li Y, et al. Preparation and characterization of mPEG-g-α-zein biohybrid micelles as a nano-carrier. J Appl Polym Sci. 2015;132:1–6. doi:10.1002/app.42555
17. Meewan J, Somani S, Laskar P, et al. Limited impact of the protein Corona on the cellular uptake of PEGylated zein micelles by melanoma cancer cells. Pharmaceutics. 2022;14:429. doi:10.3390/pharmaceutics14020439
18. Dufès C, Schätzlein AG, Tetley L, et al. Niosomes and polymeric chitosan based vesicles bearing transferrin and glucose ligands for drug targeting. Pharm Res. 2000;17:1250–1258. doi:10.1023/A:1026422915326
19. Chorny M, Fishbein I, Danenberg HD, et al. Lipophilic drug loaded nanospheres prepared by nanoprecipitation: effect of formulation variables on size, drug recovery and release kinetics. J Control Release. 2002;83:389–400. doi:10.1016/S0168-3659(02)00211-0
20. Song X, Zhao Y, Hou S, et al. Dual agents loaded PLGA nanoparticles: systematic study of particle size and drug entrapment efficiency. Eur J Pharm Biopharm. 2008;69:445–453. doi:10.1016/j.ejpb.2008.01.013
21. Yuan F, Dellian M, Fukumura D, et al. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55:3752–3756.
22. Torchilin VP, Trubetskoy VS. Which polymers can make nanoparticulate drug carriers long-circulating? Adv Drug Deliv Rev. 1995;16:141–155. doi:10.1016/0169-409X(95)00022-Y
23. Romberg B, Hennink WE, Storm G. Sheddable coatings for long-circulating nanoparticles. Pharm Res. 2008;25:55–71. doi:10.1007/s11095-007-9348-7
24. Cruz LJ, Tacken PJ, Fokkink R, et al. The influence of PEG chain length and targeting moiety on antibody-mediated delivery of nanoparticle vaccines to human dendritic cells. Biomaterials. 2011;32:6791–6803. doi:10.1016/j.biomaterials.2011.04.082
25. Gref R, Lück M, Quellec P, et al. ‘Stealth’ Corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the Corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf B Biointerfaces. 2000;18:301–313. doi:10.1016/S0927-7765(99)00156-3
26. Xu Q, Ensign LM, Boylan NJ, et al. Impact of surface Polyethylene Glycol (PEG) density on biodegradable nanoparticle transport in mucus ex vivo and distribution in vivo. ACS Nano. 2015;9:9217–9227. doi:10.1021/acsnano.5b03876
27. Suk JS, Xu Q, Kim N, et al. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51. doi:10.1016/j.addr.2015.09.012
28. Bachir ZA, Huang YK, He MY, et al. Effect of PEG surface density and chain length on the pharmacokinetics and biodistribution of methotrexate-loaded chitosan nanoparticles. Int J Nanomedicine. 2018;13:5657–5671. doi:10.2147/IJN.S167443
29. Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev. 2011;63:131–135. doi:10.1016/j.addr.2010.03.011
30. Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Deliv Rev. 2015;91:3–6. doi:10.1016/j.addr.2015.01.002
31. Du H, Chandaroy P, Hui SW. Grafted poly-(ethylene glycol) on lipid surfaces inhibits protein adsorption and cell adhesion. Biochim Biophys Acta. 1997;1326:236–248. doi:10.1016/S0005-2736(97)00027-8
32. Cruje C, Chithrani DB. Polyethylene glycol density and length affects nanoparticle uptake by cancer cells. J Nanomed Res. 2014;1:00006.
33. Pozzi D, Colapicchioni V, Caracciolo G, et al. Effect of polyethyleneglycol (PEG) chain length on the bio-nano-interactions between PEGylated lipid nanoparticles and biological fluids: from nanostructure to uptake in cancer cells. Nanoscale. 2014;6:2782–2792. doi:10.1039/c3nr05559k
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.