Back to Journals » International Journal of Nanomedicine » Volume 9 » Issue 1
Preparation and therapeutic evaluation of 188Re-thermogelling emulsion in rat model of hepatocellular carcinoma
Authors Shih Y, Lin X, Yeh C, Peng C , Shieh M, Lin W, Luo T
Received 16 April 2014
Accepted for publication 12 June 2014
Published 2 September 2014 Volume 2014:9(1) Pages 4191—4201
DOI https://doi.org/10.2147/IJN.S66346
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Ying-Hsia Shih,1,2 Xi-Zhang Lin,3 Chung-Hsin Yeh,1 Cheng-Liang Peng,1,2 Ming-Jium Shieh,2,4 Wuu-Jyh Lin,1 Tsai-Yueh Luo1,5
1Isotope Application Division, Institute of Nuclear Energy Research, Longtan, 2Institute of Biomedical Engineering, National Taiwan University, Taipei, 3Department of Internal Medicine, National Cheng Kung University, Tainan, 4Department of Oncology, National Taiwan University Hospital and College of Medicine, Taipei, 5Institute of Radiological Science, Central Taiwan University of Science and Technology, Taichung, Taiwan
Abstract: Radiolabeled Lipiodol® (Guerbet, Villepinte, France) is routinely used in hepatoma therapy. The temperature-sensitive hydrogel polyethylene glycol-b-poly-DL-lactic acid-co-glycolic acid-b-polyethylene glycol triblock copolymer is used as an embolic agent and sustained drug release system. This study attempted to combine the polyethylene glycol-b-poly-DL-lactic acid-co-glycolic acid-b-polyethylene glycol hydrogel and radio-labeled Lipiodol to form a new radio-thermogelling emulsion, rhenium-188–N,N’-1,2-ethanediylbis-L-cysteine diethyl-ester dihydrochloride–Lipiodol/hydrogel (188Re-ELH). The therapeutic potential of 188Re-ELH was evaluated in a rodent hepatoma model. Rhenium-188 chelated with N,N’-1,2-ethanediylbis-L-cysteine diethyl-ester dihydrochloride was extracted with Lipiodol to obtain rhenium-188–N,N’-1,2-ethanediylbis-L-cysteine diethyl-ester dihydrochloride–Lipiodol (188Re-EL), which was blended with the hydrogel in equal volumes to develop 188Re-ELH. The 188Re-ELH phase stability was evaluated at different temperatures. Biodistribution patterns and micro-single-photon emission computed tomography/computed tomography images in Sprague Dawley rats implanted with the rat hepatoma cell line N1-S1 were observed after in situ tumoral injection of ~3.7 MBq 188Re-ELH. The therapeutic potential of 188Re-EL (48.58±3.86 MBq/0.1 mL, n=12) was evaluated in a 2-month survival study using the same animal model. The therapeutic effects of 188Re-ELH (25.52±4.64 MBq/0.1 mL, n=12) were evaluated and compared with those of 188Re-EL. The responses were assessed by changes in tumor size and survival rates. The 188Re-ELH emulsion was stable in the gel form at 25°C–35°C for >52 hours. Biodistribution data and micro-single-photon emission computed tomography/computed tomography images of the 188Re-ELH group indicated that most activity was selectively observed in hepatomas. Long-term 188Re-ELH studies have demonstrated protracted reductions in tumor volumes and positive effects on the survival rates (75%) of N1-S1 hepatoma-bearing rats. Conversely, the 2-month survival rate was 13% in the control sham group. Therapeutic responses differed significantly between the two groups (P<0.005). Thus, the hydrogel enhanced the injection stability of 188Re-EL in an animal hepatoma model. Given the synergistic results, direct 188Re-ELH intratumoral injection is a potential therapeutic alternative for hepatoma treatment.
Keywords: 188Re-ECD-Lipiodol, hydrogel, hepatoma, thermogelling emulsion
Introduction
Hepatocellular carcinoma is a leading cause of death worldwide.1 The principal modes of cancer management are surgery, radiotherapy, and chemotherapy. In a typical hepatoma therapy, tumors are surgically removed and adjuvant radiotherapy or chemotherapy is suggested to kill the residual tumor cells. Therefore, in situ radiotherapy would be more effective when administered directly to the lesion. Great efforts have been made regarding radioisotope delivery for advancements in tumor radiotherapy. Controlled, targeted, and localized release technologies have shown great potential in cancer treatment.2–8 Recently, polymer molecular engineering has led to extensive studies on advanced polymeric materials and resulted in rather rapid progress in the field. Intelligent hydrogels or thermosensitive biodegradable polymers show great promise as implantable drug delivery systems and in tissue engineering applications.9,10 Polylactic acid, poly-ε-caprolactone, and polyglycolic acid are biocompatible, biodegradable polyesters approved by the US Food and Drug Administration for biomedical applications in humans. A synthetic triblock copolymer, polyethylene glycol-b-poly-DL-lactic acid-co-glycolic acid-b-polyethylene glycol (PEG-PLGA-PEG), drew attention as a potential injectable drug delivery depot.11,12 This polymer is reversibly thermoresponsive and undergoes phase transition from the liquid to gel form in response to an increase in temperature, characteristics that facilitate ease of preparation and administration.
Primary hepatocellular carcinoma (HCC) is the most common form of hepatic carcinoma, particularly in Asia and sub-Sahara Africa.13 Although surgical excision and ablation are usually considered the treatments of choice for focal lesions, the prognosis remains poor for many HCC patients.14 Other modalities such as transcatheter arterial embolization (TAE), chemotherapy, and external radiotherapy have demonstrated some level of efficacy. Lipiodol® (Guerbet, Villepinte, France) is a clinically available embolic agent for hepatoma treatment, and its use as a carrier for chemotherapeutic agents or radioisotopes is currently under investigation.15,16 Previously, it was shown that a 1:1 mixture of PEG-PLGA-PEG and Lipiodol could yield a stable thermogelling system for use in arterial embolization and sustained drug release. The PEG-PLGA-PEG solution had a small particle size (in the nanoscopic scale range) at low temperatures, but became larger with increasing temperature as described elsewhere.17 When the temperature exceeded transition temperature by 2°C–3°C, the PEG-PLGA-PEG solution would transform into hydrogel.18 The PEG-PLGA-PEG copolymers can self-assemble to form nanoscopic micellar structures consisting of a hydrophilic outer shell and hydrophobic inner core in aqueous medium; such polymers have been successfully used to encapsulate various poorly soluble agents as described in a previous study.19,20 The hydrophobic Lipiodol can be encapsulated in the hydrophobic inner core of micelles at low temperature. Internal radionuclide therapy can facilitate the delivery of high radionuclide doses to hepatic tumors while maintaining low activity levels in normal tissues. Radionuclides have been conjugated with monoclonal antibodies, iodized oils, and chemical compounds for the treatment of residual or unresectable HCC.15,21–24
Rhenium-188 (188Re), a γ- and β-emitter, is an emerging and promising radionuclide for clinical use in many settings. Because 188Re is available as an aqueous solution and Lipiodol is available as an oily solution, many chelating agents have been developed to link the two. Technetium-99m N,N’-1,2-ethanediylbis-L-cysteine diethyl-ester dihydrochloride (ECD) is an approved drug for clinical brain perfusion imaging.25 Previously, a method for labeling 188Re with ECD was established, followed by subsequent dissolution of the product in the Lipiodol phase to form 188Re-ECD-Lipiodol (188Re-EL).26 A report showed that 188Re-EL accumulated in hepatic tumors and was effective in treating xenotransplanted liver tumors in rats following in situ tumoral injection.27
This study attempted to combine PEG-PLGA-PEG with sol-gel characteristics with a therapeutic radiopharmaceutical to form a new radioactive thermogelling emulsion, 188Re-EL/hydrogel (188Re-ELH); it was than evaluated whether the hydrogel could potentially enhance the therapeutic effects of 188Re-ECD. A xenotransplanted rat liver tumor model was used to evaluate the therapeutic potential of 188Re-ELH when administered via in situ tumoral injection. In order to examine the effects of the hydrogel, the long-term survival effects of 188Re-ELH and 188Re-EL administered to the same animal model via the same injection method were also compared. It was found that the hydrogel synergistically enhanced the therapeutic effects of 188Re-EL in Sprague Dawley rats with N1-S1 cell hepatomas.
Materials and methods
A highly radionuclidic and radiochemically pure 188Re-perrhenate solution was eluted from a tungsten-188/188Re generator by the Institute of Nuclear Energy Research (Longtan, Taiwan).28 ECD was synthesized by the Institute of Nuclear Energy Research as described previously.29 Lipiodol, an iodized ethyl ester of a poppy seed oil fatty acid, was purchased from Guerbet. The PEG-PLGA-PEG triblock copolymer was prepared as described previously.30 All other chemicals were obtained from commercial sources.
Synthesis and characterization of PEG-PLGA-PEG triblock copolymer
Synthesis and characterizations of triblock copolymer PEG-PLGA-PEG have been described in detail elsewhere.18 The purified polymers were vacuum-dried overnight to avoid degradation.
The hydrogel solution was prepared by gradually dissolving the PEG-PLGA-PEG triblock copolymer in deionized water at a concentration of 30% (w/v). The PEG-PLGA-PEG hydrogel solution was mixed with an equal volume of 188Re-Lipiodol in a three-way extruder to obtain the radioactive thermogelling emulsions. To determine the phase diagram, sol-gel transition temperatures were recorded from 4°C–45°C according to the test tube inversion method. After thoroughly blending PEG-PLGA-PEG and Lipiodol at the designed ratio at 4°C, the vials containing the samples were immersed in water baths equilibrated at each given temperature for 15 minutes. The sol-gel transitions were examined by inverting the vials, and the samples were regarded as gels if no flow was observed within 30 seconds. The in vitro stability of the thermogelling emulsions was carefully monitored for 144 hours at room temperature.
Preparation of 188Re-ELH
188Re-EL was prepared as described previously.26 Briefly, the 188Re-ECD complex was generated by boiling ECD, tartaric acid, and stannous chloride with 188Re-perrhenate for 30 minutes. The chromatography ratio (Rf) of 188Re-ECD ranged from 0.6–0.8, and the levels of other impurities were within the normal range, as determined by thin layer chromatography (IB-F Silica-Gel [J.T. Baker, Center Valley, PA, USA], developed with ethyl acetate). Lipiodol was added to the 188Re-ECD reaction vial to extract the labeled 188Re-ECD into the Lipiodol phase. The radiochemical purity of 188Re-Lipiodol was determined according to the abovementioned method. A radio-TLC imaging scanner (AR-2000; Eckert & Ziegler Radiopharma Inc, Hopkinton, MA, USA) were used to analyze the radio thin layer chromatography plates. The PEG-PLGA-PEG hydrogel solution was mixed with an equal volume of 188Re-EL to constitute the radioactive thermogelling emulsion 188Re-ELH.
Biodistribution of 188Re-ELH in N1-S1 hepatoma-bearing rats
Animal experiments were conducted under humane conditions, with approval from the Animal Care Committee at the Institute of Nuclear Energy Research and the animal center at National Cheng Kung University (Tainan, Taiwan) and in accordance with the animal care guidelines set forth by the Agriculture Council of Taiwan.
Four-week-old male Sprague Dawley rats were fed a standard diet and given water ad libitum. Rats that had been orthotopically xenotransplanted with N1-S1 cells into the liver were used as cancer-bearing animals. The N1-S1 cell line was obtained from the Food Industry Research and Development Institute (Hsinchu, Taiwan). A cell suspension of 1.5×106 cells in a volume of 0.25 mL was injected slowly into one of the hepatic lobes under the liver capsule.
After 7 days of cell inoculations, the rats were anesthetized and laparotomized to check for tumor growth. Only rats with liver tumors and no peritoneal carcinomatosis were included in the study. The tumoral size (mm3) of each rat was recorded. A total of 32 tumor-bearing rats that received intratumoral injections of 188Re-ELH and 188Re-EL (approximately 3.7 MBq/0.1mL) were divided into three groups. At selected time points (1, 4, 24, and 48 hours), groups of three to four rats were sacrificed and radioactivity uptake in the tumor and normal tissues was measured using a gamma counter (Cobra series model 5003, Packard, CT, USA). Tissue distribution data were expressed as percentages of injected dose per gram (ID%/g).
Micro-single-photon emission computed tomography (SPECT)/computed tomography (CT) images were also obtained to evaluate the distribution and tumor targeting of 188Re-ELH in the rodent hepatoma model. The SPECT and X-ray CT images were acquired using a micro-SPECT/CT scanner system (X-SPECT®; Gamma Medica, Inc, Salem, NH, USA). The SPECT images were acquired using a low-energy, high-resolution collimator at 1 hour and 24 hours after the intratumoral injection of 188Re-ELH while mice were held in a steady position under inhaled isoflurane anesthesia (Abbott Laboratories, Abbott Park, IL, USA).
Long-term survival evaluation
Rats implanted with N1-S1 hepatoma cells (n=32) were divided into three groups to evaluate 2-month survival time; 12 rats in each group received intratumoral injections of either 188Re-ELH (25.52±4.64 MBq/0.1 mL) or 188Re-EL (48.58±3.86 MBq/0.1 mL), or were untreated (control group, n=8). These rats were closely observed for 60 days. At the end of the observation period, the tumor sizes of the surviving rats were measured and the animal livers and tumors were examined for tissue pathology.
Responses to the treatment were assessed according to the method proposed by Wang et al.31 In this method the survival period and tumor sizes before the treatment and at the end of the observation period were compared. Good response was defined as tumor disappearance or a decrease in the tumor volume by more than 10% at the end of the observation period compared to the pretreatment tumor volume. Responses that did not meet the criteria for a “good response” or did not result in a survival time of less than 60 days were defined as a “poor response”.
Statistics
Average tumor weights are expressed as mean ± standard deviation. The responses to treatment were analyzed using the chi-squared test. The survival curves for the two groups were generated using the Kaplan–Meier method. Significant survival differences between the two groups were analyzed using a nonparametric log-rank test. P≤0.05 was considered statistically significant.
Results
Preparation of 188Re-ELH
Based on the known PEG chain length (46.73 repeated units), the calculated molecular weight of PEG-PLGA-PEG was 3,927 g/mol, as measured by carbon-13 nuclear magnetic resonance, which was consistent with the data obtained using the gel permeation chromatography method (3,460 g/mole). The chemical structure of PEG-PLGA-PEG is shown in Figure 1. The property of hydrogel has been described in detail elsewhere.18 The PEG-PLGA-PEG polymer solution with high concentration of polymer presented the low gelling temperature. Phase transformation generally occurred between 20°C–30°C when polymer concentration was around 15–45 wt%. After blending with oily phase (Lipiodol) as emulsions, the formulation still preserved the thermogelling property. A sharp increase in viscosity reflected a quick gelling process. The viscosity of the formulation used in this study was about 400 cP when the temperature was kept below 15°C. It dramatically increased to more than 100,000 cP immediately once the temperature exceeded the transition temperature by 2°C–3°C.18 Therefore, this formulation was selected for use as the thermogelling emulsion system in future experiments because it gels very quickly at body temperature.
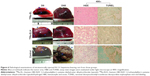
The radiochemical purities of 188Re-EL and 188Re-ELH, analyzed by the radio thin layer chromatography method, exceeded 94% in all cases. The thermogelling characteristics of the 188Re-ELH emulsion were tested from 4°C–45°C (Figure 2). The emulsion remained in the sol state from 4°C–24°C (Figure 2A). The environmental temperature was raised to the 25°C–40°C range, in which the 188Re-ELH emulsion transformed to the gel state (Figure 2B–C). When the temperature exceeded 40°C, the stability of the emulsion gradually decreased and oil separation occurred (Figure 2D). It was also found that the appearance of 188Re-ELH emulsion formulation remained stable for more than 52 hours at room temperature. There was only scant oil (Lipiodol) release from the thermogelling emulsion after 72 hours (data not shown). The 188Re release profile from 188Re-EL was evaluated as shown in Figure 2E. The 188Re release form 188Re-EL was fast at the early stage, and then followed with a low 188Re release rate after 24 hours. About 76% of 188Re was released from 188Re-EL to the phosphate-buffered saline buffer at 48 hours. However, less than 30% of the 188Re was released from the hydrogel within 60 hours. This sustained release profile of 188Re-ELH confirms the potential applicability of hydrogels as radiopharmaceutical carriers to increase the accumulation of therapeutic drug in the tumor site while at the same time minimizing the exposure of healthy tissues.
Biodistribution of 188Re-ELH in N1-S1 hepatoma-bearing rats
Biodistribution of radioactivity in tissues was determined by radioactivity counting and micro-SPECT/CT imaging after intratumoral administration of the 188Re-ELH emulsion. The micro-SPECT/CT image in Figure 3 shows that most radioactivity remained at the tumor site at 1 hour and 24 hours postinjection. The biodistribution data of the 188Re-ELH emulsion also indicated high tumor retention in hepatoma-bearing rats (Figure 4). The radioactivity level in the normal liver tissue (8.56±2.03 ID%/g at 1 hour; 2.19±0.31 ID%/g at 24 hours) was high but remained lower than that in the tumors (56.85±31.26 ID%/g at 1 hour; 37.48±41.76 ID%/g at 24 hours). Relatively high radioactivity concentrations were found in the kidneys. Some radioactivity was also detected in the spleen (1.96±0.24 ID%/g at 1 hour; 0.87±0.46 ID%/g at 24 hours) and lungs (5.33±1.44 ID%/g at 1 hour; 0.79±0.19 ID%/g at 24 hours). However, concentrations in the other organs were extremely low.
Long-term survival study
The average N1-S1 hepatoma size in rats’ livers for this experiment was 426±178 mm3. The 60-day survival study demonstrated that the 188Re-EL and 188Re-ELH groups showed protracted reductions in tumor volumes and positive effects in the xenotransplanted rat hepatoma model. In the group treated with 188Re-EL (48.58±3.86 MBq/0.1 mL, n=12), only one of the ten surviving rats had a residual tumor (16,415 mm3). The 2-month median survival time, survival rate, and good response rate were 54±14 days, 83% (10/12), and 75% (9/12), respectively (Table 1).
In the group treated with 188Re-ELH (25.52±4.64 MBq/0.1 mL, n=12), four of the nine surviving rats did not have residual tumors (residual tumor sizes were 3,899, 418, 107, and 20 mm3) and the median survival time was 49±19 days. Additionally, only one of the surviving rats treated with 188Re-ELH showed an increased tumor volume (3,899 mm3). The survival rate and good hepatoma response rate in this group were 75% (3/12) and 67% (8/12), respectively. In contrast, the survival rate and good response rate in the control group (n=8) were 13% (1/8) and 0% (0/8), respectively; in the control group, only one of the surviving rats had an increased tumor volume (7,602 mm3).
The good response of 188Re-ELH and 188Re-EL versus the control sham group showed significance difference (P<0.005). Relatively, 188Re-ELH versus 188Re-EL showed no difference between the two experiment groups (P>0.65).
The survival curves of these three groups are shown in Figure 5. The survival of N1-S1 hepatoma-bearing rats over a 60-day period in the 188Re-ELH and 188Re-EL groups versus the control sham group showed significance difference (P<0.001 and P<0.006, respectively). Nine and ten rats in the 188Re-ELH and 188Re-EL groups, respectively, survived over 60 days. At the end of the 60-day monitoring period, the surviving rats were sacrificed for pathological examination. The rats included this study all had hepatoma tumor in liver (Figure 6). Autopsies of the five surviving rats in the control sham group revealed large tumors with ascites and extensive cancer metastasis. After therapy, the 188Re-ELH and 188Re-EL groups were not found to have any tumor in liver. In contrast, rats with complete remission in the 188Re-ELH and 188Re-EL groups showed no definite residual tumors cells upon microscopic examination but were evident in the control sham group.
Discussion
Triblock PEG-PLGA-PEG copolymers are known to be biodegradable and biocompatible. Here, the PEG-PLGA-PEG hydrogel was blended with 188Re-EL to form a new radiothermosensitive gelling emulsion 188Re-ELH. The experimental results demonstrated that the long-term therapeutic effects of 188Re-EL were better than those of hydrogel used for hepatoma treatment in an animal model. However, it was also found that the therapeutic effect of 188Re-EL at the tumor site could be enhanced by the hydrogel.
Hepatoma is an important disease associated with poor treatment success rates in most cases. Therefore, the development of better treatment modalities is essential. Internal radionuclide therapy is an option for the treatment of unresectable HCC based on its ability to deliver high radiation doses to the hepatic tumor and low doses to other tissues. Yttrium-90 (90Y)-microsphere therapy is the most common approach for internal radiation therapy for primary or metastatic hepatic tumors.32–34 In this study, 188Re was complexed with ECD and then extracted in the Lipiodol/hydrogel phase. 188Re has β energy similar to that of 90Y (maximum energy: 2.12 MeV versus 2.28 MeV, respectively); it also emits γ rays (155 keV) useful for imaging. Free 90Y is known to show a predilection for accumulation in bone;31 however, perrhenate does not accumulate in the bone marrow and is rapidly cleared by the kidneys.35 Additionally, Lipiodol was found to retain the complex in the hepatoma and thus facilitate selective targeting of the tumor by 188Re, while at the same time minimizing leaching of the radioactive agent into the systemic circulation. This study has shown that 188Re-EL accumulates in the hepatic tumors and normal liver tissue following TAE. It has also shown that intratumoral injection of 188Re-EL could effectively prolong the survival rates in an animal hepatoma model.
A temperature-sensitive hydrogel PEG-PLGA-PEG presents as a free-flowing sol at room temperature; however, it becomes a transparent gel at body temperature.30 This characteristic makes it a promising drug delivery system candidate. A drug containing the PEG-PLGA-PEG solution, when directly injected into the bladder of Sprague Dawley rats, exhibited significant efficacy and few systemic side effects.36 Previous studies also reported that the thermogelling emulsion PEG-PLGA-PEG blended with Lipiodol retained the property of thermal responsiveness, which is beneficial to its use as an embolic agent and drug carrying depot.18 Here, it was shown that in situ 188Re-EL partly inhibited hepatoma growth in a Sprague Dawley rat model with an 83% survival rate compared to a 13% survival rate in rats that received normal saline treatment (P<0.05). Furthermore, the hepatoma rats treated with the radio-thermogelling emulsion 188Re-ELH had an enhanced long-term survival rate of 75%, which was also significantly better than that of the control group (P<0.05). The administered radioactivity in the 188Re-ELH group (25.52±4.64 MBq/0.1 mL) was approximately half of that in the 188Re-EL group (48.58±3.86 MBq/0.1 mL). Therefore, it was confirmed that PEG-PLGA-PEG promoted survival in N1-S1 hepatoma-bearing Sprague Dawley rats.
A previous study showed that a PEG-PLGA-PEG hydrogel provided a high hydrophilic phase proportion that allowed the drug to diffuse through the hydrogel matrix; the study also showed that the additional hydrophobic domain in the emulsion formulation resulted in a reduced burst release.18 Smaller amounts of the drug were initially released from the PEG shell, and this was followed by a combination of degradation and diffusion at a later stage.35 In the initial design, it was supposed that 188Re-EL would be stably retained in the PLGA core of the PEG-PLGA-PEG system. However, the biodistribution data of 188Re-EL (Figure 4) showed that radioactivity was rapidly washed out from the hepatoma site (4.72±2.37 ID%/g at 1 hour versus 0.33±0.26 ID%/g at 24 hours), comparing well with previous results using 188Re-ELH given via intratumoral injection (56.85±31.26 ID%/g at 1 hour versus 37.48±41.76 ID%/g at 24 hours) in the same animal model. In this study, the thermosensitive emulsion was prepared by thoroughly blending identical volumes of the hydrogel and 188Re-EL. The authors suggest that this emulsion formulation permits 188Re-EL to exist in a discontinuous oil form that is distributed within the hydrogel solution; the proposed structure is shown in Figure 3. The higher surface area of oil droplets in the emulsion would suggest an initial burst release of 188Re-ECD from the Lipiodol phase to the hydrophilic environment. This could explain the slightly better long-term survival rate for animals treated with 188Re-EL compared with animals of the same hepatoma model treated with 188Re-ELH via the same intratumoral injection method (83% versus 75%, respectively).
The estimated biodistribution of 188Re-ELH suggests that the kidneys and urinary bladder are the main organs of concern with regard to protection from radiation as most of the radioactivity was excreted through the renal system. Lipiodol is eliminated as two components: iodine, which is excreted through the renal system, and lipid, which is excreted through the biliary system.37 Although the exact mechanism has not yet been established, most of the excreted 188Re radioactivity was found in the bladder. The radioactivity to the urinary system could be reduced by hydration and more frequent bladder voiding after 188Re-ELH administration.
In this study, a single injection of 188Re-ELH was administered in a fixed volume to the tumor site. Although the rats treated with 188Re-ELH showed better response and 2-month survival rates than those of the control sham group, seven of the eight rats died due to tumors. Because the maximum β-ray penetration range of the 188Re isotope is 10.1 mm (mean 3.8 mm), the positive therapeutic effects depend on the tumor size and shape. Small tumors such as those described in this animal model might be sufficiently affected to be eliminated, even if radioactivity is applied only to the tumor center. However, tumors larger than 2 cm in diameter will not have sufficient penetration. Multiple injections might be more effective than a single injection for irregularly shaped tumors.38 Advance calculation of suitable intratumoral injection doses of 188Re-ELH will help increase good response rates for this hepatoma treatment.
While the results here are promising, investigations into other agents are also ongoing. Lipiodol, a clinically available TAE agent, has been labeled with iodine-131 and used to treat hepatoma.15,33 90Y-microsphere therapy was reported to be very promising and has been approved for hepatoma treatment.21,34,39 However, iodine-131 has the disadvantage of low β energy and high γ emission, and 90Y, if liberated into the circulatory system, could accumulate in the bones and result in high doses to the bone marrow.31
Transcatheter arterial infusion involving the use of radioisotopes such as 90Y or 188Re has shown encouraging results in several clinical trials.21,34,39–42 However, radionuclide administration via transcatheter arterial infusion has some disadvantages. The existence of arteriovenous shunts in tumors could lead to leakage of the radionuclides to the lung, which could result in pulmonary fibrosis. In one study, approximately 30% of iodine-131–Lipiodol administered via TAE accumulated in the lung region.43 A previous study also showed that if 188Re-EL is administered via hepatic artery injection, high concentration of radioactivity could be observed in the lungs at 24 hours.26 Although some radioactivity was observed in the lungs upon initial 188Re-ELH administration (Table 1), this rapidly decreased to background levels. Whether 188Re-ELH is administered via intratumoral injection or TAE, diagnostic lung scans should be performed in advance so that pulmonary fibrosis and other complications are prevented.
Conclusion
In this study, it was demonstrated that a 188Re-EL combined hydrogel could remain radionuclidic in a hepatoma tumor site and partly inhibit hepatoma growth. It was also found that 188Re-ELH could significantly enhance the therapeutic effects on survival and response rates in Sprague Dawley rats with xenotransplanted liver tumors. This new radio-thermogelling emulsion showed potential as a hepatoma treatment; thus, further validation in clinical trials is warranted.
Acknowledgments
This project was supported by grant ARA0401 from the Atomic Energy Council of Republic of China.
Disclosure
The authors report no conflicts of interest in this work.
References
Gonzalez SA, Keeffe EB. Risk assessment for hepatocellular carcinoma in chronic hepatitis B: scores and surveillance. Int J Clin Pract. 2012; 66(1):7–10. | ||
Li J, Quan H, Liu Q, Si Z, He Z, Qi H. Alterations of axis inhibition protein 1 (AXIN1) in hepatitis B virus-related hepatocellular carcinoma and overexpression of AXIN1 induces apoptosis in hepatocellular cancer cells. Oncol Res. 2013;20(7):281–288. | ||
Hong CW, Libutti SK, Wood BJ. Liposomal doxorubicin plus radio frequency ablation for complete necrosis of a hepatocellular carcinoma. Curr Oncol. 2013; 20(3):e274–e277. | ||
Dewhirst MW, Landon CD, Hofmann CL, Stauffer PR. Novel approaches to treatment of hepatocellular carcinoma and hepatic metastases using thermal ablation and thermosensitive liposomes. Surg Oncol Clin N Am. 2013;22(3):545–561. | ||
Kwon JH, Lee N, Park JY, et al. Actionable gene expression-based patient stratification for molecular targeted therapy in hepatocellular carcinoma. PLoS One. 2013;8(6):e64260. | ||
Zhang C, Wu X, Zhang M, et al. Small molecule R1498 as a well-tolerated and orally active kinase inhibitor for hepatocellular carcinoma and gastric cancer treatment via targeting angiogenesis and mitosis pathways. PLoS One. 2013;8(6):e65264. | ||
Johnson RP, Jeong YI, John JV, et al. Dual stimuli-responsive poly(N-isopropylacrylamide)-b-poly(L-histidine) chimeric materials for the controlled delivery of doxorubicin into liver carcinoma. Biomacromolecules. 2013;14(5):1434–1443. | ||
Yang R, Zhang S, Kong D, Gao X, Zhao Y, Wang Z. Biodegradable polymer–curcumin conjugate micelles enhance the loading and delivery of low-potency curcumin. Pharm Res. 2012;29(12):3512–3525. | ||
Peng CL, Shih YH, Liang KS, et al. Development of in situ forming thermosensitive hydrogel for radiotherapy combined with chemotherapy in a mouse model of hepatocellular carcinoma. Mol Pharm. 2013;10(5): 1854–1864. | ||
Zhang XJ, Ke LM, Yang J, et al. Development, characterization, and anti-tumor effect of a sequential sustained-release preparation containing ricin and cobra venom cytotoxin. Pharmazie. 2012;67(7):618–621. | ||
Jeong B, Bae YH, Kim SW. Thermoreversible gelation of PEG-PLGA-PEG triblock copolymer aqueous solutions. Macromolecules. 1999; 32(21):7064–7069. | ||
Jeong B, Bae YH, Kim SW. Drug release from biodegradable injectable thermosensitive hydrogel of PEG-PLGA-PEG triblock copolymers. J Control Release. 2000;63(1–2):155–163. | ||
Tabor E. Hepatocellular carcinoma: global epidemiology. Dig Liver Dis. 2001;33(2):115–117. | ||
Lin TY, Lee CS, Chen KM, Chen CC. Role of surgery in the treatment of primary carcinoma of the liver: a 31-year experience. Br J Surg. 1987;74(9):839–842. | ||
Park CH, Suh JH, Yoo HS, Lee JT, Kim DI. Evaluation of intrahepatic 131I ethiodol on a patient with hepatocellular carcinoma. Therapeutic feasibility study. Clin Nucl Med. 1986;11(7):514–517. | ||
Ohishi H, Uchida H, Yoshimura H, et al. Hepatocellular carcinoma detected by iodized oil. Use of anticancer agents. Radiology. 1985; 154(1):25–29. | ||
Gao X, Deng X, Wei X, et al. Novel thermosensitive hydrogel for preventing formation of abdominal adhesions. Int J Nanomedicine. 2013;8:2453–2463. | ||
Kan P, Lin XZ, Hsieh MF, Chang KY. Thermogelling emulsions for vascular embolization and sustained release of drugs. J Biomed Mater Res B Appl Biomater. 2005;75(1):185–192. | ||
Peng CL, Shieh MJ, Tsai MH, Chang CC, Lai PS. Self-assembled star-shaped chlorin-core poly(ε-caprolactone)–poly(ethylene glycol) diblock copolymer micelles for dual chemo-photodynamic therapies. Biomaterials. 2008;29(6):3599–3608. | ||
Peng CL, Lai PS, Lin FH, Yueh-Hsiu Wu S, Shieh MJ. Dual chemotherapy and photodynamic therapy in an HT-29 human colon cancer xenograft model using SN-38-loaded chlorin-core star block copolymer micelles. Biomaterials. 2009;30(21):3614–3625. | ||
Gulec SA, Mesoloras G, Dezarn WA, McNeillie P, Kennedy AS. Safety and efficacy of 90Y microsphere treatment in patients with primary and metastatic liver cancer: the tumor selectivity of the treatment as a function of tumor to liver flow ratio. J Transl Med. 2007;5:15–24. | ||
Sundram F. Radionuclide therapy of hepatocellular carcinoma. Biomed Imaging Interv J. 2006;2(3):e40. | ||
Liu L, Jiang Z, Teng GJ, et al. Clinical and experimental study on regional administration of phosphorous 32 glass microspheres in treating hepatic carcinoma. World J Gastroenterol. 1999;5(6):492–505. | ||
Häfeli UO, Casillas S, Dietz DW, et al. Hepatic tumor radioembolization in a rat model using radioactive rhenium (186Re/188Re) glass microspheres. Int J Radiat Oncol Biol Phys. 1999;44(1):189–199. | ||
Walovitch RC, Hill TC, Garrity ST, et al. Characterization of technetium-99m-L,L-ECD for brain perfusion imaging, part 1: pharmacology of technetium-99m ECD in nonhuman primates. J Nucl Med. 1989; 30(11):1892–1901. | ||
Luo TY, Hsieh BT, Wang SJ, et al. Preparation and biodistribution of rhenium-188 ECD/Lipiodol in rats following hepatic arterial injection. Nucl Med Biol. 2004;31(5):671–677. | ||
Luo TY, Shih YH, Chen CY, et al. Evaluating the potential of 188Re-ECD/Lipiodol as a therapeutic radiopharmaceutical by intratumoral injection for hepatoma treatment. Cancer Biother Radiopharm. 2009; 24(5):535–541. | ||
Luo TY, Lo AR, Hsieh BT, Lin WJ. A design for automatic preparation of highly concentrated 188Re-perrhenate solutions. Appl Radiat Isot. 2007;65(1):21–25. | ||
Blondeau P, Berse C, Gravel D. Dimerization of an intermediate during the sodium in liquid ammonium reduction of L-thiazolidine-4-carboxylic acid. Can J Chem. 1967;45:49–59. | ||
Jeong B, Bae YH, Kim SW. Biodegradable thermosensitive micelles of PEG-PLGA-PEG triblock copolymers. Colloids Surf B Biointerfaces. 1999;16(1–4):185–193. | ||
Wang SJ, Lo TY, Hsieh BT, et al. A new technique for labeling of Lipiodol with 188Re in the treatment of hepatic tumor. Journal of Radioanalytical and Nuclear Chemistry. 2004;261(1):189–193. | ||
Khodjibekova M, Szyszko T, Khan S, Nijran K, Tait P, Al-Nahhas A. Selective internal radiation therapy with yttrium-90 for unresectable liver tumors. Rev Recent Clin Trials. 2007;2(3):212–216. | ||
Raoul JL, Guyader D, Bretagne JF, et al. Prospective randomized trial of chemoembolization versus intra-arterial injection of 131I-labeled iodized oil in the treatment of hepatocellular carcinoma. Hepatology. 1997;26(5):1156–1161. | ||
Salem R, Lewandowski R, Roberts C, et al. Use of yttrium-90 glass microspheres (TheraSphere) for the treatment of unresectable hepatocellular carcinoma in patients with portal vein thrombosis. J Vasc Interv Radiol. 2004;15(4):335–345. | ||
Hayes RL. Chemistry and radiochemistry of metal-ion nuclides commonly employed in radiopharmaceuticals. In: Heindel ND, Burns HD, Honda T, Brady LW, editors. The Chemistry of Radiopharmaceuticals. New York, NY: Masson; 1978:155–168. | ||
Tyagi P, Li Z, Chancellor M, De Groat WC, Yoshimara N, Huang L. Sustained intravesical drug delivery using thermosensitive hydrogel. Pharm Res. 2004;21(5):832–837. | ||
Raoul JL, Bourguet P, Bretagne JF, et al. Hepatic artery injection of 131I-labeled Lipiodol. Part I. Biodistribution study results in patients with hepatocellular carcinoma and liver metastases. Radiology. 1988;168(2):541–545. | ||
Junfeng Y, Ruping Z, Xinlan D, et al. Intratumoral injection with (188Re)rhenium sulfide suspension for treatment of transplanted human liver carcinoma in nude mice. Nucl Med Biol. 2000;27(4):347–352. | ||
Gray B, Van Hazel G, Hope M, et al. Randomized trial of SIR-Spheres plus chemotherapy vs chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol. 2001; 21(12):1711–1720. | ||
Kumar A, Srivastava DN, Chau TT, et al. Inoperable hepatocellular carcinoma: transarterial 188Re HDD-labeled iodized oil for treatment – prospective multicenter clinical trial. Radiology. 2007;243(2):509–519. | ||
Sundram FX, Jeong JM, Zanzonico P, et al. Trans-arterial rhenium-188 Lipiodol in the treatment of inoperable hepatocellular carcinoma: an IAEA sponsored multi-center Phase I study. World J Nucl Med. 2002; 1(1):5–11. | ||
Bernal P, Raoul JL, Vidmar G, et al. Intra-arterial rhenium-188 Lipiodol in the treatment of inoperable hepatocellular carcinoma: results of an IAEA-sponsored multination study. Int J Radiat Oncol Biol Phys. 2007; 69(5):1448–1455. | ||
Nakajo M, Kobayashi H, Shimabukuro K, et al. Biodistribution and in vivo kinetics of iodine-131 Lipiodol infused via hepatic artery of patients with hepatic cancer. J Nucl Med. 1988;29(6):1066–1077. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.