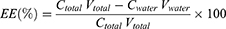Back to Journals » International Journal of Nanomedicine » Volume 16
Preparation and Evaluation of Lipid Emulsion Containing 13 Vitamins for Injection Without Anaphylactoid Reactions
Authors Hui MQ, Mi YN, Ma YF, Chen T, Cao YX
Received 24 November 2020
Accepted for publication 4 March 2021
Published 12 May 2021 Volume 2021:16 Pages 3317—3327
DOI https://doi.org/10.2147/IJN.S289596
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yan Shen
Min-Quan Hui,1,2,* Yan-Ni Mi,1,* Yu-Fan Ma,2 Tao Chen,2 Yong-Xiao Cao1
1Department of Pharmacology, Health Science Center, Xi’an Jiaotong University, Xi’an, People’s Republic of China; 2Xi’an Libang Pharmaceutical, Xi’an, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yong-Xiao Cao
Department of Pharmacology, Health Science Center, Xi’an Jiaotong University, 76 Yanta West Road, Xi’an, 710061, People’s Republic of China
Tel +86-29-8265-5140
Email [email protected]
Objective: Multivitamins containing Tween 80 can cause anaphylactoid reactions. The objective of this study was to develop a new lipid emulsion containing 13 fat- and water-soluble vitamins for injection (13V-LE) that were simultaneously dissolved in one bottle and to evaluate the stability of and anaphylactoid reactions to 13V-LE.
Methods: Particle size, ζ-potential, and polydispersity of 13V-LE were assayed with a Zetasizer Nano ZS. Entrapment efficiency of 13V-LE was determined with HPLC. Behavior, histamine, and blood pressure of beagle dogs were investigated by observation, fluorospectrophotometry, and sphygmomanometry.
Results: The 13V-LE with the smallest particles and highest entrapment efficiency with stable ζ-potential was composed of soybean oil, glycerin (2.25%, w:v), egg lecithin (1.2%, w:v), and purified water. There was no obvious change in characteristics of the 13V-LE samples in terms of appearance, size distribution, ζ-potential, pH value, or concentration over 6 months. In anaphylactoid reactions tests, when being administered with the multivitamin Infuvite Adult containing Tween 80, six beagles showed grade IV symptoms (P< 0.01 vs control), low blood pressure, and high plasma-histamine concentrations (P< 0.05 or P< 0.01). However, there were no significant differences in behavior, blood pressure, or histamine concentration in the dogs before and after administration in the 13V-LE group.
Conclusion: The 13V-LE formulation is a suitable intravenous lipid emulsion without anaphylactoid reactions.
Keywords: anaphylactoid reaction, multivitamins, lipid emulsion, beagle dog
Introduction
Adverse drug reactions cause increased patient morbidity and mortality, are a considerable source of financial burden to the health-care system, and remain a huge challenge in modern health care.1 Adverse drug reactions occur in approximately 17% of patients.2,3 Hypersensitivity reactions comprise approximately a third of adverse drug reactions. Anaphylactoid reactions represent >60% of hypersensitivity reactions.4 Avoiding adverse drug reactions, especially anaphylactoid reactions, is essential from both an economic and ethical standpoint.
Nutrition is the source of healthy growth and organism activity, and indispensable for the recovery of patients. The prevalence of malnutrition in patients can be as high as 50%.5–8 Malnutrition has become an independent risk factor of mortality, morbidity, and hospitalization in patients.9–14 Multivitamins are conducive to the ingestion of essential vitamins to maintain the body’s resistance and repair functions. Multivitamins are the most common supplement, and their use is increasing.15 Thirteen vitamins needed by the human body are mainly used in multivitamin preparations for parenteral nutrition support.16
The multivitamin Infuvite Adult is an injection containing 13 vitamins that has been widely used for years. The product comprises two bottles. Bottle 1 contains vitamins A, D, E, K, C, B1, B2, B6, nicotinamide, and dexpanthenol. Tween 80 (1.4%) is used to solubilize the fat-soluble vitamins A, D, E, and K, in bottle 1. Bottle 2 contains vitamin B12, biotin, folic acid, using 30% propylene glycol as solvent. The two bottles are mixed before clinical injection. However, a problem occurs with the use of Tween 80. There is increasing evidence that it can cause anaphylactoid reactions.17–19 Not surprisingly, multivitamins can produce severe and fatal anaphylactoid reactions.20–23 Tween 80 is not suitable as a solubilizing agent for intravenous preparations. In addition to Tween 80–induced anaphylactoid reactions, there are some difficulties in the study of multivitamin preparation. Fat-soluble vitamins are insoluble in water. It is difficult to dissolve fat- and water-soluble vitamins in the same system.
To solve these problems, a new lipid emulsion containing 13 fat- and water-soluble vitamins in one bottle (13V-LE) was developed in this study. Intravenous lipid emulsions were used to supply essential fatty-acids to patients with intestinal failure who needed parenteral nutrition in the US in the 1970s.24 Over the last 50 years, new lipid emulsions have been continuously produced with the goal of improving security and efficacy and realizing physiologically optimal formulations.24 In the present day, lipid emulsions have become a tool for systemic delivery of poorly soluble and cytotoxic drugs.25,26 13V-LE combines water-soluble and fat-soluble vitamins without Tween 80 and forms a cosoluble single freeze-dried compound, which could improve vitamins stability and avert hypersensitivity. The objective of this study was to evaluate the stability and the anaphylactoid reactions of 13V-LE.
Methods
Materials
Vitamins were purchased from Disiman Vitamin (Shanghai, China). Poloxamer 188 (Pluronic F68) was purchased from Shanghai Qingyuanxing Chemical Technology (Shanghai, China). Glycerol, oleate, soybean oil, and egg lecithin (PC 98%) was supplied by Xi’an Libang Pharmaceutical (Shaanxi, China) and α-Tocopherol purchased from Sigma-Aldrich (MO, USA).
Preparation
Formulation and optimized composition of 13V-LE were begun with filtration sterilization and ultrasonic treatment. Briefly, in the case of 13V-LE, fat-soluble vitamins and soybean oil were considered as oil phase. Egg lecithin and oleate acid were chosen as emulsifier. Water-soluble vitamins, glycerin, and purified water were considered as a water phase.
The new multivitamin 13V-LE contains nine water-soluble vitamins (B1, B2, B6, B12, C, nicotinamide, dexpanthenol, biotin, and folic acid) and four kinds of liposoluble vitamins (including vitamins A, D3, E, and K1). These are shown in Table 1. In order to solve the problem of the cosolubility of water-soluble and liposoluble vitamins in the same system, 16 emulsifier formulations were designed, as presented in Table 2. Various proportions of lecithin, F68, oleate, and Tween 80 were used as emulsifiers for the preparation of 13V-LE. Soybean oil was considered as a solvent of the oil phase. Glycerin (2.25%, w:v) and purified water were considered as a solvent of the water phase in the preparation of the different formulations. Based on physical appearance, particle size, ζ-potential, and entrapment efficiency (EE), an optimized formulation of emulsifiers was chosen.
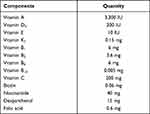 |
Table 1 Composition of 13V-LE |
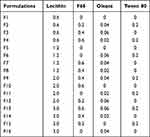 |
Table 2 Emulsifiers for 13V-LE (w:v, %) |
To prepare 13V-LE, egg lecithin was dissolved into dehydrated alcohol and agitated until dissolved with the addition of fat-soluble vitamins. Then, soybean oil was added in the dissolved solution and heated at 70°C until the mixture had become a clear and stable oil phase containing emulsifier. Glycerin and oleate acid were dissolved in water and heated at 70°C to form a water phase. After that, the water-phase solution was added slowly to the oil-phase solution containing the emulsifier. The coarse emulsion was prepared by high-speed shearing for 15 minutes at 70°C under the protection of nitrogen. The coarse emulsion was cooled to room temperature and adjusted to pH 5–7 with NaOH. The final emulsion was prepared with high-pressure homogenization (Niro Soavi NS1001L) at 1,000 bar for eight cycles at 40°C. After homogenization, the water-soluble vitamins were added to form 13V-LE, then freeze-dried after sterilization through a 0.22 µm bacteria-retentive filter.
Characterization of Formulations
Particle size, polydispersity, and ζ-potential of the 13V-LE were assayed with a Zetasizer Nano ZS (Malvern Instruments). The samples of 13V-LE were redissolved in 5 mL water, then diluted to 1:1,000 with water before measurement. The pH value of the 13V-LE was tested with a pH meter (PP-50 Professional Meter; Sartorius).
Entrapment Efficiency
The EE of the emulsions was assayed by vitamin concentration in the dispersion phase. 13V-LE was centrifuged at 50,000 rpm for 1.5 hours at 4°C by ultracentrifugation (OPTIMA L-100XP; Beckman Coulter), then collected in the water phase. Vitamin concentrations in the water phase were measured with HPLC. EE was calculated:
where Ctotal is the concentration of vitamin in the emulsion, Vtotal the volume of the emulsion, Cwater the concentration of vitamin in the water phase after ultracentrifugation, and Vwater the volume of water phase collected after ultracentrifugation.
Vitamin A was the most abundant and unstable in the oil phase among the vitamins of 13V-LE, so the stability of the formulation was determined by examining the encapsulation rate of vitamin A. The concentration of fat-soluble vitamin A was measured with HPLC using a Waters 2996 UV detector. The column was an Agilent XDB-C18 (4.6×150 mm, 5 μm), and the mobile phase was a mixture (methanol : isopropanol 95:5 v:v). The wavelength was 326 nm and the injection volume 10 µL. The flow rate of the mobile phase was 1.2 mL/min. The column temperature was 25°C. All measurements were repeated in triplicate.
Stability
13V-LE was stored at 40±2°C and at room temperature for 6 months. After that, the physical and chemical stability of these samples were evaluated with assays of appearance, polydispersity, particle-size distribution, ζ-potential, pH value, and drug remaining. All measurements were repeated three times. For appearance evaluation, if a lipid emulsion is stratified, aggregated, flocculated, produces small oil droplets on the surface, or shows other anomalous changes, it can be defined as “bad”. If a lipid emulsion is homogeneous and shows no anomalous changes, it can be defined as “good”.
Compatibility
Compatibility of the 13 vitamins with pharmaceutical excipients and that of 13V-LE with different infusion solutions were investigated. Vitamins were mixed with different pharmaceutical excipients, as shown in Supplementary Table 1. These mixtures were exposed to high temperature (60°C), damp (90%±5%), light of 4,500±500 lx for 10 days. The content of vitamins and related substances were tested with HPLC. To test compatibility with different infusion solutions, samples of 13V-LE were redissolved in 5 mL water for injection, then diluted to 500 mL with 0.9% sodium chloride solution, 5% glucose injection, and fatemulsion injection. The osmotic pressure of these mixtures was measured with an Osmomat-3000D (Gonotec).
Anaphylactoid Reaction Tests
Animals and Treatments
Six beagle dogs (three male, three female) weighing 8–12 kg were purchased from Xi’an Dilepu Biology Resources (Shaanxi, China). They were housed in separate cages and allowed access to food and water ad libitum in an environment at room temperature with 40%–70% humidity, and a day–night cycle of 12–12 hours. The dogs were handled in accordance with the criteria outlined in the Guide for Care and Use of Laboratory Animals. All experimental protocols were approved by the Animal Ethics Committee at Xi’an Jiaotong University (2017–0045).
13V-LE and Infuvite Adult are compound vitamin injections. The content of each vitamin in the injections is different and the units of measurement inconsistent. For the convenience of research and calculation, vitamin C was used as a marker of 13V-LE and Infuvite Adult to calculate the dose. The dose of vitamin C in 13V-LE and Infuvite Adult for an adult is 200 mg/day. When human body weight is set at 70 kg, the dose of vitamin C is 2.9 mg/kg. The dose-converse factor of dog:person - is 1.85. Therefore, the equivalent dose of vitamin C for a dog is 5.3 mg/kg. In this study, 6 mg/kg vitamin C was selected as the dose for the dogs.
There were three groups in the anaphylactoid reaction tests: control (normal saline), 13V-LE, and Infuvite Adult. On the first day of the dosing regimen, all six beagles received intravenous saline injections as the control group. Then, all dogs were divided into two groups of three animals each. Two groups of dogs were cross-injected with Infuvite Adult and 13V-LE on the second and fourth days. On the second day, dogs 1–3 were intravenously injected with Infuvite Adult and dogs 4–6 intravenously injected 13V-LE. On the fourth day, dogs 1–3 were intravenously injected with 13V-LE dogs 4–6 dogs intravenously injected with Infuvite Adult (Table 3). Blood samples were collected in tubes with heparin before and 10 minutes after administration. Samples were centrifuged for 10 minutes, then plasma collected and stored at –20°C until analysis.
 |
Table 3 Modes of administration in anaphylactoid reaction tests |
Behavioral Observation
Before and after administration, dogs’ eyes, mouth, neck, abdomen, limbs, skin, behavior, gait, and body coordination were observed for 30 minutes for anaphylactoid reactions. Levels of reaction were assessed using the criterion in Supplementary Table 2.
Plasma-Histamine Concentration
Concentrations of plasma histamine were measured with fluorescence spectroscopy. Ethyl alcohol (300 µL) was added to plasma samples of 100 µL for deproteinization. The supernatant was tested after vortex mixing and centrifugation at 10,000 g. Sample supernatants (100 μL) were added to 0.5 M NaOH solution (40 μL) and 2.5 μg/mL o-phthalaldehyde (20 μL) and incubated at 37°C for 30 minutes. Subsequently, 10 μL 3 M HCl solution was added to terminate the reaction. The excitation wavelength was 365 nm and the emission wavelength 465 nm. The fluorescence intensity of samples was detected with a fluorescence plate reader, and the standard curve was drawn, and then histamine concentrations of samples were calculated.
Blood-Pressure Detection
Blood pressure was measured with an OMRON HEM-8611 at 0, 10, 20, and 30 minutes after administration of drugs. Dogs were put in the decubitus position without anesthesia and the blood-pressure monitor put on the arm. Measurements were repeated in triplicate at each time point.
Statistical Analysis
SPSS 11.5 was used for statistical analyses. All data are shown as means ± SE. P<0.05 was considered statistically significant. ANOVA with least significant difference was used to analyze quantitative data in multiple groups, including blood pressure at different time points. Student’s t-test was used to analyze quantitative data between two groups, such as concentrations of histamine. The rank-sum test was used to analyze ordinal data, such as grades of behavior, in the anaphylactoid-reaction tests.
Results and Discussion
Composition and Stability of 13V-LE
HPLC
Representative chromatograms were shown in Supplementary Figure 1. The peak of vitamin A was eluted within about 33 minutes under the analytical conditions described. The reproducibility of the detection was studied with repeated injections in triplicate samples containing vitamin A at three concentrations (2, 3, and 4 µg/mL). Relative standard deviation was <0.3%. The relative recovery of vitamin A at 2, 3, and 4 µg/mL was 101.8%±0.0%, 102.3%±0.0%, and 103.0%±0.0%, respectively (n=3). The standard curve of vitamin A concentration was obtained: y=3.401x+73.986, R2=0.9994.
Composition of 13V-LE
In this study, a new formulation of intravenous lipid emulsions was developed to solve the problem of cosolvability of water-soluble vitamins (B1, B2, B6, B12, C, nicotinamide, dexpanthenol, biotin, and folic acid) and liposoluble vitamins (A, D3, E, and K1) in the same system. The appearance, ζ-potential, particle-size distribution, and EE of 13V-LE in the different emulsifiers (lecithin, F68, oleate, and Tween 80) were assessed to select the optimal formulation of 13V-LE. In the emulsified state, 13V-LE was in an oil-in-water state, and the four liposoluble vitamins (A, D3, E, and K1) were encased in oil in the lipid emulsion. Commonly, only the EE of encased vitamins needs to be evaluated. Vitamin A was the most abundant and unstable vitamin in the oil phase in 13V-LE. Therefore, it was was chosen as a representative to evaluate the stability of the formulation by examining its EE. As shown in Table 4, F1, F4, F6, F9, F10, F13, and F14 were inferior formulations with poor appearance, such as oil drops, stratification, or flocculation. 13V-LE particle size increased with increasing lecithin content. When emulsifiers contained 0.6% lecithin, F68, oleate, and Tween 80 in F2 and F3, this contributed to emulsion formation, but with low EE. Particles of F7, F8, F11, F12, F13 and F14 containing F68 or oleate were larger than those of F5 containing lecithin. When an emulsifier contained only 1.2% lecithin, the emulsion had the smallest particles and highest EE of vitamin A with stable ζ-potential (F5). Therefore, egg lecithin 1.2% (w:v) was chosen as an emulsifier.
 |
Table 4 Effects of different emulsions on characterization of 13V-LE |
Before freeze-drying, 13V-LE is an oil-in-water lipid emulsion. The new multivitamin 13V-LE contains 3,300 IU (990 μg) vitamin A, 200 IU vitamin D3, 10 IU (9.1 mg) vitamin E, 0.15 mg vitamin K1, 200 mg vitamin C, 6 mg vitamin B1, 3.6 mg vitamin B2, 6 mg vitamin B6, 40 mg nicotinamide, 15 mg dexpanthenol, 0.005 mg vitamin B12, 0.06 mg biotin, 0.6 mg folic acid, soybean oil, egg lecithin (1.2%, w:v), glycerin (2.25%, w:v), NaOH, and water. Fat-soluble vitamins and soybean oil were considered as oil phase, egg lecithin was chosen as an emulsifier, and water-soluble vitamins, glycerin, and water were considered as water phase in 13V-LE.
Characterization of Lipid Emulsions
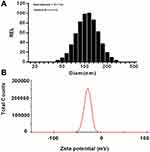
The 13V-LE dispersion, composed of soybean oil, glycerin, egg lecithin, 13 vitamins, and purified water, was analyzed in terms of size and polydispersity. As shown in Figure 1A, the mean diameter of 13V-LE was <200 nm, which is suitable for parenteral administration. Polydispersity is a dimensionless parameter of size distribution for broadness. The lower the polydispersity, the narrower the distribution of lipid emulsion. The mean polydispersity of 13V-LE was <0.2, showing that 13V-LE had proper delivery.
ζ-potential is a crucial parameter of emulsion stability. It determines the size of electrostatic repulsion among particles, which affects the aggregation of particles and the stability of a lipid emulsion. Generally, ζ-potential ranges from –20 mV to –45 mV, while that of 13V-LE was –27.7±2.1 mV, suggesting that the emulsions were stable (Figure 1B).
Stability of 13V-LE
13V-LE was stored at 25°C and 40°C for 6 months, then its stability was studied by monitoring size and polydispersity. Table 5 shows that the appearance, particle-size distribution, pH value, ζ-potential, and vitamin A content of the 13V-LE samples underwent no significant change in 6 months, suggesting that 13V-LE had favorable stability. Severe test, accelerated, and long-term storage tests also showed that 13V-LE had favorable stability (Supplementary Table 3–5).
 |
Table 5 Physical stability of 13V-LE during 6 months (n=3) |
Multivitamins/minerals refers to any supplement containing three or more vitamins or minerals, but no herbs, hormones, or drugs, with each component at a dose less than the tolerable upper level determined by the US Food and Nutrition Board, ie, the maximum daily intake likely to pose no risk of adverse health effects.27 The content of the vitamins in 13V-LE is the same as that in Infuvite Adult. The differences between 13V-LE and Infuvite Adult are their form and pharmaceutic adjuvants. Tween 80 is used to solubilize the fat-soluble vitamins in Infuvite Adult. In order to avoid Tween 80–induced anaphylactoid reactions, a lipid emulsion was chosen to be the drug-carrier system for 13V-LE. Lipid emulsions have been a significant component of parenteral nutrition and play a crucial role in supplying calories, providing necessary fatty acids that cannot be endogenously synthesized, and more importantly improving safety and efficacy and achieving physiologically optimal formulations.24 Soybean oil, which has high phytosterol content, was the triglyceride source of the first lipid emulsion manufactured and successfully administered intravenouly.28 Egg lecithin, a natural phospholipid, is used in oral, dermal, and parenteral products, including oil-in-water emulsions as an emulsifier.29 Soybean oil, egg lecithin, and glycerin formed a stable and homogeneous drug-carrier system for 13V-LE, with high ζ-potential and low polydispersity. Many vitamins, such as vitamin C, are sensitive and easily lost during handling, processing, and storage.30 Loss of vitamin C during storage is lower in freeze-dried powder than vacuum, tunnel-dried, or fresh powder.31,32 Cernevit is also a cosoluble single freeze-dried compound containing nine water-soluble vitamins and three liposoluble vitamins without vitamin K. Freeze-dried technology is conducive to the stability of vitamins. Our testing suggested that 13V-LE had good stability.
Compatibility of 13V-LE
The compatibility of vitamins with the pharmaceutical excipients soybean oil, egg lecithin, glycerin, F68, Tween 80, and oleate was investigated. The content of each vitamin and the related substances did not change significantly and was within the range of standard requirements, suggesting that the vitamins had good compatibility with these excipients (Supplementary Table 6). The 13V-LE was mixed with 0.9% sodium chloride solution, 5% glucose injection, and fat-emulsion injection. None of these mixtures showed anomalous changes in appearance, such as stratification, aggregation, and flocculation. The osmotic pressure of these mixtures were 290.3±0.9 Osm/kg, 292.7±0.9 Osm/kg, and 301.7±1.8 Osm/kg, respectively (Table 6). The osmotic pressure of human blood is 285–310 Osm/kg. The range of osmotic pressure required for intravenous preparations should be the same as the osmotic pressure of blood in the body. The results of osmotic pressure suggested that 13V-LE had good compatibility with different infusion solutions. The drug-carrier system of 13V-LE consisting of soybean oil, egg lecithin, and glycerin is similar to that of propofol, which is an intravenous anesthetic and Vitalipid, which is a fat-soluble injection containing vitamins A, D, E, and K1.33 These products have good stability and compatibility with parenteral nutrition admixtures.
 |
Table 6 Osmotic pressure of 13V-LE mixed with different infusion solutions (n=3) |
Anaphylactoid Reactions
Alterations in Behavior
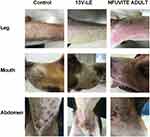
The behavior of the dogs was observed in anaphylactoid reaction tests. The six beagles of the saline group did not show any abnormal behavior within 30 minutes after administration (Table 7 and Figure 2). In the 13V-LE group, there was no abnormal behavior after intravenous infusion of 13V-LE. Within 30 minutes after intravenous administration of Infuvite Adult, the six dogs displayed grade IV symptoms, such as nose and head scratching and ear flicking (>3 minutes), multiple skin and mucous rubeosis, skin rash, tachypnea, instability of gait, diarrhea, pawing the ground, rubbing the ground, rollingover, somnolence, hypodynamia, and wheezing. Figure 2 show the influence of 13V-LE on the skin of the dogs.There was multiple skin and mucous rubeosis and skin rash in dogs who had been administered Infuvite Adult. Anaphylactoid reactions were grade IV with a mean score of 25.5±3.1, which was obviously higher than the control and 13V-LE groups (P<0.01, Table 7). These results indicated that 13V-LE did not induce any anaphylactoid reaction, but Infuvite Adult did induce anaphylactoid reactions triggered by Tween 80.
 |
Table 7 Anaphylactoid reaction tests |
Alterations in Blood Pressure
Before administration of Infuvite Adult, systolic and diastolic blood pressure of the dogs were 122±3 mmHg and 69±3 mmHg, respectively. Systolic pressure decreased to 93±4 mmHg at 10 minutes after drug administration, which was significantly lower than systolic pressure before administration (P<0.01). Diastolic pressure was 58±5 mmHg, and there was a declining trend. Systolic pressure at 20 and 30 minutes after administration were 86±7 mmHg and 81±8 mmHg, respectively, which significantly decreased (P<0.01). Diastolic pressure was also lower than before administration (P<0.01). Obviously, Infuvite Adult caused a decline of blood pressure.
There was no obvious alteration in blood pressure within 30 minutes after the intravenous administration of 13V-LE. In the 13V-LE group, systolic and diastolic pressure were 119±2 mmHg and 68±3 mmHg, respectively, before administration. Systolic pressure was 117±2 mmHg, 112±4 mmHg, and 112±3 mmHg at 10, 20, and 30 minutes, respectively, after intravenous administration of 13V-LE. Diastolic pressure was 71±2 mmHg, 68±4 mmHg, and 64±4 mmHg, respectively. Systolic and diastolic pressure did not show significant differences when compared with before administration (Figure 3). These results further demonstrated that 13V-LE had no influence on blood pressure, but Infuvite Adult did.
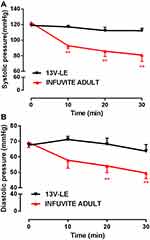 |
Figure 3 Effect of compound vitamins on blood pressure in beagle dogs. (A) Systolic pressure; (B) diastolic pressure. Means ± SE, n=6. **P<0.01 vs before administration. |
Alterations in Plasma-Histamine Concentration
In the Infuvite Adult group, plasma-histamine concentration increased from 3.1±0.5 μg/mL to 5.7±0.9 μg/mL after administration, an increase of 45.6% (P<0.05). In the 13V-LE group, there was no significant difference in plasma histamine concentration between before and after administration (P>0.05, Table 8). This demonstrated that 13V-LE did not increase plasma histamine, but Infuvite Adult induced an increase in plasma histamine, which is involved in the inflammatory response and itching.
 |
Table 8 Effect of multivitamins on plasma histamine |
Anaphylactoid reaction, also referred to pseudoallergy or nonallergic drug hypersensitivity, has clinical manifestations that are indistinguishable from anaphylaxis.34 Behavioris considered an important visual and intuitive method to determine anaphylactoid reactions. Beagle dogs are more sensitive and anaphylactoid reactions in them more easily observable than in mice and guinea pigs.35 This is the reason that Beagle dogs were used to investigate anaphylactoid reactions to 13V-LE. From the perspective of animal ethics, we try to minimize the number of animals. Therefore, we reused the dogs on the premise that the accuracy of the results would not be affected. From a pharmacokinetic perspective, 97% of a medicine can be eliminated after five elimination half-lives. In fact, multivitamins cannot be sufficiently cleared from the body after 48 hours, because some vitamins have a long half-life. For example, vitamins C, D3, E have half-lives of approximately 10, 15, and 70–90 hours, respectively.36,37 However, this had no influence on the anaphylactoid reaction results in this study. Firstly, anaphylactoid reaction is an immediate and transient reaction, and symptoms last for only a few hours. Anaphylactoid reaction is characterized by immediate and transient degranulation of mast cells. Clinical manifestations usually occur within 15 minutes of parenteral injection of the causative agent, reach the maximum usually within 60 minutes, and usually disappear after a few hours.38,39 Anaphylactoid reactions to Infuvite Adult disappeared after about 30 minutes. Secondly, the anaphylactoid reactions to Infuvite Adult were induced by Tween 80, but not by the various vitamins. The plasma concentration of Tween 80 was <0.05% (v:v) within 15 minutes after drug administration.40 From a pharmacokinetic perspective, Tween 80 can be sufficiently cleared in the body after 48 hours. Thirdly, in the same group, the results of behavioral observation and plasma histamine concentration on the second day were consistent with the results on the fourth day. Therefore, reuse of dogs after 48 hours had no influence on the anaphylactoid reaction test.
Manifestations of anaphylaxis and anaphylactoid reactions are continuous. Weak cutaneous signs, such as rhinorrhea, flushing, and pruritus, often occur at the start.41 These symptoms in dogs are scratching the nose or head, flinging the ears, sneezing, coughing, skin or mucous rubeosis, and sialism. The manifestations further aggravate with gastrointestinal symptoms, such as nausea, vomiting, and diarrhea.41 In more serious cases, hypotension, laryngeal obstruction, severe bronchospasm, shock, and asphyxiation occur.41 Anaphylactoid reaction is related to the activation of mast cells via non-IgE–mediated degranulation.34 Mast cells can immediately respond to stimulants by degranulation releasing granule-associated mediators, such as histamine, lysosomes, and TNF, into the extracellular milieu, inducing immediate allergic reactions.42 Increases in plasma- or tissue-histamine levels have been noted during anaphylaxis and anaphylactoid reactions.41 Infuvite Adult containing Tween 80 induced anaphylactoid reactions, with serious symptoms (such as pruritus, skin rash, tachypnea, instability of gait, diarrhea, somnolence, hypodynamia, and wheezing), hypotension and high plasma-histamine levels. These reactions were similar to vitamin K1 injections, with both containing Tween 80.17 However, behavior, blood pressure, and plasma histamine did not show obvious change in the dogs following administration of 13V-LE, suggesting that it did not induce anaphylactoid reactions.
Conclusion
13V-LE is a novel multivitamin. In this formulation, fat-soluble vitamins and soybean oil were considered as oil phase, egg lecithin was chosen as an emulsifying agent, and water-soluble vitamins, glycerin, and water were considered as water phase. The formulation of 13V-LE displayed high EE with stable ζ-potential, uniform distribution, and small particles, suitable for parenteral administration. Moreover, 13V-LE did not induce anaphylactoid reactions. This formulation of 13V-LE has promise as a new approach to avert hypersensitivity to multivitamins, and to simultaneously improve the stability of vitamins and dissolve water-soluble vitamins and fat-soluble vitamins.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (81803647), China Postdoctoral Science Foundation (2017M613155), and Shaanxi Province Postdoctoral Science Foundation (2018BSHEDZZ94). We would like to thank Jing Liu and Pingping Yan at the Department of Pharmacology, Xi’an Jiaotong University College of Medicine for their kind help and support with the experiments.
Disclosure
The authors report no conflicts of interest.
References
1. Coleman J, Pontefract SK. Adverse drug reactions. Clin Med (Northfield Il). 2016;16(5):481–485. doi:10.7861/clinmedicine.16-5-481
2. Bouvy JC, De Bruin ML, Koopmanschap MA. Epidemiology of adverse drug reactions in Europe: a review of recent observational studies. Drug Saf. 2015;38(5):437–453. doi:10.1007/s40264-015-0281-0
3. Lisha J, Annalakshmi V, Maria J, Padmini D. Adverse drug reactions in critical care settings: a systematic review. Curr Drug Saf. 2017;12(3):147–161. doi:10.2174/1574886312666170710192409
4. Bohm R, Cascorbi I. Pharmacogenetics and predictive testing of drug hypersensitivity reactions. Front Pharmacol. 2016;7:396. doi:10.3389/fphar.2016.00396
5. Niedert KC. Position of the American Dietetic Association: liberalization of the diet prescription improves quality of life for older adults in long-term care. J Am Diet Assoc. 2005;105(12):1955–1965.
6. Bharadwaj S, Ginoya S, Tandon P, et al. Malnutrition: laboratory markers vs nutritional assessment. Gastroenterol Rep. 2016;4(4):272–280. doi:10.1093/gastro/gow013
7. Malone A, Hamilton C. The Academy of Nutrition and Dietetics/the American Society for Parenteral and Enteral Nutrition consensus malnutrition characteristics: application in practice. Nutr Clin Pract. 2013;28(6):639–650. doi:10.1177/0884533613508435
8. Mulasi U, Kuchnia AJ, Cole AJ, Earthman CP. Bioimpedance at the bedside: current applications, limitations, and opportunities. Nutr Clin Pract. 2015;30(2):180–193. doi:10.1177/0884533614568155
9. Benoist S, Brouquet A. Nutritional assessment and screening for malnutrition. J Visc Surg. 2015;152(Suppl 1):S3–S7. doi:10.1016/s1878-7886(15)30003-5
10. Hu W-H, Cajas-Monson LC, Eisenstein S, Parry L, Cosman B, Ramamoorthy S. Preoperative malnutrition assessments as predictors of postoperative mortality and morbidity in colorectal cancer: an analysis of ACS-NSQIP. Nutr J. 2015;14. doi:10.1186/s12937-015-0081-5
11. Mahgoub HM, Adam I. Morbidity and mortality of severe malnutrition among Sudanese children in New Haifa Hospital, Eastern Sudan. Trans R Soc Trop Med Hyg. 2012;106(1):66–68. doi:10.1016/j.trstmh.2011.09.003
12. Martin AN, Das D, Turrentine FE, Bauer TW, Adams RB, Zaydfudim VM. Morbidity and mortality after gastrectomy: identification of modifiable risk factors. J Gastrointestinal Surg. 2016;20(9):1554–1564. doi:10.1007/s11605-016-3195-y
13. Munthali T, Jacobs C, Sitali L, Dambe R, Michelo C. Mortality and morbidity patterns in under-five children with severe acute malnutrition (SAM) in Zambia: a five-year retrospective review of hospital-based records (2009–2013). Arch Public Health. 2015;73. doi:10.1186/s13690-015-0072-1
14. Caccialanza R, Cereda E, Klersy C. Malnutrition, age and inhospital mortality. CMAJ. 2011;183(7):826. doi:10.1503/cmaj.111-2038
15. Macpherson H, Pipingas A, Pase MP. Multivitamin-multimineral supplementation and mortality: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2013;97(2):437–444. doi:10.3945/ajcn.112.049304
16. American Medical Association.Multivitamin preparations for parenteral use - statement by the nutrition advisory group. J Parenteral Enteral Nutr. 1979;3(4):258–262. doi:10.1177/014860717900300410
17. Mi YN, Ping NN, Xiao X, Zhu YB, Liu J, Cao YX. The severe adverse reaction to vitamin k1 injection is anaphylactoid reaction but not anaphylaxis. PLoS One. 2014;9(3):e90199. doi:10.1371/journal.pone.0090199
18. Sun WW, Li YK, Zhang JY. [Anaphylactoid reactions inducing effect of polysorbate 80 and polysorbate 80 contained Houttuynia cordata injection on beagle]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2011;31(1):90–93. Chinese.
19. Coors EA, Seybold H, Merk HF, Mahler V. Polysorbate 80 in medical products and nonimmunologic anaphylactoid reactions. Ann Allergy Asthma Immunol. 2005;95(6):593–599. doi:10.1016/S1081-1206(10)61024-1
20. Hernandez CR, Ponce EC, Busquets FB, et al. Hypersensitivity reaction to components of parenteral nutrition in pediatrics. Nutrition. 2016;32(11–12):1303–1305. doi:10.1016/j.nut.2016.04.010
21. Bartels CL, Sanz C, Stec R, Coulter DW. Parenteral nutrition-induced hypersensitivity in an adolescent. JPEN J Parenter Enteral Nutr. 2012;36(1):117–121. doi:10.1177/0148607111399288
22. Orfan NA, Dykewicz MS, Rosand J, Bertino J. anaphylaxis induced by folate in a multivitamin. J Allergy Clin Immunol. 1995;95(1):371.
23. Christian VJ, Tallar M, Walia CLS, Sieracki R, Goday PS. Systematic review of hypersensitivity to parenteral nutrition. J Parenteral Enteral Nutr. 2018;42(8):1222–1229. doi:10.1002/jpen.1169
24. Fell GL, Nandivada P, Gura KM, Puder M. Intravenous lipid emulsions in parenteral nutrition. Adv Nutr. 2015;6(5):600–610. doi:10.3945/an.115.009084
25. Zhang X, Wu B. Submicron lipid emulsions: a versatile platform for drug delivery. Curr Drug Metab. 2015;16(3):211–220. doi:10.2174/138920021603150812124221
26. Tayeb HH, Sainsbury F. Nanoemulsions in drug delivery: formulation to medical application. Nanomedicine. 2018;13(19):2507–2525. doi:10.2217/nnm-2018-0088
27. Health N. National Institutes of Health State-of-the-Science Conference Statement: multivitamin/mineral supplements and chronic disease prevention. Am J Clin Nutr. 2007;85:257S–264S.
28. O S, W A. Intravenous infusion of fat emulsions, phosphatides and emulsifying agents. Acta Chir Scand Suppl. 1961;278(1–12).
29. van Hoogevest P, Wendel A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur J Lipid Sci Technol. 2014;116(9):1088–1107. doi:10.1002/ejlt.201400219
30. Sk L, Aa K. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol Technol. 2000;20:207–220. doi:10.1016/S0925-5214(00)00133-2
31. M V, S J, K D, M V, Gk R. Effects of various dehydration methods and storage on physicochemical properties of guava powder. J Food Sci Technol. 1993. doi:10.1007/s13197-013-1020-0
32. Rahman MS, Al-Rizeiqi MH, Guizani N, Al-Ruzaiqi MS, Al-Aamri AH, Zainab S. Stability of vitamin C in fresh and freeze-dried capsicum stored at different temperatures. J Food Sci Technol. 2015;52(3):1691–1697. doi:10.1007/s13197-013-1173-x
33. Folino TB, Muco E, Safadi AO, Parks LJ. Propofol, in StatPearls. Treasure Island (FL); 2020.
34. Farnam K, Chang C, Teuber S, Gershwin ME. Nonallergic drug hypersensitivity reactions. Int Arch Allergy Immunol. 2012;159(4):327–345. doi:10.1159/000339690
35. Mi YN, Ping NN, Xiao X, Wang C, Zhu YJ, Cao YX. A sensitive beagle dog model to evaluate anaphylactoid reactions. Allergologie. 2015;38(10):491–497. doi:10.5414/ALX011794
36. Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88(2):582S–586S. doi:10.1093/ajcn/88.2.582S
37. Schwedhelm E, Maas R, Troost R, Boger RH. Clinical pharmacokinetics of antioxidants and their impact on systemic oxidative stress. Clin Pharmacokinet. 2003;42(5):437–459. doi:10.2165/00003088-200342050-00003
38. Levy JH, Roizen MF, Morris JM. Anaphylactic and anaphylactoid reactions. A review. Spine. 1986;11(3):282–291. doi:10.1097/00007632-198604000-00017
39. Ring J, Behrendt H. Anaphylaxis and anaphylactoid reactions. Classification and pathophysiology. Clin Rev Allergy Immunol. 1999;17(4):387–399. doi:10.1007/BF02737644
40. van Tellingen O, Beijnen JH, Verweij J, Scherrenburg EJ, Nooijen WJ, Sparreboom A. Rapid esterase-sensitive breakdown of polysorbate 80 and its impact on the plasma pharmacokinetics of docetaxel and metabolites in mice. Clin Cancer Res. 1999;5(10):2918–2924.
41. Rusznak C, Peebles RS
42. Wernersson S, Pejler G. Mast cell secretory granules: armed for battle. Nat Rev Immunol. 2014;14(7):478–494. doi:10.1038/nri3690
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.