Back to Journals » Journal of Inflammation Research » Volume 16
Preoperative Systemic Immune-Inflammation Index is a Potential Biomarker in Adult Patients with High-Grade Gliomas Undergoing Radical Resection
Authors Jiang YT, Wang TC , Zhang W
Received 20 June 2023
Accepted for publication 3 August 2023
Published 16 August 2023 Volume 2023:16 Pages 3479—3490
DOI https://doi.org/10.2147/JIR.S423488
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Yu-Ting Jiang,1 Tian-Cheng Wang,2,* Wei Zhang1,*
1Department of Radiology, Brain Hospital of Hunan Province, the School of Clinical Medicine, Hunan University of Chinese Medicine, Changsha, People’s Republic of China; 2Department of Radiology, the Second Xiangya Hospital of Central South University, Changsha, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tian-Cheng Wang; Wei Zhang, Email [email protected]; [email protected]
Background: Increasing evidence has highlighted that systemic immune-inflammation index (SII), a recently developed prognostic biomarker that utilizes peripheral platelet, lymphocyte and neutrophil counts, is associated with unfavorable prognosis in various tumors. Nevertheless, the prognostic significance of SII in high-grade gliomas patients undergoing radical resection remains unclear. Therefore, the present study aimed to assess the potential of SII as a prognostic biomarker in this patient population.
Methods: A total of 111 adult patients with high-grade gliomas who underwent radical resection were consecutively enrolled in this investigation. The study involved the categorization of patients into high and low SII groups using predetermined cut-off values. Subsequently, forward stepwise logistic regression was employed to identify autonomous predictors for early gliomas recurrence. To mitigate the impact of confounding factors, a propensity score matching (PSM) analysis was performed between high and low SII patients. Finally, the Kaplan-Meier approach was utilized to compare the progression-free survival (PFS) and overall survival (OS) of the two groups.
Results: The study involved the categorization of patients into two groups based on their SII levels, namely high SII (> 604.8) and low SII (≤ 604.8) groups. Forward stepwise logistic regression revealed that high SII (p < 0.001) and tumor size ≥ 50 mm (p < 0.001) were significantly related to early recurrence of gliomas. Furthermore, the results indicate that PFS and OS were significantly shorter in the high SII group compared to the low SII group, both before and after PSM (p < 0.05).
Conclusion: Preoperative biomarker SII can serve as a prognostic biomarker for early recurrence and prognosis in patients with high-grade gliomas undergoing radical resection. Furthermore, the combination of tumor size and SII demonstrates a robust predictive capacity for early recurrence and prognosis in this patient population.
Keywords: systemic immune-inflammation index, high-grade gliomas, resection, survival, prognosis
Introduction
Gliomas represent the most prevalent malignancies of the central nervous system (CNS), comprising over 50% of CNS malignancies in adults.1,2 Traditionally, gliomas are classified into two primary types based on histological characterization: low-grade gliomas (I–II) and high-grade (III–IV) gliomas.3,4 The high-grade gliomas pose significant challenges in treatment and have a poor prognosis due to their high recurrence rate, despite initial maximal radical resection.5,6 One of the main challenges is that gliomas exhibit invasive growth, and the determination of the tumor boundary through conventional radiology is typically challenging.7 Consequently, the extent of tumor resection is frequently confined to the boundary identified by preoperative radiological assessment, rather than the precise histopathological boundary and this limitation may contribute significantly to the propensity for gliomas recurrence following radical resection. Overall, patients with high-grade gliomas who undergo radical resection exhibit varying outcomes with 5-year survival rate fluctuates between 5.5% and 75.2%, which can be attributed to the differences in tumor burdens, histopathological status, and immune response status.8–10 Consequently, to enhance risk stratification and improve prognostic accuracy, effective prognostic biomarkers are required for this population of high-grade gliomas patients who undergo radical resection.
In the context of cancer progression, the hallmarks of inflammatory response and immunoregulation are widely recognized as crucial factors that contribute to processes such as priming, proliferation, angiogenesis, and migration.11,12 It is reported that high-grade gliomas lead to a inflammatory and immunosuppressive environment both locally and systemically, which presents significant challenges that can negatively impact clinical outcomes and the effectiveness of therapeutic interventions.13,14 For example, elevated levels of myeloid-derived suppressor cells have been found in the tumor microenvironment of gliomas while higher proportions of circulating regulatory T cells have been found in the peripheral blood of gliomas patients.13 To date, peripheral blood inflammatory mediators, including the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and monocyte-to-lymphocyte ratio (MLR), have already been reported to be effective predictors for predicting the clinical outcomes of several malignancies.15–17 Furthermore, the novel biomarker systemic immune-inflammation index (SII), comprising platelets, neutrophils, and lymphocytes, has been identified as a promising prognostic biomarker in various malignancies.17
Given the uncertain prognostic significance of preoperative SII in high-grade gliomas patients undergoing radical resection, the present study endeavors to assess the prognostic value of SII in this patient cohort and to further evaluate whether SII can be used as an ideal biomarker for the prognosis of gliomas.
Method
Patient Selection
This study reports on a cohort of 111 patients diagnosed with high-grade gliomas who underwent radical resection (Radical resection is defined as complete resection of enhancing nodule and maximal resection of peritumoral edema area.) as their initial therapy between April 2018 and September 2022 at our institution. To ensure the comparability of data and minimize the influence of subjective factors on inflammatory indicators, inclusion and exclusion criteria were established. The inclusion criteria were as follows: patients were/had (1) aged >18 years; (2) an Eastern Cooperative Oncology Group performance status of 0, 1; (3) no diagnosis of other tumors at the time of diagnosis; (4) with a solitary lesion; (5) no fever/infection at the time of admission. The exclusion criteria were as follows: (1) incomplete follow-up data (n=81); (2) received adjuvant chemotherapy or radiotherapy before surgery (n=15); (3) did not received adjuvant chemotherapy or radiotherapy after surgery (n=12); (4) had a history of inflammatory diseases in the past month (n=5). Finally, there were 111 patients included in this study. The flowchart of the study population is shown in Figure 1.
 |
Figure 1 Diagram of the study population. |
The institutional review boards of the Brain Hospital of Hunan Province (Hunan Second People’s Hospital) approved this retrospective study, which adhered to the Declaration of Helsinki. As the study was retrospective and patient data was anonymized and de-identified prior to analysis, the requirement for written consent was waived.
Data Collection
Clinical information pertaining to patients, such as age, gender, pathology grade (WHO classification in 2016), tumor size, and degree of edema, was collected, as were peripheral blood results, including neutrophil count, lymphocyte count, and platelet count within 1 week prior to radical resection. To calculate SIRI and SII, the following formula was employed: 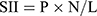 ,
, 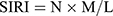 (Note: P=platelet count; N=neutrophil count; L=lymphocyte count; M=monocyte count).
(Note: P=platelet count; N=neutrophil count; L=lymphocyte count; M=monocyte count).
Early Recurrence Evaluation and Follow-Up
Magnetic resonance imaging (MRI) was screened by two radiologists who had either 9 years or 12 years of experience in neuroimaging radiology. Early recurrence of gliomas was defined as new lesions appearing within six months after radical resection. The patients with gliomas who underwent radical resection were segregated into two groups: those who experienced early recurrence and those who did not. The duration of PFS was determined from the time of diagnosis to the point of tumor recurrence. Similarly, OS was calculated from the date of diagnosis to the date of death from any cause or the last follow-up. Both PFS and OS were computed both prior to and following one-to-one PSM.
Statistical Analysis
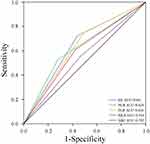
The present study utilized the receiver operating characteristic (ROC) curve to ascertain the optimal cut-off value for SII, SIRI, NLR, MLR, and PLR in the screening of gliomas patients with a higher likelihood of recurrence within six months. The data was presented as either the median with interquartile (IQR) or as frequencies. Numerical variables (non-normal distribution) were compared using the Mann–Whitney U-test, while categorical variables were compared using Pearson’s chi-squared test or Fisher’s exact test. To evaluate the inter-reader agreement of radiological data between the two neuroimaging radiologists, the Kappa test (for categorical data) was performed. Agreement was classified as poor (Kappa value, 0–0.40), fair to good (Kappa value, 0.40–0.75), and excellent (Kappa value, > 0.75). A forward stepwise logistic regression model was employed to identify the most effective marker through univariate and multivariate analyses. Additionally, a one-to-one PSM analysis was employed to mitigate the influence of confounding variables between the high and low SII groups. The Kaplan-Meier method and Log rank test were utilized to compare the differences in OS and PFS between the high and low SII groups prior to and following PSM. The discriminatory ability of the combination of SII and tumor size in predicting early gliomas recurrence was evaluated by ROC curves.
Statistical analyses were conducted using SPSS 26.0 statistical software, and a two-tailed P value of < 0.05 was deemed statistically significant. The effect size and statistical power of this study was analyzed by G-power 3.1.9.7 software.
Result
Demographic, Radiological and Laboratorial Characteristics
A total of 111 patients were included (65 males and 46 females) in the present study. The statistical power calculated by G-power software for this sample size was 0.80 (Type of power analysis: Post-hoc power analysis; Parameter: effect size = 0.265; α err prob = 0.05; n = 111). All the diagnoses of high-grade gliomas were based on pathology after radical resection. There were 53 (47.7%) patients with WHO class III gliomas and 58 (52.3%) patients with WHO class IV gliomas. There were 54 patients (48.6%) with slight peritumoral edema and 57 patients (51.4%) with severe peritumoral edema. In this study, 38 patients (34.2%) had tumor size less than 50 mm, while 73 patients (65.8%) had tumor size greater than or equal to 50 mm. The inter-reader agreements of radiological data between two radiologists were all excellent with Kappa values of 0.910 (peritumoral edema) and 0.955 (tumor size). Table 1 and Table 2 provide detailed demographic, radiological, and laboratory characteristics.
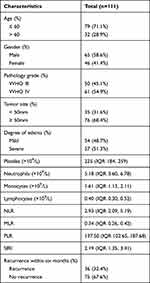 |
Table 1 The Demographic, Radiological and Laboratorial Characteristics of the Patients |
 |
Table 2 The Demographic, Radiological and Laboratorial Characteristics of the Low SII and High SII Patients |
Potential Predictive Factors for Early Gliomas Recurrence
Early recurrence of gliomas was defined as a recurrence of gliomas within 6 months after radical surgery. Thirty-six (32.4%) gliomas patients recurred within six months after radical resection while 75 (67.6%) patients did not recur within six months. ROC curves were performed to decide the diagnostic efficacy of biomarker and their respective cutoff points. The cut-off point of the ROC is the threshold that optimizes the performance of the classification model and the cut-off points of NLR, MLR, PLR, SII and SIRI were 3.6, 0.3, 126.2, 604.8 and 1.3, respectively. The area under curves (AUCs) of NLR, MLR, PLR, SII and SIRI were determined to be 0.624, 0.544, 0.628, 0.641 and 0.592, respectively (Figure 2). In univariate analysis, early recurrence of gliomas was correlated with pathology grade WHO IV (p = 0.001), severe peritumoral edema (p = 0.025), NLR > 3.6 (p = 0.011), PLR > 126.2 (p = 0.010), SII > 604.8 (p = 0.005), tumor size ≥ 50 mm (p < 0.001). A forward stepwise multivariate analysis was then performed using the significant risk factors identified in the univariate analysis. The analysis revealed that tumor size ≥ 50mm and SII > 604.8 were independent biomarkers that correlated with early recurrence of gliomas. The odds ratios and 95% confidence intervals for these biomarkers are presented in Table 3.
 |
Table 3 Assessment of Potential Risk Factors for Early Recurrence of Gliomas |
Impact of Low and High SII on the PFS and OS
The findings derived from the logistic regression model indicate that NLR, PLR, and SII were linked to early recurrence of gliomas in the univariate analysis. However, only SII demonstrated a significant association with early recurrence of gliomas in the multivariate analysis. As a result, the Log rank test was utilized to ascertain the dissimilarity in PFS and OS between patients with low and high SII. Compared to the low SII cohort (52, 46.8%), the patients of the high SII cohort (59, 53.2%) had higher pathological grade, larger tumor size and higher degree of edema. The Kaplan-Meier survival curves for PFS and OS in patients with low and high SII were depicted in Figure 3. Compared with the high SII group, the low SII group had a significantly longer median PFS (low SII, 442.0 days [95% CI 364.949–519.051] vs high SII, 225.0 days [95% CI 150.691–299.309]; p < 0.001) (Figure 3A) and OS (low SII, 1017.0 days [95% CI 562.372–1471.628] vs high SII, 449.0 days [95% CI 316.194–581.806]; p < 0.001) (Figure 3B). Additionally, to mitigate selection bias arising from confounding factors between different groups, a one-to-one PSM analysis was conducted. The PSM study enrolled 64 patients, comprising 32 with low SII and 32 with high SII. The PSM study was successfully conducted and there were no discernible differences in baseline characteristics including age, gender, pathology grade, tumor size and edema degree between the two groups (Table 4). Additionally, the low SII group exhibited a significantly higher median PFS (low SII, 330.0 days [95% CI 193.678–466.322] vs high SII, 180.0 days [95% CI 107.562–252.438]; p = 0.005) (Figure 4A) and OS (low SII, 762.0 days [95% CI 345.770–1178.230] vs high SII, 449.0 days [95% CI 316.079–581.921]; p =0.011).
 |
Table 4 Demographic, Radiological and Laboratorial Characteristics of the Patients After Propensity Score Matching |
Combining SII and Clinical Markers to Predict Early Recurrence of Gliomas
Figure 5 displays the ROC curves for SII, tumor size, and the combination of SII and tumor size in predicting early recurrence of gliomas, with corresponding AUCs of 0.641, 0.651, and 0.714, respectively. A higher AUC value indicates a heightened capacity for predicting the early recurrence of glioma. In the present study, the combination of SII and tumor size demonstrated superior discriminatory power in predicting early recurrence of gliomas when compared to either SII or tumor size alone.
Discussion
Gliomas are the most commonly occurring brain tumor in adults, and despite therapeutic advancements, high-grade gliomas have a near-universal fatality rate.18,19 As such, it is imperative to identify effective prognostic biomarkers that can accurately predict the prognosis of high-grade gliomas patients. To date, the role of systemic inflammatory biomarkers in predicting the prognosis of malignancies has been reported.20,21 Some studies demonstrated that NLR and PLR had predictive value in gliomas patients.22,23 Furthermore, recent research has identified a novel biomarker, SII, which combines the strengths of NLR and PLR and has demonstrated potential as a more effective prognostic indicator for various malignancies.24,25 In this study, the inflammatory biomarkers NLR, MLR, PLR, SIRI, and SII were evaluated using ROC curves. The cut-off value for SII was determined to be 604.8, and the AUC for SII was found to be superior to that of NLR, MLR, PLR, and SIRI across the entire study population. The results of both univariate and multivariate analyses indicated a significant correlation between elevated SII and early gliomas recurrence, as well as larger tumor size and higher pathology grade. Furthermore, Kaplan-Meier survival analysis demonstrated that high SII remained an unfavorable biomarker for both OS and PFS before and after PSM. While the cut-off values for SII may differ across various cancer types, our findings are consistent with prior research demonstrating that a high preoperative level of SII is an independent unfavorable biomarker for cancer.23,25,26
The promotion of tumors by inflammation is a significant characteristic of malignancies, as reported in literature.27 Recent studies have highlighted the complex interplay between cancer cells and their microenvironments, which play a crucial role in regulating tumor proliferation, migration, and immune evasion.28–30 During the inflammatory process, tumor cells and immune cells within tumor microenvironments are capable of producing numerous cytokines, including pro-inflammatory cytokines, growth factors, and chemokines, all of which contribute to the progression of tumors.31,32 The progression of tumors is linked to inflammation, which is initiated by a range of blood factors, including neutrophils, lymphocytes, and platelets.33,34 Neutrophils have been identified as possessing tumor-promoting characteristics, as they are able to migrate to the tumor microenvironment and secrete a variety of pro-angiogenic factors, such as Bv8, VEGFA, and MMP9, thereby promoting tumor progression.35,36 Additionally, neutrophils are capable of secreting pro-inflammatory factors, which can result in genetic instability and cellular DNA damage, ultimately leading to carcinogenesis.36 Lymphocytes play a crucial role in the pathogenesis and progression of tumors and it has been reported that augmented infiltration of lymphocytes in tumors is linked with prolonged survival of high-grade gliomas patients.13 Peripheral blood lymphocytes can be recruited to the tumor microenvironment and differentiate into various subtypes of lymphocytes. For instance, CD8+ cytotoxic lymphocytes can recognize tumor antigens and induce apoptosis in cancer cells by producing cytotoxins such as perforin and granzyme while γδ T cells can recognize and promptly respond to a diverse range of tumor antigens.37 Peripheral platelets are crucial in primary hemostasis, but they also perform a diverse range of other functions. It is reported that platelets secrete pro-angiogenic cytokines, including vascular endothelial growth factor and endothelial cell growth factor, which promote tumor angiogenesis.38,39 Furthermore, peripheral platelets facilitate the invasiveness of circulating tumor cells by covering them in a P-selectin-dependent mechanism, thereby preventing natural killer (NK) cell-mediated tumor cell apoptosis.39
Prior researches have indicated that levels of NLR, PLR, and MLR are linked to the prognosis of patients with gliomas. Specifically, Chim et al found that elevated NLR was significantly correlated with larger tumor size, while higher MLR was identified as a predictor for poorer OS in gliomas patients.40 Qi et al demonstrated that high NLR and derived NLR were associated with unfavorable prognoses in gliomas patients.41 He et al reported that lower PLR was significantly associated with prolonged OS in patients with grade III gliomas.42 However, the aforementioned variables are limited to one or two cell types, whereas the SII comprises three biomarkers, and may exhibit superior predictive capabilities in high-grade gliomas patients who have undergone radical resection. SII has been found to possess favorable predictive value in various cancers. For instance, Chen et al established SII as an independent risk factor of OS and PFS in patients with colorectal cancer.26 He et al demonstrated that SII is a straightforward yet potent index with a high predictive value for survival outcomes in patients with gastric cancer.43 Hu’s study demonstrated the potency of SII as a prognostic indicator for unfavorable outcomes in hepatocellular carcinoma (HCC) patients, and its potential as a tool for determining treatment strategies for HCC.25 Our findings align with previous study, indicating that high-grade gliomas patients who undergo radical resection and have an SII > 604.8 exhibit a higher six-month recurrence rate and a poorer prognosis. Moreover, in our study, the prediction ability of SII is superior to that of NLR, PLR and MLR. The clinical significance of this study lies in the provision of a relatively convenient and non-invasive biomarker for predicting the six-month recurrence rate and long-term survival of gliomas patients who undergo radical resection. Moreover, it has been reported that models integrating multiple factors demonstrate enhanced predictive capabilities.44,45 Similarly, the utilization of SII in conjunction with other clinical markers can enhance their predictive efficacy in this study. Collectively, the prognostic value of the SII in patients with high-grade gliomas can aid clinicians in identifying individuals with a heightened likelihood of recurrence. Consequently, this knowledge can facilitate the implementation of tailored adjuvant therapy following radical surgical procedures.
However, in the present study, there are some limitations should be taken into account. Firstly, this is a retrospective study with a relatively small number of patients included and thus may be subject to selection and statistical bias. Therefore, further studies with a larger sample size should be conducted to validate this hypothesis. Secondly, our analysis did not incorporate alternative confounders that could impact inflammatory biomarkers, such as genetic mutations in the tumor, as a result, these confounders have the potential to either expand or restrict the correlation between SII and glioma prognosis. Thirdly, SII was solely computed prior to radical resection in this study. However, the dynamic alteration of SII between pre- and post-surgery could potentially yield more comprehensive information.
Conclusion
In short, our research demonstrates that a high preoperative SII level is an independent risk factor for early tumor recurrence and poorer prognosis in gliomas patients undergoing radical resection. Taken together, the biomarker SII or the combination of SII with other clinical markers can easily be implemented in clinical practice and may help clinicians formulate a more reasonable individualized treatment plan.
Data Sharing Statement
The data of this study are available from the corresponding author (Zhang Wei).
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Almenawer SA, Badhiwala JH, Alhazzani W, et al. Biopsy versus partial versus gross total resection in older patients with high-grade gliomas: a systematic review and meta-analysis. Neuro Oncol. 2015;17(6):868–881. doi:10.1093/neuonc/nou349
2. Yang Y, Yao M, Long S, et al. Prognostic nomograms for primary high-grade gliomas patients in adult: a retrospective study based on the SEER database. Biomed Res Int. 2020;2020:1346340. doi:10.1155/2020/1346340
3. Morshed RA, Young JS, Hervey-Jumper SL, Berger MS. The management of low-grade gliomas in adults. J Neurosurg Sci. 2019;63(4):450–457. doi:10.23736/S0390-5616.19.04701-5
4. Wang Y, Liu R, Zhang Q, et al. Charged particle therapy for high-grade gliomas in adults: a systematic review. Radiat Oncol. 2023;18(1):29. doi:10.1186/s13014-022-02187-z
5. Vargas LA, Fernandez CC, Valera MM, Rodriguez-Boto G. Survival analysis in high-grade gliomas: the role of salvage surgery. Neurologia. 2023;38(1):21–28. doi:10.1016/j.nrleng.2020.04.032
6. Zhao Y, Chen Y, Wang L, et al. The clinicopathological features and prognosis of multifocal high-grade gliomas in adults with H3F3A mutation. Neurosciences. 2023;28(1):42–47. doi:10.17712/nsj.2023.1.20220080
7. Du P, Yang X, Shen L, et al. Nomogram model for predicting the prognosis of high-grade glioma in adults receiving standard treatment: a retrospective cohort study. J Clin Med. 2022;12(1):196. doi:10.3390/jcm12010196
8. Alvarez S, Alvarez-Vega MA, Balbin M, et al. Prognostic factors and survival study in high-grade gliomas in the elderly. BRIT J NEUROSURG. 2016;30(3):330–336. doi:10.3109/02688697.2016.1139049
9. Ostrom QT, Price M, Neff C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015–2019. Neuro Oncol. 2022;24(Suppl 5):v1–v95. doi:10.1093/neuonc/noac202
10. Mirimanoff RO. High-grade gliomas: reality and hopes. Chin J Cancer. 2014;33(1):1–3. doi:10.5732/cjc.013.10215
11. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. doi:10.1016/j.immuni.2019.06.025
12. Murata M. Inflammation and cancer. Environ Health Prev. 2018;23(1):50. doi:10.1186/s12199-018-0740-1
13. Grabowski MM, Sankey EW, Ryan KJ, et al. Immune suppression in gliomas. J Neuro-Oncol. 2021;151(1):3–12. doi:10.1007/s11060-020-03483-y
14. Medikonda R, Dunn G, Rahman M, Fecci P, Lim M. A review of glioblastoma immunotherapy. J Neuro-Oncol. 2021;151(1):41–53. doi:10.1007/s11060-020-03448-1
15. Saputra HM, Hidayatullah F, Kloping YP, et al. Prognostic value of neutrophil-to-lymphocyte ratio (NLR) in penile cancer: a systematic review and meta-analysis. Ann Med Surg. 2022;81:104335. doi:10.1016/j.amsu.2022.104335
16. Wang TC, An TZ, Li JX, Pang PF. Systemic inflammation response index is a prognostic risk factor in patients with hepatocellular carcinoma undergoing TACE. Risk Manag Healthc P. 2021;14:2589–2600. doi:10.2147/RMHP.S316740
17. Yamamoto T, Kawada K, Obama K. Inflammation-related biomarkers for the prediction of prognosis in colorectal cancer patients. Int J Mol Sci. 2021;22(15):8002. doi:10.3390/ijms22158002
18. Sim HW, Morgan ER, Mason WP. Contemporary management of high-grade gliomas. CNS Oncol. 2018;7(1):51–65. doi:10.2217/cns-2017-0026
19. Zhou Q, Xue C, Ke X, Zhou J. Treatment response and prognosis evaluation in high-grade glioma: an imaging review based on MRI. J Magn Reson Imaging. 2022;56(2):325–340. doi:10.1002/jmri.28103
20. Fang L, Yan FH, Liu C, et al. Systemic inflammatory biomarkers, especially fibrinogen to albumin ratio, predict prognosis in patients with pancreatic cancer. Cancer Res Treat. 2021;53(1):131–139. doi:10.4143/crt.2020.330
21. Uludag SS, Sanli AN, Zengin AK, Ozcelik MF. Systemic inflammatory biomarkers as surrogate markers for stage in colon cancer. Am Surgeon. 2022;88(6):1256–1262. doi:10.1177/0003134821995059
22. Diem S, Schmid S, Krapf M, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–181. doi:10.1016/j.lungcan.2017.07.024
23. Liu J, Li S, Zhang S, et al. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J Clin Lab Anal. 2019;33(8):e22964. doi:10.1002/jcla.22964
24. Ding P, Guo H, Sun C, et al. Combined systemic immune-inflammatory index (SII) and prognostic nutritional index (PNI) predicts chemotherapy response and prognosis in locally advanced gastric cancer patients receiving neoadjuvant chemotherapy with PD-1 antibody sintilimab and XELOX: a prospective study. BMC Gastroenterol. 2022;22(1):121. doi:10.1186/s12876-022-02199-9
25. Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin cancer res. 2014;20(23):6212–6222. doi:10.1158/1078-0432.CCR-14-0442
26. Chen JH, Zhai ET, Yuan YJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroentero. 2017;23(34):6261–6272. doi:10.3748/wjg.v23.i34.6261
27. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–e503. doi:10.1016/S1470-2045(14)70263-3
28. Fane M, Weeraratna AT. How the ageing microenvironment influences tumour progression. Nat Rev Cancer. 2020;20(2):89–106. doi:10.1038/s41568-019-0222-9
29. Ren B, Cui M, Yang G, et al. Tumor microenvironment participates in metastasis of pancreatic cancer. Mol Cancer. 2018;17(1):108. doi:10.1186/s12943-018-0858-1
30. Bianconi A, Aruta G, Rizzo F, et al. Systematic review on tumor microenvironment in glial neoplasm: from understanding pathogenesis to future therapeutic perspectives. Int J Mol Sci. 2022;23(8):4166. doi:10.3390/ijms23084166
31. Habanjar O, Bingula R, Decombat C, et al. Crosstalk of inflammatory cytokines within the breast tumor microenvironment. Int J Mol Sci. 2023;24(4):4002. doi:10.3390/ijms24044002
32. Ozga AJ, Chow MT, Luster AD. Chemokines and the immune response to cancer. Immunity. 2021;54(5):859–874. doi:10.1016/j.immuni.2021.01.012
33. Guan Y, Xiong H, Feng Y, et al. Revealing the prognostic landscape of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in metastatic castration-resistant prostate cancer patients treated with Abiraterone or enzalutamide: a meta-analysis. Prostate Cancer P D. 2020;23(2):220–231. doi:10.1038/s41391-020-0209-3
34. Zhang CL, Jiang XC, Li Y, et al. Independent predictive value of blood inflammatory composite markers in ovarian cancer: recent clinical evidence and perspective focusing on NLR and PLR. J Ovarian Res. 2023;16(1):36. doi:10.1186/s13048-023-01116-2
35. Mizuno R, Kawada K, Itatani Y, et al. The role of tumor-associated neutrophils in colorectal cancer. Int J Mol Sci. 2019;20(3):529. doi:10.3390/ijms20030529
36. Que H, Fu Q, Lan T, Tian X, Wei X. Tumor-associated neutrophils and neutrophil-targeted cancer therapies. BBA Rev Cancer. 2022;1877:188762.
37. Wang H, Zhou H, Xu J, et al. Different T-cell subsets in glioblastoma multiforme and targeted immunotherapy. Cancer Lett. 2021;496:134–143. doi:10.1016/j.canlet.2020.09.028
38. Filippelli A, Del GC, Simonis V, et al. Scoping review on platelets and tumor angiogenesis: do we need more evidence or better analysis? Int J Mol Sci. 2022;23(21):13401. doi:10.3390/ijms232113401
39. Marx S, Xiao Y, Baschin M, et al. The role of platelets in cancer pathophysiology: focus on malignant gliomas. Cancers. 2019;11(4):569. doi:10.3390/cancers11040569
40. Chim ST, Sanfilippo P, O’Brien TJ, Drummond KJ, Monif M. Pretreatment neutrophil-to-lymphocyte/monocyte-to-lymphocyte ratio as prognostic biomarkers in gliomas patients. J Neuroimmunol. 2021;361:577754. doi:10.1016/j.jneuroim.2021.577754
41. Qi Z, Cai J, Meng X, et al. Prognostic value of preoperative inflammatory markers among different molecular subtypes of lower-grade gliomas. J Clin Neurosci. 2022;96:180–186. doi:10.1016/j.jocn.2021.10.006
42. He Q, Li L, Ren Q. The prognostic value of preoperative Systemic Inflammatory Response Index (SIRI) in patients with high-grade gliomas and the establishment of a nomogram. Front Oncol. 2021;11:671811. doi:10.3389/fonc.2021.671811
43. He K, Si L, Pan X, et al. Preoperative Systemic Immune-Inflammation Index (SII) as a superior predictor of long-term survival outcome in patients with stage I–II gastric cancer after radical surgery. Front Oncol. 2022;12:829689. doi:10.3389/fonc.2022.829689
44. Le VH, Kha QH, Minh TNT, et al. Development and validation of CT-based radiomics signature for overall survival prediction in multi-organ cancer. J Digit Imaging. 2023;36(3):911–922. doi:10.1007/s10278-023-00778-0
45. Le VH, Kha QH, Hung TNK, et al. Risk score generated from CT-based radiomics signatures for overall survival prediction in non-small cell lung cancer. Cancers. 2021;13(14):3616. doi:10.3390/cancers13143616
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.