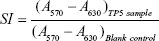Back to Journals » International Journal of Nanomedicine » Volume 14
Preparation, intestinal segment stability, and mucoadhesion properties of novel thymopentin-loaded chitosan derivatives coated with poly (n-butyl) cyanoacrylate nanoparticles
Authors Xu Y, Lu S, Liu Q, Hong Y, Xu B, Ping Q, Jin X, Shen Y, Webster TJ , Rao Y
Received 14 November 2018
Accepted for publication 31 January 2019
Published 4 March 2019 Volume 2019:14 Pages 1659—1668
DOI https://doi.org/10.2147/IJN.S194529
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Ying Xu,1 Shengzhe Lu,1 Qi Liu,2 Yun Hong,3 Bohui Xu,4 Qineng Ping,5 Xuefeng Jin,5 Yan Shen,5 Thomas J Webster,6 Yuefeng Rao3,7
1Department of Pharmaceutics, School of Pharmacy, Jiangsu University, Zhenjiang, 212013, China; 2Department of Dermatology, Johns Hopkins University School of Medicine, Baltimore, MD 21231, USA; 3Department of Pharmacy, School of Medicine, The First Affiliated Hospital, Zhejiang University, Hangzhou 310003, China; 4Department of Pharmacy, School of Pharmacy, Nantong University, Nantong 226001, China; 5Department of Pharmaceutics, School of Pharmacy, China Pharmaceutical University, Nanjing 210009, China; 6Department of Chemical Engineering, Northeastern University, Boston, MA 02115, USA; 7Department of Pharmaceutics, College of Pharmaceutical Sciences, Zhejiang University, Hangzhou 310058, China
Background: In order to develop a promising carrier for the oral delivery of proteins and peptide drugs, a novel bioadhesive nanocarrier of chitosan (CTS) derivatives coated with poly (n-butyl) cyanoacrylate nanoparticles (PBCA-NPs) was prepared in this study.
Methods: Three different thymopentin (TP5)-loaded nanoparticles were prepared in the present study. TP5-PBCA-NPs were developed by modifying an emulsion polymerization method, and CTS and chitosan–glutathione (CG) derivative-coated PBCA nanoparticles were obtained from the electrostatic interactions between CTS or CG with negatively charged PBCA nanoparticles.
Results: The particle sizes of TP5-PBCA-NPs, TP5-CTS-PBCA-NPs, and TP5-CG-PBCA-NPs were 212.3±6.9, 274.6±8.2, and 310.4±7.5 nm, respectively, while the respective zeta potentials were –22.6±0.76, 23.3±1.2, and 34.6±1.6 mV with encapsulation efficiencies of 79.37%±2.15%, 74.21%±2.13%, and 72.65%±1.48%, respectively. An everted intestinal ring method indicated that drug stability was remarkably improved after incorporation into the nanoparticles, especially the CG-coated nanoparticles. The mucus layer retention rates for CTS- and CG-coated nanoparticles were 1.43 and 1.83 times that of the uncoated nanoparticles, respectively, using ex vivo mucosa. The in vivo mucoadhesion study illustrated that the transfer of uncoated PBCA-NPs from the stomach to the intestine was faster than that of CTS-PBCA-NPs and CG-PBCA-NPs, while the CG-PBCA-NPs presented the best intestinal retentive characteristic.
Conclusion: In summary, this study demonstrated the feasibility and benefit of orally delivering peptide drugs using novel CTS derivative-coated nanoparticles with optimal stability and bioadhesive properties.
Keywords: chitosan derivatives, PBCA nanoparticles, thymopentin, stability, bioadhesive
Introduction
The oral delivery of peptides and proteins is of high research value in pharmaceuticals due to their high patient compliance.1,2 Today, most peptide and protein drugs are still delivered via parenteral routes because they can be degraded by the acidic environment of the stomach and enzymes in the gastrointestinal tract (GIT), and their absorption is obstructed by intestinal physical barriers, thereby leading to poor oral bioavailability. However, the oral administration of drugs still presents some challenges to pharmaceutical scientists. Some attempts have been made to improve the oral bioavailability of peptides and proteins, focusing on how to ameliorate the stability and absorption of peptides and proteins in the GIT.3,4 As a promising carrier for the oral delivery of protein and peptide drugs, nanoparticles can protect the bioactive components from degradation and promote their permeability in the intestine.5,6
Thymopentin (TP5), a synthetic pentapeptide consisting of five amino acids (H2N-Arg-Lys-Asp-Val-Tyr-OH), is well known for its activity as an immune-modulating drug that can induce T cell differentiation and proliferation as well as the maturation of T lymphocyte precursors. TP5 is used clinically to treat a variety of diseases, including primary and secondary immune deficiency, autoimmune diseases, type II diabetes mellitus, infection, and cancer.7,8 However, TP5 exhibits a very short half-life of about 30 seconds in plasma due to extensive hepatic metabolization and enzymatic degradation in the GIT, and repeated intramuscular or intravenous injection is inconvenient and painful for patients during the first 6 months of clinical treatment.9 Therefore, the development of a new oral delivery system for TP5 has a great clinical value.
In the early 1980s, Couvreur et al successfully used poly α-cyanoacrylate (PBCA) to prepare nanoparticles, which has attracted a great deal of attention as of late.10 PBCA has significant qualities, such as low toxicity, biocompatibility, and biodegradability.11 In recent years, PBCA nanoparticles have been extensively used as an effective drug delivery system for targeting tumor tissue, crossing the blood-brain barrier, and protecting proteins and peptides when encountering the harsh environment of the stomach.12–15
Bioadhesive polymers have been used in various pharmaceutical formulations as a means to extend the amount of time the drug resides at the absorption site and enhance their absorption by achieving intimate contact with the mucosa.16 Bioadhesive polymers exhibit valuable characteristics such as a larger molecular weight, longer molecular chain, more hydrophilic groups, flexibility of the segment, as well as an appropriate degree of cross-linking and swelling.17 More importantly, bioadhesive polymers can reduce the degradation of drugs when they encounter the digestive enzymes in the GIT and provide for a steep concentration gradient in the absorption membrane. In addition, they could be used for some localized disease treatments.
Along these lines, chitosan (CTS) is a widely used bioadhesive polymer with properties such as biocompatibility, biodegradability, and the permeation enhancing effect via the opening of tight junctions between the intestinal epithelial cells.18,19 Thiolated chitosan (Thio-CTS) was obtained by introducing mercapto groups to the molecular structure of CTS,20,21 such as the chitosan–glutathione (CG) derivative. Thio-CTS can enhance the mucosal adhesion ability by forming disulfide bonds with cysteine-rich domains of mucus glycoproteins.22 In addition, these materials develop additional mucosal permeation-enhancing properties through a glutathione regeneration mechanism. Further, they demonstrate potential antiprotease activity due to their ability to bind divalent cations, such as Zn2+ or Mg2+, which are cofactors of many proteases.20 The combination of such characteristics makes Thio-CTS a promising material for the oral administration of peptides and proteins.
Here, a novel bioadhesive drug delivery system for CG derivative-coated TP5 poly (n-butyl) cyanoacrylate nanoparticles (PBCA-NPs) with core–shell structures was constructed. The preparation conditions of CTS- or CG-coated PBCA NPs were further optimized. The intestinal segment stability of CTS- or CG-coated TP5 PBCA nanoparticles was also investigated. The ex vivo and in vivo bioadhesive properties of CTS- or CG-coated PBCA nanoparticles were evaluated with fluorescent yellow light as the probe in this present study, showing much promise for future investigations.
Materials and methods
Materials and animals
An α-butyl cyanoacrylate (BCA) monomer was purchased from Beijing Suncon Medical Adhesive Co., Ltd. (Beijing, China). Poloxamer 188 (BASF) was obtained from Beijing Fengli Jingqiu Trade Limited Liability Co., Ltd. (Beijing, China). CTS was purchased from Zhenjiang Aoxing Biological Technology Co., Ltd. (Zhenjiang, China). The CG was synthesized by our laboratory. Dextran-70 (BR) was purchased from Shanghai Crystal Pure Reagent Co., Ltd. (Shanghai, China). TP5 was purchased from Shanghai Soho-Yiming Pharmaceuticals Co., Ltd (Shanghai, China). Pepsin (1:3,000, USP grade) and trypsin (1:250, >1,000 N.F.U/mG, EC 3.4.21.4, Amresco) were obtained from Shanghai Technical Service Industry and Biological Engineering Co., Ltd. (Shanghai, China). All other chemicals used in this study were of analytical reagent grade without further purification. Deionized water was used for the preparation of all solutions.
Male Sprague Dawley rats (190±10 g) were provided by The Experimental Animal Center of Zhejiang Province (Hangzhou, China). All animal experiments were approved by the Animal Ethical Committee of Jiangsu University and complied with the Guidelines for Care and Use of Laboratory Animals.
Preparation of TP5-PBCA-NPs, TP5-CTS-PBCA-NPs, TP5-CG-PBCA-NPs, and fluorescent yellow-labeled nanoparticles
TP5-PBCA-NPs were prepared by modifying the emulsion polymerization method as previously described.23 In brief, dextran-70 (0.6%), poloxamer188 (0.7%), and sodium metabisulfite (0.1%) were dissolved in deionized water, and the pH was adjusted to 2.0 by adding 1 N HCl. A butyl cyanoacrylate monomer (100 μL) was dropped slowly into this mixed solution while stirring, then 1 mL of the TP5 solution was added and stirring was maintained for at least 4 hours or until the polymerization was completed. The colloidal suspension was obtained by adjusting the pH to 4.5 with 0.1 M NaOH while continuously stirring for 1 hour. The suspension was filtered through a 0.8-μm microfiltration membrane to remove agglomerates.
CTS or CG was coated on PBCA-NPs by electrostatic interaction. A TP5-PBCA-NP suspension was added into an equal volume of a CTS or CG solution drop by drop while stirring and was then incubated for a period of time. CTS-coated TP5-PBCA-NPs (TP5-CTS-PBCA-NPs) or CG derivative-coated TP5-PBCA-NPs (TP5-CG-PBCA-NPs) were obtained.
In addition, to observe and evaluate the stability and the adhesion of the nanoparticles in the intestinal loop, fluorescent yellow loaded nanoparticles were prepared using the above method by replacing TP5 with fluorescent yellow. By adding 1 mg of fluorescent yellow to the BCA acetone solution, fluorescent yellow (Flu) labeled nanoparticles (Flu-PBCA-NPs, Flu-CTS-PBCA-NPs, and Flu-CG-PBCA-NPs) were obtained.
Factor optimization and thiol groups detection
The optimal incubation conditions for CTS and CG were determined in this study. The effect of incubation time (15, 30, or 60 minutes) on particle size distribution, zeta potential, and encapsulation efficiency of the nanoparticles was investigated.
Ellman’s reagent (DTNB, 0.03% in PBS) was used to determine the concentration of thiol groups. The TP5-CG-PBCA-NPs were centrifuged at 15,000 rpm for 30 minutes. The thiol groups in the TP5-CG-PBCA-NPs suspension before and after centrifugation were determined with Ellman’s reagent as well as the difference expressed in the surface thiol group ratios of TP5-CG-PBCA-NPs.
Characterization of TP5 nanoparticles
Transmission electron microscopy
The morphology of PBCA and CTS- or CG-coated nanoparticles was observed by transmission electron microscopy (H-700; Hitachi, Japan) using a negative-staining method. Particle size, size distribution, and the zeta potential of the nanoparticles were determined with a Zetasizer (3000HS, Malvern Instruments, Malvern, UK).
Entrapment efficiency of TP5 nanoparticles
The drug entrapment efficiency (DEE, given in %) of TP5 was calculated as the mass ratio of the amount of TP5 loaded in TP5-CTS-PBCA-NPs and TP5-CG-PBCA-NPs. Briefly, 200 μL of the nanoparticle suspension was diluted with double distilled water and centrifuged at 15,000 rpm for 30 minutes. The unloaded drug in the supernatant was separated and detected using HPLC (1100 LC, Agilent, Santa Clara, CA, USA). The total drug amount was determined with the same HPLC method; however, tetrahydrofuran was used instead of DD water to dissolve the nanoparticles. The HPLC chromatographic conditions consisted of an RPC18 column (150×6.0 mm, 5 μm, Shim-pack, Tokyo, Japan). The mobile phase consisted of PBS (0.02 mol/L, pH 7.0)–MeOH (87:13, v/v), and the flow rate was 1 mL/minute with a column temperature of 30°C, while the detection wavelength was 275 nm. The DEE was calculated as follows:
|
In vitro release studies and bioactivity assay
The in vitro release of TP5 from TP5-CG-PBCA-NPs in different pH conditions was performed as follows. One milliliter of the TP5-CG-PBCA-NP suspension was transferred into a dialysis bag (MWCO 8,000–12,000) that was tied and dipped into 80 mL of 0.05 mol/L PBS (pH 6.8), 0.05 mol/L PBS (pH 5.0), or 0.1 M HCl (pH 1.2), respectively, at 37°C with 60 rpm stirring. At predetermined intervals, 1 mL of the release medium was withdrawn for the HPLC assay and the same amount of fresh media was added. The release of fluorescent yellow (Flu) from Flu-PBCA-NPs, Flu-CTS-PBCA-NPs, and Flu-CG-PBCA-NPs was performed in 0.05 mol/L phosphate buffer (pH 6.8) in the same way. However, the HPLC chromatographic condition of Flu was PBS (0.1 mol/L, pH 4.4)–MeOH (30:70, v/v) in the mobile phase, and the flow rate was 1 mL/minute. The HPLC system consisted of a pump (LC-10ATVP) and a fluorescence detector (Shimadzu, Tokyo, Japan) set at Ex 465 nm/Em 502 nm and an RP C18 column (150×6.0 mm, 5 μm, Shim-pack, Tokyo, Japan) was used at 30°C; the detection wavelength was 275 nm. The cumulative release of TP5 or Flu from the nanoparticles was calculated. The studies were performed in triplicate.
After the in vitro release study in pH 1.2 medium, a TP5-CG-PBCA-NP suspension was removed from the dialysis bag and centrifuged at 15,000 rpm for 30 minutes, and then the supernatant was discarded and the precipitate was washed with DD water and centrifuged once more. After that, the precipitate was resuspended in PBS (pH 6.8), and the release study was carried out in 0.05 mol/L PBS (pH 6.8) as above. The bioactivity of TP5 in TP5-CG-PBCA-NPs after the in vitro release study in pH 1.2 medium was detected by a T cell proliferation assay method.24 In brief, the release medium was withdrawn and diluted to 1 μg/mL of the TP5 test sample. The TP5 solution (1 μg/mL) was freshly prepared as a reference solution. Spleen cells were collected from BALB/c mice and treated with the test sample or TP5 reference solution with or without 10 μL concanavalin A (ConA, 50 μg/mL) for 48 hours. The cells without TP5 treatment were used as a blank control. Cell proliferation was examined by an MTT assay. The TP5 bioactivity was expressed as the stimulation index (SI) calculated as follows. The studies were performed in triplicate.
|
Stability of TP5 and TP5 nanoparticles in everted intestinal rings
To investigate the stability of free TP5 and TP5 nanoparticles in different bowels, the everted intestinal ring method was used.25 In brief, the duodenum, jejunum, ileum, and colon were cut into rings with a length of about 0.5 cm immediately after the Sprague Dawley rats were deeply anesthetized and sacrificed. The intestinal rings were rinsed with physiological saline and then placed into 10 mL of PBS (0.05 mol/L, pH 6.8) after reversing at 37°C for 20 minutes. After that, TP5 or TP5-loaded nanoparticles were added to the intestinal rings and incubated. At specified time intervals (5, 10, 20, 30, 45, and 60 minutes), 200 μL of the samples were withdrawn and mixed with equal volumes of 1 M perchloric acid and then assayed by HPLC to determine the amount of TP5 in the incubation sample compared with the initial TP5 concentration to calculate the residual percentage.
Ex vivo intestinal mucosa adhesion study
The Sprague Dawley rats were fasted for 24 hours but were allowed access to water freely, and they were then sacrificed after being anesthetized. The jejunum was rinsed with physiological saline and divided into several segments about 3 cm in length. The mucosa segments were cut lengthwise along the mesentery and spread on an aluminum plate. Then, the segments were fixed tightly to another aluminum plate with a 1.5 cm2 hole in the center to obtain the in vitro intestinal mucosa sample.
An aliquot (200 μL) of the fluorescent yellow-labeled nanoparticle suspension (Flu-PBCA-NPs, Flu-CTS-PBCA-NPs, and Flu-CG-PBCA-NPs) was accordingly added into the hole of the in vitro intestinal mucosa samples prepared above and maintained for 2 hours at room temperature while protected from light. Then, the nanoparticle suspension was removed gently, and the mucosal samples were rinsed thrice with 1 mL of physiological saline to remove unabsorbed nanoparticles. The mucous layer was scraped and dispersed into a 1% NaOH–2% sodium dodecyl sulfate mixed solution, after ultrasonication for 2 hours, and the samples were incubated at room temperature overnight until the mucous and nanoparticles dissolved completely. HPLC was used to detect the Flu content to compare the bioadhesion of the three nanoparticles in the intestinal mucosa. All operations were performed in triplicate.
In vivo mucoadhesion study
The mucoadhesion of uncoated PBCA NPs or CTS- and CG-coated NPs in the stomach and different segments of the intestinal tract was also estimated with fluorescent yellow as a probe. Eighteen Sprague Dawley rats (220±20 g) were divided into three groups randomly and fasted for 24 hours but had free access to water. An aliquot (1 mL) of fluorescent yellow-labeled nanoparticle suspensions (Flu-PBCA-NP, Flu-CTS-PBCA-NP, or Flu-CG-PBCA-NP) was orally administered accordingly. After oral administration, three rats from each of the groups were sacrificed at 1 and 2 hours, respectively, after anesthesia. The intestinal rings about 3 cm in length were cut from each intestinal segment (ie, the duodenum, upper jejunum, middle jejunum, lower jejunum, upper ileum, and lower ileum), and 2 mL of physiological saline was added and homogenized while the stomach was cutoff and placed in 10 mL of physiological saline. Five milliliters of the homogenate were dispersed into a 1% NaOH–2% SDS mixed solution and ultrasonicated for 2 hours. After dilution with the mobile phase, the samples were filtered through 0.22 μm of Millipore filtration. The content of the fluorescent yellow was determined by HPLC.
Statistical analysis
Mean values and standard deviations of the recorded parameters were calculated. The ANOVA followed by a post hoc Student–Newman–Keuls test (SPSS 13; SPSS Int., Chicago, IL, USA) was applied to compare the experimental groups and the corresponding controls. Significant differences between or among groups were indicated by *P<0.05, **P<0.01, and ***P<0.001.
Results and discussion
Preparation of TP5 nanoparticles
The TP5-PBCA-NPs prepared by modifying the emulsion polymerization of BCA monomers had a uniform appearance with blue opalescence. In our previous study, it was proved that the polymerization process of TP5-PBCA-NPs had no influence on the bioactivity of TP5 in the nanoparticles.26 CTS and CG have a positive charge which can adsorb to the surface of the TP5-PBCA-NPs that have a negative charge from electrostatic force. As shown in Figure 1, the incubation time affected the size, zeta potential, and encapsulation efficiency of the TP5-CTS-PBCA-NPs and TP5-CG-PBCA-NPs. The particle size became larger as the zeta potential increased when the TP5-PBCA-NPs were incubated with CTS or CG for a period of time. The particle sizes of both TP5-CTS-PBCA-NPs and TP5-CG-PBCA-NPs after a 30-minutes incubation period were apparently larger than those that were incubated for 15 minutes, but the encapsulation efficiency was reduced. However, when the incubation time was >30 minutes, the particle size, zeta potential, and encapsulation efficiency changed minimally. This result suggested that the adsorption balance was not achieved until a stable coating layer formed around the nanoparticles, indicating that a 30-minute incubation time was the optimized condition.
The thiol group percentage on the surface of the TP5-CG-PBCA-NPs was determined using Ellman’s reagent. The amount of free thiol groups was determined to be 277.4±12.1 μmol per gram conjugate. The thiol groups could form disulfide bonds with the mucoprotein, thus, enhancing the adhesive effect of the nanoparticles on the mucosa. According to the literature, a CTS–thiobutylamidine conjugate displaying 264 μmol thiol groups per gram of polymer presented a much higher mucoadhesion property in comparison to unmodified CTS;27 the higher the amount of immobilized thiol groups, the higher the mucoadhesive properties will be.28
Characteristics of TP5 nanoparticles
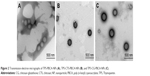
Transmission electron micrographs of TP5-PBCA-NPs, TP5-CTS-PBCA-NPs, and TP5-CG-PBCA-NPs are shown in Figure 2. The nanoparticles appeared as monodispersed spheres with a solid and consistent structure but were different in terms of their surface properties. The TP5-PBCA-NPs had a smooth surface, but the surfaces of the TP5-CTS-PBCA-NPs and TP5-CG-PBCA-NPs were rough with a black shadow layer, like a core–shell structure, which indicated that some of the soft material adhered to the surface of the TP5-PBCA-NPs.
The particle size and zeta potential were ascertained with a Malvern Mastersizer via dynamic light scattering, and the encapsulation efficiency was determined by the HPLC method. After being coated with the CTS or CG, the mean diameter increased from 212.3±6.9 nm (TP5-PBCA-NPs) to 274.6±8.2 and 310.4±7.5 nm for the TP5-CTS-PBCA-NPs and TP5-CG-PBCA-NPs, respectively, and the respective zeta potential changed from −22.6±0.76 mV (TP5-PBCA-NPs) to 23.3±1.2 and 34.6±1.6 mV, respectively. The results indicated that the positively charged CTS and CTS derivatives were successfully coated with the negatively charged PBCA-NPs, while the encapsulation efficiency of TP5-PBCA-NPs, TP5-CTS-PBCA-NPs, and TP5-CG-PBCA-NPs were 79.37%±2.15%, 74.21%±2.13%, and 72.65%±1.48%, respectively.
In vitro release studies and bioactivity assay
The cumulative release of TP5 from the TP5-CG-PBCA-NPs in different pH conditions is shown in Figure 3A. The in vitro release of TP5 from the nanoparticles presented pH dependence. In simulated gastric fluid (pH 1.2 HCl), the release of TP5 was very slow, only about 20% of the drug released from the nanoparticles at 2 hours, which was mainly attributed to the high affinity of TP5 with NPs under a low pH condition. The in vitro release of TP5 from the nanoparticles in a pH 1.2 medium was detected until 3 hours, because the gastric residence time of an oral dosage form is usually not >3 hours. The bioactivity of TP5 entrapped in the nanoparticles after the release experiment in pH 1.2 medium was determined by the classical T cell proliferation method. TP5 had a synergistic effect on the stimulation function of ConA to T cell proliferation. As shown in Figure 3B, the TP5 test sample had the same enhanced effect on the SI with a freshly prepared reference solution (P>0.05). The results indicated that TP5 entrapped in the nanoparticles could be protected from degradation in an acidic condition and maintain its bioactivity in the lower parts of the GIT. The TP5 release profiles from the nanoparticles at pH 5.0 and 6.8 were much faster. Especially in pH 6.8, about 67% of the TP5 was released at 3 hours and over an 80% cargo was released at 6 hours. The results indicated that TP5 could remain stable in the nanoparticles in an acidic condition, which could protect the drug from degradation when they pass through the stomach, and then the drug could release in the intestinal tract for absorption.
The cumulative release of fluorescein from the three kinds of nanoparticles is indicated in Figure 3C. The release of fluorescein from the uncoated nanoparticles was faster than that from CTS- or CG-coated nanoparticles, which indicated that a coating with CTS or CG slowed down the release of the drug loaded in the nanoparticles.
Stability of TP5 in the everted intestinal rings
The percentage of TP5 remaining after the incubation of free TP5, TP5-loaded PBCA nanoparticles, or CTS- or CG-coated TP5-PBCA nanoparticles with the everted intestinal rings at different time points is presented in Figure 4. TP5 was unstable in the GIT because of the abundance of digestive enzymes that exist in the intestinal mucosa layer, which would degrade the peptide. The free TP5 demonstrated a dissimilar degradation rate in different intestinal segments. As shown in Figure 4, free TP5 degraded almost completely after 30 minutes in the duodenum, but only about 50% of the TP5 degraded after 60 minutes in the colon. All formulations were more stable in the colon. The TP5 formulation stability in the different intestinal segments was as follows: colon > ileum > jejunum > duodenum. The results accounted for the variety and the quantity of digestive enzymes in the duodenum and jejunum being greater than in the ileum and colon. Additionally, the residual percentage of TP5 entrapped in the PBCA nanoparticles was higher than that of the aqueous solution, which indicates that using PBCA, a biocompatible and biodegradable polymer, to prepare nanoparticles can effectively improve the stability of TP5 in the GIT. Furthermore, when CTS or CG was used to coat the surface of the PBCA nanoparticles, the drug stability was further increased. This implies that CTS can form a layer around the nanoparticles, thereby providing better protection for the nanoparticles; more importantly, CG with antiprotease activity can effectively inhibit TP5 degradation.29
Ex vivo mucoadhesion study of TP5 NPs
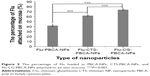
The percentage of fluorescein-labeled TP5-PBCA nanoparticles that adhered to ex vivo mucosa before or after coating with CTS or CG is shown in Figure 5. After 2 hours of attachment to the mucus layer, the mucus layer retention rate of CTS-coated nanoparticles was 1.48 times that of the uncoated nanoparticles, while the CG-coated nanoparticles had a higher retention rate (1.83 fold) than the uncoated nanoformulation. The release profile of Flu nanoparticles showed that some Flu may release from the uncoated nanoparticles (Figure 5) after 2 hours; however, the free Flu was easy to rinse out and had little impact on the results.
In vivo mucoadhesion study
The retentive characteristics of PBCA-NPs, CTS-PBCA-NPs, and CG-PBCA-NPs in the stomach and intestine after intragastrical administration are shown in Figure 6. Most nanoparticles were detained in the stomach at 1 hour and transported to the intestine at 2 hours. When compared to the 1 hour time interval, about 33% of PBCA-NPs, 24% of CTS-PBCA-NPs, and 21% of CG-PBCA-NPs were transferred from the stomach to the intestine after 2 hours. Uncoated PBCA-NPs were significantly faster than CTS-PBCA-NPs and CG-PBCA-NPs (P<0.05). However, the retentive characteristics of CTS-PBCA-NPs and CG-PBCA-NPs in the stomach had a small difference. Such differences were mainly caused by disulfide bonds formed by sulfhydryl groups in the molecular structure of CG, with the sticky protein thiol being weaker under the acidic conditions of the stomach and adhesion being based on the positively charged amino in the CTS main chain. Therefore, the CG-PBCA-NPs presented similar retentive characteristics as CTS-PBCA-NPs in the stomach.
The retentive characteristics of PBCA-NPs, CTS-PBCA-NPs, and CG-PBCA-NPs in the intestinal tract after intragastrical administration are depicted in Figure 7. One hour after intragastrical administration, CTS-PBCA-NPs and CG-PBCA-NPs were enriched from the duodenum to the middle portion of the jejunum, while the former stayed more in the middle jejunum, the latter was found in the upper jejunum. However, PBCA-NPs were mainly located in the lower jejunum at 1 hour. At 2 hours, the uncoated PBCA-NPs were delivered to the ileum and were mostly distributed in the upper ileum. At the same time, the CTS-PBCA-NPs were mainly located in the latter jejunum and upper ileum, and the CG-PBCA-NPs were mostly located in the middle and lower jejunum. The retentive distributions of the three nanoparticles showed marked differences. These results illustrated that bioadhesive materials coated onto the surface of the nanoparticles can enhance the retention effect of nanoparticles on the intestinal mucosa and delay the delivery rate. Moreover, the retention capacity of nanoparticles in the mucous could be further enhanced via the disulfide bonds formed between the sulfhydryls of CG and mucin. Therefore, CG-PBCA-NPs had the best retention capacity in the intestine.
Conclusion
In this study, a new bioadhesive nano-drug delivery system loaded with TP5 was prepared. The surfaces of the PBCA-NPs were coated with CTS or a CG derivative (Thio-CTS) via electrostatic adsorption to achieve a core–shell structure bioadhesive nanocarrier design. The particle size and zeta potential of the PBCA-NPs were obviously increased after being coated with CTS or its derivative. CG-coated nanoparticles can effectively improve the stability of peptide drugs in the digestive tract as well as enhance the mucosal adhesion ability and retention capacity of the nanoparticles in the intestinal tract, which could provide a promising nanocarrier for oral peptide drug delivery.
Acknowledgments
This research was supported by The National Natural Science Foundation of China (No 81872213, No 81202930), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (No 12KJB350002), the Research Foundation for Advanced Scholars of Jiangsu University (No 11JDG122), the China Postdoctoral Science Foundation (2017M610309), and the Zhejiang Provincial Natural Science Foundation of China (No LY17H310002). The Training Program for the Young Scholar of Jiangsu University and the Postgraduate Research and Practice Innovation Program of Jiangsu Province (Project number SJCX17_0584) are also acknowledged.
Disclosure
The authors report no conflicts of interest in this work.
References
Namdev N, Upadhyay S. Challenges and approaches for oral protein and peptide drug delivery. Res J Pharm Technol. 2016;9(3):305. | ||
Chu C, Tong SS, Xu Y, et al. Proliposomes for oral delivery of dehydrosilymarin: preparation and evaluation in vitro and in vivo. Acta Pharmacol Sin. 2011;32(7):973–980. | ||
Chen Y, Yuan L, Congyan L, et al. Antitumor activity of tripterine via cell-penetrating peptide-coated nanostructured lipid carriers in a prostate cancer model. Int J Nanomed. 2013;8:4339–4350. | ||
Zhang JX, Wang K, Mao ZF, et al. Application of liposomes in drug development – focus on gastroenterological targets. Int J Nanomedicine. 2013;8:1325–1334. | ||
Niu Z, Samaridou E, Jaumain E, et al. PEG-PGA enveloped octaarginine-peptide nanocomplexes: an oral peptide delivery strategy. J Control Release. 2018;276:125–139. | ||
Zhang HY, Firempong CK, Wang YW, et al. Ergosterol-loaded poly(lactide-co-glycolide) nanoparticles with enhanced in vitro antitumor activity and oral bioavailability. Acta Pharmacol Sin. 2016;37(6):834–844. | ||
Clumeck N, Cran S, van de Perre P, Mascart-Lemone F, Duchateau J, Bolla K. Thymopentin treatment in AIDS and pre-AIDS patients. Surv Immunol Res. 1985;4(Suppl 1):58–62. | ||
Singh VK, Biswas S, Mathur KB, Haq W, Garg SK, Agarwal SS. Thymopentin and splenopentin as immunomodulators. Current status. Immunol Res. 1998;17(3):345–368. | ||
Tischio JP, Patrick JE, Weintraub HS, Chasin M, Goldstein G. Short in vitro half-life of thymopoietin 32–36 pentapeptide in human plasma. Chem Biol Drug Des. 2010;14(5):479–484. | ||
Couvreur P. Polyalkylcyanoacrylates as colloidal drug carriers. Crit Rev Ther Drug Carrier Syst. 1988;5(1):1–20. | ||
Zhang MX, Hu J. Studies on aclacinomycin-A nanocapsules. J Chin Pharm Sci. 1994;3(2):103–109. | ||
Kolter M, Ott M, Hauer C, Reimold I, Fricker G. Nanotoxicity of poly(n-butylcyano-acrylate) nanoparticles at the blood-brain barrier, in human whole blood and in vivo. J Control Release. 2015;197:165–179. | ||
Kuo Y-C, Chung C-Y. Transcytosis of CRM197-grafted polybutylcyanoacrylate nanoparticles for delivering zidovudine across human brain-microvascular endothelial cells. Colloids Surf B Biointerfaces. 2012;91:242–249. | ||
Lin Y, Pan Y, Shi Y, Huang X, Jia N, Jiang JY. Delivery of large molecules via poly(butyl cyanoacrylate) nanoparticles into the injured rat brain. Nanotechnology. 2012;23(16):165101. | ||
Zhou Y, Peng Z, Seven ES, Leblanc RM. Crossing the blood-brain barrier with nanoparticles. J Control Release. 2018;270:290–303. | ||
Mohideen M, Quijano E, Song E, et al. Degradable bioadhesive nanoparticles for prolonged intravaginal delivery and retention of elvitegravir. Biomaterials. 2017;144:144–154. | ||
Ling Y, Zhen-Hai Z, Cao W, et al. Effect of cell-penetrating peptide-coated nanostructured lipid carriers on the oral absorption of tripterine. Int J Nanomed. 2012;7:4581–4591. | ||
Patel MP, Patel RR, Patel JK. Chitosan mediated targeted drug delivery system: a review. J Pharm Pharm Sci. 2010;13(4):536–557. | ||
Liu J, Li H, Chen D, et al. In vivo evaluation of novel chitosan graft polymeric micelles for delivery of paclitaxel. Drug Deliv. 2011;18(3):181–189. | ||
Bernkop-Schnürch A, Guggi D, Pinter Y. Thiolated chitosans: development and in vitro evaluation of a mucoadhesive, permeation enhancing oral drug delivery system. J Control Release. 2004;94(1):177–186. | ||
Werle M, Bernkop-Schnürch A. Thiolated chitosans: useful excipients for oral drug delivery. J Pharm Pharmacol. 2008;60(3):273–281. | ||
Bravo-Osuna I, Vauthier C, Farabollini A, Palmieri GF, Ponchel G. Mucoadhesion mechanism of chitosan and thiolated chitosan-poly(isobutyl cyanoacrylate) core-shell nanoparticles. Biomaterials. 2007;28(13):2233–2243. | ||
Jin X, Huang A, Ping Q, Cao F, Su Z. Box-Behnken optimization design and enhanced oral bioavailability of thymopentin-loaded poly (butyl cyanoacrylate) nanoparticles. Pharmazie. 2011;66(5):339. | ||
Chi Q, Xie X, Zhang J, Yang Y, Li Z, Mei X. Synthesis, characterization and biological activities of thymopentin ethyl ester. Pharmazie. 2008;63(11):784–787. | ||
Heizmann J, Langguth P, Biber A, Oschmann R, Merkle HP, Wolffram S. Enzymatic cleavage of thymopoietin oligopeptides by pancreatic and intestinal brush-border enzymes. Peptides. 1996;17(7):1083–1089. | ||
Jin X, Xu Y, Shen J, Ping Q, Su Z, You W. Chitosan–glutathione conjugate-coated poly(butyl cyanoacrylate) nanoparticles: promising carriers for oral thymopentin delivery. Carbohydr Polym. 2011;86(1):51–57. | ||
Roldo M, Hornof M, Caliceti P, et al. Mucoadhesive thiolated chitosans as platforms for oral controlled drug delivery: synthesis and in vitro evaluation. Eur J Pharm Biopharm. 2004;57(1):115–121. | ||
Bernkop-Schnürch A. Thiomers: a new generation of mucoadhesive polymers. Adv Drug Deliv Rev. 2005;57(11):1569–1582. | ||
Kafedjiiski K, Föger F, Werle M, Bernkop-Schnürch A. Synthesis and in vitro evaluation of a novel chitosan-glutathione conjugate. Pharm Res. 2005;22(9):1480–1488. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.