Back to Journals » International Journal of Nanomedicine » Volume 11
Preparation and evaluation of a self-nanoemulsifying drug delivery system loaded with Akebia saponin D–phospholipid complex
Authors Shen J, Bi J, Tian H, Jin Y, Wang Y, Yang X, Yang Z, Kou J, Li F
Received 18 March 2016
Accepted for publication 12 July 2016
Published 26 September 2016 Volume 2016:11 Pages 4919—4929
DOI https://doi.org/10.2147/IJN.S108765
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
Jinyang Shen,1 Jianping Bi,2 Hongli Tian,1 Ye Jin,1 Yuan Wang,3 Xiaolin Yang,4 Zhonglin Yang,1 Junping Kou,5 Fei Li1
1State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing, 2Shandong Provincial Traditional Chinese Medical Hospital & Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, 3Traditional Chinese Medical Hospital of Pukou District, 4Key Laboratory of Pharmaceutical and Biological Marine Resources Research and Development of Jiangsu Province, Nanjing University of Chinese Medicine, 5Jiangsu Key Laboratory of TCM Evaluation and Translational Research, Department of Complex Prescription of TCM, China Pharmaceutical University, Nanjing, People’s Republic of China
Background: Akebia saponin D (ASD) exerts various pharmacological activities but with poor oral bioavailability. In this study, a self-nanoemulsifying drug delivery system (SNEDDS) based on the drug–phospholipid complex technique was developed to improve the oral absorption of ASD.
Methods: ASD–phospholipid complex (APC) was prepared using a solvent-evaporation method and characterized by infrared spectroscopy, differential scanning calorimetry, morphology observation, and solubility test. Oil and cosurfactant were selected according to their ability to dissolve APC, while surfactant was chosen based on its emulsification efficiency in SNEDDS. Pseudoternary phase diagrams were constructed to determine the optimized APC-SNEDDS formulation, which was characterized by droplet size determination, zeta potential determination, and morphology observation. Robustness to dilution and thermodynamic stability of optimized formulation were also evaluated. Subsequently, pharmacokinetic parameters and oral bioavailability of ASD, APC, and APC-SNEDDS were investigated in rats.
Results: The liposolubility significantly increased 11.4-fold after formation of APC, which was verified by the solubility test in n-octanol. Peceol (Glyceryl monooleate [type 40]), Cremophor® EL (Polyoxyl 35 castor oil), and Transcutol HP (Diethylene glycol monoethyl ether) were selected as oil, surfactant, and cosurfactant, respectively. The optimal formulation was composed of Glyceryl monooleate (type 40), Polyoxyl 35 castor oil, Diethylene glycol monoethyl ether, and APC (1:4.5:4.5:1.74, w/w/w/w), which showed a particle size of 148.0±2.7 nm and a zeta potential of -13.7±0.92 mV after dilution with distilled water at a ratio of 1:100 (w/w) and good colloidal stability. Pharmacokinetic studies showed that APC-SNEDDS exhibited a significantly greater Cmax1 (733.4±203.8 ng/mL) than ASD (437.2±174.2 ng/mL), and a greater Cmax2 (985.8±366.6 ng/mL) than ASD (180.5±75.1 ng/mL) and APC (549.7±113.5 ng/mL). Compared with ASD, Tmax1 and Tmax2 were both remarkably shortened by APC-SNEDDS. The oral bioavailability in rats was enhanced significantly to 183.8% and 431.8% by APC and APC-SNEDDS, respectively.
Conclusion: These results indicated that APC-SNEDDS was a promising drug delivery system to enhance the oral bioavailability of ASD.
Keywords: Akebia saponin D, phospholipid complex, self-nanoemulsifying drug delivery systems, oral bioavailability
Introduction
Akebia saponin D (ASD; Figure 1) is a triterpenoid saponin isolated from the rhizome of Dipsacus asper Wall. It has been reported to possess anti-osteoporotic, hepatoprotective, neuroprotective, and cardioprotective effects.1–4
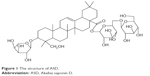  | Figure 1 The structure of ASD. |
However, ASD is poorly absorbed when administrated orally. The oral bioavailability of ASD in rats is only 0.13%.5 The poor oral absorption of ASD could mainly result from the low intestinal permeability,6 efflux mediated by MRP,6 metabolism in the intestine,7 excretion from the bile,8 and elimination in the liver. Therefore, it is necessary to develop a dosage form to overcome these absorbing barriers.
Some oral drug delivery systems have been employed to improve oral absorption of hydrophilic saponins, including water-in-oil (W/O) microemulsion9 and oils.10,11 It seemed that a W/O microemulsion was suitable for ASD. However, phase inversion happened frequently in vivo when the W/O microemulsion formulation was diluted with gastrointestinal fluid, leading to the leakage of compounds into the aqueous media and loss of absorption enhancement.12 In oils, some hydrophobic esters and their hydrolysis products by pancreatic lipase could significantly promote intestinal absorption of poorly absorbed drugs.10,11,13 Nevertheless, emulsions would form when these hydrophobic esters are diluted with gastrointestinal fluid, leading to a longer release time and a delayed absorption of drugs in vivo,10,11 which is undesired for any drug.
Until now, some phytochemicals have been loaded into oral nanoparticles to improve their aqueous solubility, permeation, stability, circulation time, target specificity, and bioavailability.14,15 The common biodegradable and biocompatible nanoparticles include nanoemulsions, nanoliposomes, micelles, lipid nanocarriers, and poly(lactic-co-glycolic acid) nanoparticles.16–20 Self-nanoemulsifying drug delivery system (SNEDDS) is an anhydrous homogenous liquid mixture consisting of oil, surfactant, cosurfactant, and drug, which spontaneously forms oil-in-water nanoemulsion of <200 nm in size upon dilution with water under gentle stirring.21 SNEDDS improves drug delivery by improving permeability or transport of poorly permeable drugs, attenuating the activity of intestinal efflux transporters, preventing degradation of drugs in physiological milieu, and facilitating absorption of drugs via intestinal lymphatic pathway.21 Due to these advantages, SNEDDS was applied to improve oral absorption of ASD in this study.
Until now, some pharmaceutical excipients, which are commonly used in pharmaceutical formulations, have been identified as potential inhibitors of MRP. For example, Cremophor® RH 40 (Polyoxyl 40 hydrogenated castor oil) and Cremophor® EL (Polyoxyl 35 castor oil) have been proved to possess inhibitory effects on MRP.22 Polyoxyl 40 hydrogenated castor oil and Polyoxyl 35 castor oil are nonionic surfactants which are considered as less toxic than other types of surfactants and less influenced by ionic strength and pH change. Therefore, these two excipients were considered for incorporation in SNEDDS to increase the intestinal absorption of ASD.
SNEDDS has been widely employed as a delivery system for poorly water-soluble drugs.23–26 As a hydrophilic compound, ASD cannot be directly entrapped into SNEDDS without any change. Previously, a phospholipid-based strategy was established to develop SNEDDS for several hydrophilic drugs (matrine and insulin), in which phospholipid complexes were formulated to significantly enhance the liposolubility of water-soluble drugs and drastically facilitate their incorporation into SNEDDS.12,27 Oral absorption of matrine and insulin was significantly improved by this special formulation. In this study, a phospholipid complex was employed to improve the liposolubility of ASD and facilitate the incorporation of ASD into SNEDDS.
Therefore, the objective of the present study was to develop a novel SNEDDS based on the phospholipid complex technique to enhance the oral bioavailability of ASD. Hopefully, this study could provide not only a promising oral formulation of ASD for its clinical application but also references for further study of other hydrophilic saponins.
Materials and methods
Materials
ASD (95.23%, high-performance liquid chromatography purity) was a pilot product prepared by our laboratory. Its purity was calibrated by the standard substance purchased from the National Institutes for Food and Drug Control (Beijing, People’s Republic of China). Phospholipid (91.4% phosphatidylcholine) was purchased from Beijing Yuan Hua Mei Lecithin Sci-Tech Co., Ltd. (Beijing, People’s Republic of China). Labrafac Lipophile WL 1349, Labrafil M 1944 CS, Peceol (Glyceryl monooleate [type 40]), Maisine 35-1, Capryol 90, and Transcutol HP (Diethylene glycol monoethyl ether) were kindly supplied by Gattefossé (Saint-Priest Cedex, France). Olive oil, oleic acid, ethyl oleate, polyethylene glycol 400, and 1,2-propanediol were purchased from Nanjing Chemical Reagent Co., LTD. (Nanjing, People’s Republic of China). Polyoxyl 40 hydrogenated castor oil and Polyoxyl 35 castor oil were donated by BASF (Ludwigshafen, Germany). Diazepam (purity ≥98%) was purchased from Aladdin Industrial Corporation (Shanghai, People’s Republic of China). All the other chemicals were of analytical or chromatography grade.
Preparation of ASD–phospholipid complex
The ASD–phospholipid complex (APC) was prepared with ASD and phospholipids at a molar ratio of 1:3. Weighed amount of ASD (0.6500 g) and phospholipids (1.5750 g) was put in a 100 mL conical flask, and 50 mL of ethanol was added. The mixture was stirred with a magnetic agitator (Wanfeng Instrument Co., Ltd., Jintan, People’s Republic of China) at 50°C for 2 hours. The resultant clear solution was concentrated to dry under nitrogen at room temperature. Later, the residue was dried under vacuum for 36 hours to obtain APC, and APC was transferred into a desiccator and stored at room temperature.
Characterization of APC
The interactions between ASD and phospholipids were studied using Fourier transform infrared (FT-IR) spectroscopy and differential scanning calorimetry (DSC). The FT-IR spectra of ASD, phospholipid, APC, and physical mixture of ASD and phospholipids were recorded in the range of 4,000–400 cm−1 with a resolution of 4 cm−1 using a Bruker tensor 27 FT-IR spectrometer (Bruker Corporation, Billerica, MA, USA). DSC analyses were carried out on a Netzsch DSC 204 F1 phoenix differential scanning calorimeter (Netzsch, Selb, Germany). The samples were heated from 40°C to 350°C at a heating rate of 10°C/min under a nitrogen purge gas flow of 20 mL/min.
The morphology and surface characteristics of ASD and APC were examined by a Hitachi S-3000N scanning electron microscope (Hitachi Ltd., Tokyo, Japan) at an accelerated voltage of 20 kV.
Determination of ASD content in APC
The content of ASD in APC was determined by HPLC method. The analysis was performed on an Agilent 1260 HPLC system equipped with an ODS-C18 column (150×4.6 mm, 5 μm) (Agilent Technologies, Santa Clara, CA, USA). The mobile phase was acetonitrile:water (35:65, v/v) at a flow rate of 1 mL/min. Column temperature was maintained at 30°C, and detection wavelength was set at 212 nm.
Solubility studies
Solubility studies in water and n-octanol were conducted to verify whether the liposolubility of ASD was improved after formation of APC. Briefly, excess amount of ASD, APC, and physical mixture of ASD and phospholipids was added to 2 mL water or n-octanol in sealed penicillin bottles at 37°C, respectively. The samples were shaken for 24 hours and then centrifuged at 10,012× g for 10 minutes. Later, the supernatants were collected and diluted with methanol. The concentration of ASD was assayed by the aforementioned HPLC method.
Solubility studies of APC in eight kinds of oils, two types of nonionic surfactants, and three types of cosurfactants were carried out to optimize proper excipients with maximum solubilization, and to obtain optimum drug loading. Briefly, an excess amount of APC was introduced into 1 mL of each excipient, and the mixtures were kept in sealed penicillin bottles. The samples were shaken for 24 hours at 37°C and then centrifuged at 10,012× g for 10 minutes. The concentration of ASD in supernatant was determined by the aforementioned HPLC method.
Selection of surfactant
The emulsification efficiency was investigated to select the proper surfactant between Polyoxyl 40 hydrogenated castor oil and Polyoxyl 35 castor oil. At a certain weight of oil (Glyceryl monooleate [type 40]), each of the surfactants was mixed with selected cosurfactant (Diethylene glycol monoethyl ether) at ratios of 4:1, 3:1, and 2:1. The experimental arrangement of formulations is shown in Table 1. Each formulation was dispersed into the distilled water at a ratio of 1:100 (w/w) with gentle magnetic stirring. The spontaneous self-emulsifying properties, appearance, and dispersibility of each formulation were visually assessed based on the five grading systems28 presented in Table 2.
  | Table 1 Experimental arrangement of formulations |
  | Table 2 Visual assessment of efficiency of self-emulsification |
Construction of pseudoternary phase diagram
The pseudoternary phase diagram was used to identify the self-nanoemulsifying region of the selected system with different drug loading. On the basis of studies conducted, Glyceryl monooleate (type 40), Polyoxyl 35 castor oil, and Diethylene glycol monoethyl ether were selected as the oil, surfactant, and cosurfactant, respectively. A series of formulations with varying concentrations of Glyceryl monooleate (type 40) (0%–20%, w/w), Polyoxyl 35 castor oil (20%–50%, w/w), Diethylene glycol monoethyl ether (20%–50%, w/w), and APC (0%–15%, w/w) were prepared. Each sample was sonicated until clear liquid mixture appeared and then added dropwise into distilled water at a ratio of 1:100 (w/w) with gentle magnetic stirring. Through investigating the tendency to emulsify and observing the dispersibility and appearance, it was easy to differentiate between the nanoemulsion and the simple emulsion. The self-nanoemulsifying regions were plotted on pseudoternary phase diagrams.
Characterization of APC-SNEDDS
The droplet size (number-weighted) and zeta potential of APC-SNEDDS diluted with distilled water (1:100, w/w) were measured by dynamic light scattering using a BI-90 Plus particle size analyzer (Brookhaven Instruments Corporation, Holtsville, NY, USA). These parameters were repeatedly measured for three times.
The morphology of APC-SNEDDS was observed by a Hitachi H-600 transmission electron microscope (Hitachi Ltd.). Briefly, APC-SNEDDS was diluted with distilled water at a ratio of 1:100 (w/w), and a drop of sample was placed on a copper grid. After removal of excess liquid, the sample was negatively stained by 2% phosphomolybdic acid and then subjected to observation under a transmission electron microscope.
APC-SNEDDS stability analysis
The influences of pH and volume of the diluents on the droplet size of APC-SNEDDS were evaluated by diluting with deionized water, pH 1.2 simulated gastric fluid (0.1 M HCl), and pH 6.8 simulated intestinal fluid (phosphate-buffered saline) to 50-, 100-, and 1,000-fold, respectively. The droplet size was measured using a BI-90 Plus particle size analyzer (Brookhaven Instruments Corporation) after 10 minutes of dilution.
The thermodynamic stability of APC-SNEDDS was evaluated using a heating–cooling test at 40°C and −4°C for 48 hours and six refrigerator cycles. The formulation was assessed for the changes in particle size, cracking, creaming, and phase separation.
All these experiments were repeated for three times.
Pharmacokinetic studies in rats
The animal studies were approved by the Animal Ethics Committee of China Pharmaceutical University (Nanjing, People’s Republic of China). Sprague Dawley rats (body weight 200–220 g, n=18) were obtained from the Zhejiang Animal Breeding Center (Hangzhou, People’s Republic of China). All rats were divided randomly into three groups (half male and half female in each group) and fasted for 12 hours but granted free access to water prior to experiment. ASD, APC, and APC-SNEDDS at a dose equivalent to 90 mg/kg of ASD were dispersed in distilled water and orally administrated to the three groups of rats (n=6), respectively.
Blood samples (250 μL) were collected from the eye ground veins pre-dose and at the predetermined time points (0.167, 0.333, 0.5, 1, 3, 5, 7, 9, 11, 14, 18, 24, 30, and 36 hours post-dosing). The plasma samples were collected after centrifugation (1,112× g, 10 minutes) and stored at −20°C until analysis.
An aliquot of 100 μL rat plasma was mixed with 280 μL methanol and 20 μL internal standard (IS; diazepam, 700 ng/mL). The mixture was vortex-mixed for 5 minutes and then centrifuged at 10,012× g for 10 minutes. The supernatant (300 μL) was transferred to a centrifuge tube and evaporated to dryness. The obtained residue was reconstituted in 150 μL methanol, and an aliquot of 8 μL was used for HPLC–tandem mass spectrometry (MS/MS) analysis.
The HPLC–MS/MS analysis was carried out on an Agilent 1260 liquid chromatograph (Agilent Technologies) with an Agilent 6420 triple quadrupole mass spectrometer (Agilent Technologies) equipped with an electrospray source (model G1956B). Chromatographic separation was performed on a Hedera ODS-2 column (2.1×150 mm, 5 μm) (Hanbon Science and Technology, Huaian, People’s Republic of China) with a security Guard-C18 column (4.6×12.5 mm, 5 μm) (Agilent Technologies). The mobile phase, consisting of methanol–10 mM ammonium acetate buffer solution containing 0.05% acetic acid (76:24, v/v), was delivered at a flow rate of 0.39 mL/min. The column temperature was maintained at 30°C. The electrospray ionization-MS/MS operation parameters were set as follows: positive ion electrospray; drying gas (N2) temperature, 350°C; drying gas (N2) flow rate, 8 L/min; capillary voltage, 4.0 kV; nebulizer pressure, 15 psig; fragmentor voltage, 156 V for ASD and 140 V for the IS; and collision energy, 20 eV for ASD and 30 eV for the IS. Multiple-reaction monitoring used the transitions of m/z 946.4→455.3 for ASD and m/z 285.1→192.9 for the IS.
The pharmacokinetic parameters were analyzed with a non-compartmental model (DAS, Version 2.1.1; Chinese Pharmacological Association, Shanghai, People’s Republic of China). The relative bioavailability (F) was calculated by the following equation:
F (%) = AUCtest/AUCASD ×100 | (1) |
where AUC is the area under the plasma concentration–time curve from 0 to 36 hours.
Statistical analysis
Statistical analyses were performed using Student’s t-test, and a value of P<0.05 was considered to be significantly different.
Results
Characterization of APC
The interactions between ASD and phospholipids were studied using FT-IR spectroscopy (Figure 2A–D) and DSC (Figure 3A–D). There were some differences between the ASD FT-IR spectrum (Figure 2A) and APC FT-IR spectrum (Figure 2D). The characteristic absorption peak of ASD at 3,406.7 cm−1 (for O–H stretch vibration) shifted to 3,333.2 cm−1 in the spectrum of APC, and the characteristic absorption peak of ASD at 2,944.7 cm−1 (for C–H stretch vibration) could not be found in the spectrum of APC, indicating interactions between ASD and phospholipids. Moreover, no new peaks were observed in the spectra of APC and physical mixture (Figure 2C).
  | Figure 2 Infrared spectra. |
DSC is a reliable method to explore the possible interactions between drug and excipients.27 In DSC, a possible interaction can be concluded by appearance of new peak, elimination of endothermic peak, changes in peak shape and its onset, melting point, and relative peak area or enthalpy.27 Thermogram of ASD (Figure 3A) exhibited two endothermic peaks with onset temperature at 59.4°C and 198.8°C, respectively. The second peak (198.8°C) might have been caused by melting of ASD. Thermogram of phospholipids (Figure 3B) showed two endothermic peaks (50.2°C and 167.7°C) and one exothermic peak (310.4°C). The physical mixture of ASD and phospholipids (Figure 3C) showed three endothermic peaks (49°C, 164.8°C, and 266.6°C) and one exothermic peak (315.4°C). When the thermograms of ASD and phospholipids were compared, we noted that the endothermic peak (198.8°C) caused by melting of ASD was missing, and found a new endothermic peak (266.6°C) in the thermogram of mixture. This might have been due to the speculation that when temperature increased, phospholipids melted and then ASD dissolved in the phospholipids partly forming the complex. The formation of phospholipid complex changed the melting temperature of ASD from 198.8°C to 266.6°C. The thermogram of APC (Figure 3D) was in accordance with the thermogram of physical mixture.
The morphologies of ASD and APC under scanning electron microscope are shown in Figure 4. ASD (Figure 4A) appeared as an irregular granule with edges and corners. APC (Figure 4B) did not show the appearance of ASD but appeared to be spherical with a smooth surface.
  | Figure 4 Scanning electron micrographs. |
The content of ASD in the phospholipid complex determined by HPLC was 26.8%±0.52%. The value was concordant with the reactant ratio of ASD and phospholipids.
Solubility studies
The change of liposolubility was studied by the solubility tests in water and n-octanol. As shown in Table 3, APC exhibited much higher solubility in n-octanol and water compared with ASD and physical mixture. The results indicated that the hydrophilicity and liposolubility of ASD were both significantly enhanced after the formation of APC.
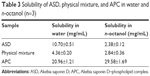  | Table 3 Solubility of ASD, physical mixture, and APC in water and n-octanol (n=3) |
In order to obtain a large drug loading, solubility studies in various carriers were carried out. Figure 5A shows that Glyceryl monooleate (type 40) had the maximum drug solubility (6.53±0.22 mg/mL) among the oils tested. Glyceryl monooleate (type 40), a readily dispersible and lymphotropic solubilizing agent composed mainly of a mixture of mono- and diglycerides of oleic acid, had been used in the self-emulsifying drug delivery system by other researchers.29,30 Moreover, Diethylene glycol monoethyl ether showed the largest solubility (236.29±21 mg/mL) as compared with the other cosurfactants (Figure 5B). Diethylene glycol monoethyl ether, a highly purified diethylene glycol monoethyl ether, was often employed in the SNEDDS formulation as a co-emulsifier or solubilizer for actives.31 Therefore, Glyceryl monooleate (type 40) and Diethylene glycol monoethyl ether were selected as the oil and cosurfactant, respectively. Both nonionic surfactants (Polyoxyl 40 hydrogenated castor oil and Polyoxyl 35 castor oil) had very less solubility (<0.6 mg/mL), so further study is needed to select the proper surfactant.
Selection of surfactant
Emulsification efficiency is more important than the ability to solubilize the drug during the selection of surfactants for using in an SNEDDS formulation.26 Grade of different weight ratios of surfactant/cosurfactant is shown in Table 1. The results clearly indicated that Polyoxyl 35 castor oil was more effective in making a grade I nanoemulsion, and it was preliminarily chosen as the surfactant in the system.
Development of APC-SNEDDS
The pseudoternary phase diagram was widely used to optimize the formulation during the development of SNEDDS.23,24 The self-nanoemulsifying regions of the selected SNEDDS with varying drug loading (from 0% to 5%) were present on the pseudoternary phase diagrams. Through observation, the self-nanoemulsifying region was found to decrease when the drug loading increased. The droplet size of the SNEDDS determined the bioavailability and stability of the formulated drugs,32,33 so it was adopted as a key criterion to select the proper formulation. Emulsion with a higher zeta potential will be more stable,21 so zeta potential was another important selection criterion. Table 4 shows that particle size increased and zeta potential decreased with the increase in drug loading. Although larger drug loading was preferred, the formulation with 5% drug loading had the smallest self-nanoemulsifying region, lowest zeta potential, and largest particle size, indicating an unstable drug delivery system. To obtain a good balance between drug loading and efficient emulsification, the formulation composed of Glyceryl monooleate (type 40), Polyoxyl 35 castor oil, Diethylene glycol monoethyl ether, and APC (1:4.5:4.5:1.74, w/w/w/w) with a drug loading of 4% (w/w) was selected as the best one of its kind. The pseudoternary phase diagram of the selected SNEDDS is shown in Figure 6.
Characterization of APC-SNEDDS
The prepared APC-SNEDDS exhibited a mean particle size of 148.0±2.7 nm and a zeta potential of −13.7±0.92 mV as shown in Table 4. As shown in Figure 7, APC-SNEDDS had a uniform and spherical morphology with a narrow size distribution.
APC-SNEDDS stability
Nanoemulsions, colloidal dispersions of nanosized oil droplets in aqueous mediums, are thermodynamically unstable and kinetically stable systems.34 It is necessary to evaluate their robustness to dilution and thermodynamic stability. In this study, APC-SNEDDS showed no significant changes of the droplet size in different pH diluents (Table 5). Additionally, heating–cooling cycles also had no significant effect on the droplet size (Table 5) and physical appearance of APC-SNEDDS.
Pharmacokinetic results
The pharmacokinetic data were processed using a non-compartmental model. The plasma concentration–time curves obtained after oral administration are shown in Figure 8. The main pharmacokinetic parameters are listed in Table 6.
Compared with ASD group, Tmax1 was found to be significantly shortened from 2.0±1.1 to 0.195±0.068 and 0.195±0.068 hours for the APC and APC-SNEDDS groups, respectively. Meanwhile, Cmax2 significantly increased from 180.5±75.1 to 549.7±113.5 and 985.8±366.6 ng/mL for the APC and APC-SNEDDS groups, respectively. APC-SNEDDS group’s Cmax1 was significantly higher and Tmax2 was significantly shorter compared with ASD group. The relative bioavailability of APC and APC-SNEDDS was 183.8% and 431.8% with ASD group as control group, respectively.
Compared with APC group, Cmax1 and Cmax2 of APC-SNEDDS group were found to be significantly improved. AUC0–36 h increased from 5,551.1±1,357.4 to 13,038.4±5,121.7 ng·h/mL with statistical difference. The results indicated that the oral absorption was further improved by the formation of SNEDDS compared with phospholipid complex.
In conclusion, the oral absorption of ASD was profoundly increased by APC-SNEDDS with an outstanding F of 431.8%.
Discussion
Many naturally occurring hydrophilic saponins exert various pharmacological effects but with unsatisfied oral absorption.35,36 Poor bioavailability has been the major challenge in developing hydrophilic saponins as clinically useful drugs. In this study, an SNEDDS based on phospholipid complex technique was designed to improve the oral bioavailability of ASD.
There were two major obstacles in the development of APC-SNEDDS formulation. The first one was to entrap hydrophilic ASD into SNEDDS which was widely employed for poorly water-soluble drugs.24–26 Previous studies demonstrated that phospholipid complexes could improve the liposolubility and oral absorption of the hydrophilic drug.10,12,27 Thus, APC was prepared, and the liposolubility significantly increased 11.4-fold after formation of APC, which was verified by the solubility test in n-octanol. Different from other studies, the hydrophilicity increased ~0.96-fold compared with ASD. This result might have been caused by the transformation of polymorphs37 or solubilization by liposome micelles formed by APC after dissolving in water.38 The exact mechanism(s) needs to be further studied. In addition, different hydrophilic drug-loaded phospholipid complexes had different lipid solubility,10,27 so it was difficult to select appropriate absorption-enhancing oils which could dissolve a large amount of APC. In this study, Glyceryl monooleate (type 40) was found to have the largest dissolving capacity for APC among the oils tested. Moreover, Glyceryl monooleate (type 40) was a readily dispersible and absorbable solubilizing agent which could provide a triglyceridogenic substrate for efficient chylomicron synthesis and subsequent lymphatic transport.29 Thus, Glyceryl monooleate (type 40) was selected as the oil phase for the APC-SNEDDS to obtain an optimum drug loading and improve lymphatic transport of ASD.
According to the characterizations of APC-SNEDDS and definition of SNEDDS, a schematic diagram of APC-SNEDDS after diluting with distilled water is proposed in Figure 9. Based on the studies conducted, we propose that APC could be completely embedded into the oil phase (Glyceryl monooleate [type 40]) and cosurfactant phase (Diethylene glycol monoethyl ether) to form an aggregate. Polyoxyl 35 castor oil mainly aided in the formation of fine droplets and improved the stability of the nanoemulsion through reducing the interfacial tension. The APC-SNEDDS with such a structure was proved to improve oral absorption of ASD via the pharmacokinetic studies in rats.
  | Figure 9 Proposed schematic diagram of APC-SNEDDS dissolved in distilled water. |
ASD was an amphipathic saponin which could self-assemble as micelles in gastrointestinal fluid with a large aggregate size.11 The formation of self-micelles might reduce the absorption rate and amount of ASD absorbed from gastrointestinal tract.11 APC and APC-SNEDDS were likely to prevent ASD from aggregation and increase its absorption rate and absorbed dose. This might be the reason that Tmax was significantly shortened by the two new preparations. The oral absorption enhancement of APC probably resulted from the increase of liposolubility, effect on the biological membrane by phospholipids as a surfactant, and destruction of the self-micelles formed by ASD in gastrointestinal fluid.39 It was supposed that APC-SNEDDS enhanced the oral bioavailability of ASD via increasing intestinal permeability, inhibiting MRP-mediated efflux, preventing degradation by intestinal bacteria, and enhancing the lymphatic transport of ASD. The definite absorption mechanisms are rather complicated, and further investigations are in need.
Although APC-SNEDDS could significantly improve the oral absorption of ASD, the drug loading is limited. Screening for new absorption-enhancing oils which could dissolve more APC and adopting different technologies to entrap hydrophilic ASD into SNEDDS are promising strategies.
Conclusion
The present study demonstrated that hydrophilic ASD was successfully loaded into SNEDDS using the phospholipid complex technique. The oral absorption of ASD was significantly improved by APC and APC-SNEDDS. The exact absorption mechanisms were complicated and would be investigated in future studies. This study provided a new strategy to enhance the oral bioavailability of highly water-soluble but poorly absorbed saponins.
Acknowledgments
This project was financially supported by the National Natural Science Foundation of China (No 81403080 and 30730113) and the Natural Science Foundation of Jiangsu Province (No BK20140674).
Disclosure
The authors report no conflicts of interest in this work.
References
Zhou YQ, Yang ZL, Xu L, Li P, Hu YZ. Akebia saponin D, a saponin component from Dipsacus asper Wall, protects PC 12 cells against amyloid-β induced cytotoxicity. Cell Biol Int. 2009;33(10):1102–1110. | ||
Niu Y, Li Y, Huang H, et al. Asperosaponin VI, a saponin component from Dipsacus asper Wall, induces osteoblast differentiation through bone morphogenetic protein-2/p38 and extracellular signal-regulated kinase 1/2 pathway. Phytother Res. 2011;25(11):1700–1706. | ||
Li C, Tian J, Li G, et al. Asperosaponin VI protects cardiac myocytes from hypoxia-induced apoptosis via activation of the PI3K/Akt and CREB pathways. Eur J Pharmacol. 2010;649(1–3):100–107. | ||
Gong LL, Wang ZH, Li GR, Liu LH. Protective effects of Akebia saponin D against rotenone-induced hepatic mitochondria dysfunction. J Pharmacol Sci. 2014;126(3):243–252. | ||
Wu C, Ma P, Li PF. LC-MS/MS determination of oral bioavailability of Akebia saponin D in rat. J Beijing Normal Univ. 2014;50(1):62–65. | ||
Zhou Y, Li W, Chen L, Ma S, Ping L, Yang Z. Enhancement of intestinal absorption of Akebia saponin D by borneol and probenecid in situ and in vitro. Environ Toxicol Pharmacol. 2010;29(3):229–234. | ||
Yan L, Yang X, Meng Z, et al. Simultaneous quantification of Akebia saponin D and its five metabolites in human intestinal bacteria using ultraperformance liquid chromatography triple quadrupole mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;971(15):81–88. | ||
Wang L, Liu EW, Han LF. Study on bile excretion of effective component asperosaponin VI in Dipsacus asperoides. Tianjin J Trad Chin Med. 2012;30(11):681–684. | ||
Han M, Fu S, Gao JQ, Fang XL. Evaluation of intestinal absorption of ginsenoside Rg1 incorporated in microemulsion using parallel artificial membrane permeability assay. Biol Pharm Bull. 2009;32(6):1069–1074. | ||
Xiong J, Guo J, Huang L, et al. The use of lipid-based formulations to increase the oral bioavailability of Panax notoginseng saponins following a single oral gavage to rats. Drug Dev Ind Pharm. 2008;34(1):65–72. | ||
Xiong J, Guo J, Huang L, Meng B, Ping Q. Self-micelle formation and the incorporation of lipid in the formulation affect the intestinal absorption of Panax notoginseng. Int J Pharm. 2008;360(1–2):191–196. | ||
Zhang Q, He N, Zhang L, et al. The in vitro and in vivo study on self-nanoemulsifying drug delivery system (SNEDDS) based on insulin-phospholipid complex. J Biomed Nanotechnol. 2012;8(1):90–97. | ||
Li CL, Deng YJ. Oil-based formulations for oral delivery of insulin. J Pharm Pharmacol. 2004;56(9):1101–1107. | ||
Wang S, Su R, Nie S, et al. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J Nutr Biochem. 2014;25(4):363–376. | ||
Li C, Zhang J, Zu YJ, et al. Biocompatible and biodegradable nanoparticles for enhancement of anti-cancer activities of phytochemicals. Chin J Nat Med. 2015;13(9):641–652. | ||
Qhattal HSS, Wang S, Salihima T, Srivastava SK, Liu X. Nanoemulsions of cancer chemopreventive agent benzyl isothiocyanate display enhanced solubility, dissolution, and permeability. J Agric Food Chem. 2011;59(23):12396–12404. | ||
Voruganti S, Qin JJ, Sarkar S, et al. Oral nano-delivery of anticancer ginsenoside 25-OCH3-PPD, a natural inhibitor of the MDM2 oncogene: nanoparticle preparation, characterization, in vitro and in vivo anti-prostate cancer activity, and mechanisms of action. Oncotarget. 2015;6(25):21379–21394. | ||
de Pace RC, Liu X, Sun M, et al. Anticancer activities of (−)-epigallocatechin-3-gallate encapsulated nanoliposomes in MCF7 breast cancer cells. J Liposome Res. 2013;23(3):187–196. | ||
Sun M, Nie S, Pan X, Zhang R, Fan Z, Wang S. Quercetin-nanostructured lipid carriers: characteristics and anti-breast cancer activities in vitro. Colloids Surf B Biointerfaces. 2014;113(13):15–24. | ||
Zhang J, Nie S, Wang S. Nanoencapsulation enhances epigallocatechin-3-gallate stability and its antiatherogenic bioactivities in macrophages. J Agric Food Chem. 2013;61(38):9200–9209. | ||
Date AA, Desai N, Dixit R, Nagarsenker M. Self-nanoemulsifying drug delivery systems: formulation insights, applications and advances. Nanomedicine. 2010;5(10):1595–1616. | ||
Hanke U, May K, Rozehnal V, Nagel S, Siegmund W, Weitschies W. Commonly used nonionic surfactants interact differently with the human efflux transporters ABCB1 (p-glycoprotein) and ABCC2 (MRP2). Eur J Pharm Biopharm. 2010;76(2):260–268. | ||
Zhang J, Peng Q, Shi S, et al. Preparation, characterization, and in vivo evaluation of a self-nanoemulsifying drug delivery system (SNEDDS) loaded with morin-phospholipid complex. Int J Nanomedicine. 2011;2011(6):3405–3414. | ||
Rashid R, Kim DW, Yousaf AM, et al. Comparative study on solid self-nanoemulsifying drug delivery and solid dispersion system for enhanced solubility and bioavailability of ezetimibe. Int J Nanomedicine. 2015;10(1):6147–6159. | ||
Chen CH, Chang CC, Shih TH, Aljuffali IA, Yeh TS, Fang JY. Self-nanoemulsifying drug delivery systems ameliorate the oral delivery of silymarin in rats with Roux-en-Y gastric bypass surgery. Int J Nanomedicine. 2015;10(1):2403–2416. | ||
Zhang L, Zhang L, Zhang M, et al. Self-emulsifying drug delivery system and the applications in herbal drugs. Drug Deliv. 2015;22(4):475–486. | ||
Ruan J, Liu J, Zhu D, et al. Preparation and evaluation of self-nanoemulsified drug delivery systems (SNEDDSs) of matrine based on drug-phospholipid complex technique. Int J Pharm. 2010;386(1–2):282–290. | ||
Heshmati N, Cheng X, Eisenbrand G, Fricker G. Enhancement of oral bioavailability of E804 by self-nanoemulsifying drug delivery system (SNEDDS) in rats. J Pharm Sci. 2013;102(10):3793–3799. | ||
Hauss DJ, Fogal SE, Ficorilli JV, et al. Lipid-based delivery systems for improving the bioavailability and lymphatic transport of a poorly water-soluble LTB4 inhibitor. J Pharm Sci. 1998;87(2):164–169. | ||
Wasan EK, Bartlett K, Gershkovich P, et al. Development and characterization of oral lipid-based Amphotericin B formulations with enhanced drug solubility, stability and antifungal activity in rats infected with Aspergillus fumigatus or Candida albicans. Int J Pharm. 2009;372(1–2):76–84. | ||
Zhou H, Wan J, Wu L, et al. A new strategy for enhancing the oral bioavailability of drugs with poor water-solubility and low liposolubility based on phospholipid complex and supersaturated SEDDS. PLoS One. 2013;8(12):e84530. | ||
Nielsen FS, Petersen KB, Mullertz A. Bioavailability of probucol from lipid and surfactant based formulations in minipigs: influence of droplet size and dietary state. Eur J Pharm Biopharm. 2008;69(2):553–562. | ||
Gao Z, Choi H, Shin H, et al. Physicochemical characterization and evaluation of a microemulsion system for oral delivery of cyclosporine A. Int J Pharm. 1998;161(1):75–86. | ||
Anton N, Vandamme TF. Nanoemulsions and microemulsions: clarifications of the critical differences. Pharm Res. 2011;28(5):978–985. | ||
Gao S, Basu S, Yang Z, Deb A, Hu M. Bioavailability challenges associated with development of saponins as therapeutic and chemopreventive agents. Curr Drug Targets. 2012;13(14):1885–1899. | ||
Sparg SG, Light ME, van Staden J. Biological activities and distribution of plant saponins. J Ethnopharmacol. 2004;94(2–3):219–243. | ||
Wang QH, Yang XL, Xiao W, et al. Microcrystalline preparation of akebia saponin D for its bioavailability enhancement in rats. Am J Chin Med. 2015;43(3):513–528. | ||
Song Y, Zhuang J, Guo J, Xiao Y, Ping Q. Preparation and properties of a silybin-phospholipid complex. Pharmazie. 2008;63(1):35–42. | ||
Li J, Wang XL, Zhang T, et al. A review on phospholipids and their main applications in drug delivery systems. Asian J Pharm Sci. 2015;10(2):81–98. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.