Back to Journals » Cancer Management and Research » Volume 10
Preoperative PROSTATE scoring system: a potential predictive tool for the risk of biochemical recurrence after radical prostatectomy
Authors Zhang ZN, Luo C , Xu B, Song HF, Ma BL, Zhang Q
Received 31 May 2018
Accepted for publication 20 August 2018
Published 16 October 2018 Volume 2018:10 Pages 4671—4677
DOI https://doi.org/10.2147/CMAR.S175869
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Beicheng Sun
Zhe-Nan Zhang,1,2,* Cheng Luo,1,2,* Ben Xu,1,2 Hai-Feng Song,1,2 Bing-Lei Ma,1,2 Qian Zhang1,2
1Department of Urology, Peking University First Hospital, Beijing 100034, People’s Republic of China; 2Institute of Urology, Peking University, National Urological Cancer Center, Beijing 100034, People’s Republic of China
*These authors contributed equally to this work
Purpose: To study the association between the preoperative PROSTATE scoring system and the prediction of biochemical recurrence (BCR) risk, after radical prostatectomy (RP) in prostate cancer patients.
Patients and methods: A total of 340 patients who underwent a laparoscopic radical prostatectomy in Peking University First Hospital between November 2007 and March 2016 were included in the study. The preoperative PROSTATE scoring system was measured and calculated. The performance of the scoring system to predict BCR risk was estimated using the receiver operating characteristic curve (ROC curve). BCR-free survival was analyzed using the Kaplan–Meier method, and the log-rank test was applied to compare the differences in risk among the patient groups. The Cox proportional hazards regression was used to analyze the performance of the grouped PROSTATE scores.
Results: Of the total population, 91 (26.8%) patients had BCR. The PROSTATE score was significantly different between the BCR-developed and BCR-free groups (P<0.001). The ROC curve analysis of the scoring system showed an accuracy of 70.7% (95% CI 0.643–0.771) (P<0.001). The percentage of BCR in the high-risk (10–15), moderate-risk (5–9) and low-risk (0–4) groups was 63.3%, 24.6% and 10.3% respectively (P<0.001). The Cox proportional hazards regression analysis revealed that the grouped score was an independent predictor of BCR after RP (HR=2.002; 95% CI 1.222–3.280) (P=0.006).
Conclusion: The PROSTATE scoring system performed adequately in predicting the risk of BCR after RP. The scoring system can assist in decision-making about the operation and postoperative follow-up for patients with high-risk.
Keywords: PROSTATE scoring system, biochemical recurrence, radical prostatectomy, predictive, prostate cancer, preoperative
Introduction
PCa is nowadays a common non-cutaneous malignant tumor. According to the National Central Cancer Registry, the incidence of PCa in People’s Republic of China ranked seventh in male cancer patients.1 PCa is characterized by indolent, slow growing, low to high-grade tumors, but may become aggressive neoplasms with metastasis.2 In clinical practice, RP is the recommended surgical method for operable PCa patients. Though treated with RP, patients still face the risk of BCR and local PCa recurrence in the long term. Nearly 30% of RP patients, have an isolated BCR during a long-term follow-up, often without clinical or radiological evidence of PCa.3 To deal with BCR after RP, adjuvant therapy for PCa is given for those who have a high-risk of BCR soon after RP. What is more, neo-adjuvant therapy is considered to improve the clinical outcome for patients with localized high-risk PCa.4 Adjuvant therapy includes hormonal therapy, radiotherapy, chemotherapy, targeted therapy and immunotherapy.
It is important and beneficial to predict whether PCa patients after RP suffer from BCR or not. There are many established preoperative and postoperative parameters used in different ways to predict BCR after RP, including the biopsy score of Gleason, preoperative serum PSA level, clinical TNM stage,3 prostate tumor volume,5 perineural invasion,6 PSM, pathological Gleason sum, pathological stage, early period for a surgeon,7 pretreatment neutrophil-to-lymphocyte ratio,8 preoperative serum sex hormone-binding globulin9 and susceptibility at an advanced age10. Nevertheless, some of the parameters may not be independent factors for predicting the risk of BCR statistically. Additionally, there are risk assessment instruments which involve multiple preoperative and postoperative factors, rather than a single factor, to generate a score to prognosticate BCR after RP, such as the CAPRA-S score developed by the UCSF and UCSF-CAPRA score.11,12
The PROSTATE scoring system was first proposed for the prediction of PSM after RP in 2016 and was proved to be an effective instrument for predicting PSM and a helpful predictor in underpinning clinical decision making.13 The present study aims to test the hypothesis that the PROSTATE scoring system can serve as a potential predicative tool in predicating the risk of BCR after RP in clinical practice.
Patients and methods
PROSTATE scoring system
The novel scoring system for PROSTATE was initially used to predict PSM after RP in 2016. The detailed variables incorporated in PROSTATE scores are PSA level (P), ratio of positive biopsy needles (R), obesity (O), scores of Gleason (S), T stage by preoperative MRI scan (T), age (A), total prostate volume (T) and experience of surgeon (E), yet without postoperative variables.
As shown in Table 1, the total PROSTATE scores are determined by the scores from each variable. The PSA level (P) was the total PSA level measured 3 days before the operation. The scores of Gleason (S) were calculated according to the pathological results of biopsy performed by experienced urological pathologists in the Department of Pathology, Institute of Urology of Peking University, based on the 2014 International Society of Urological Pathology Modified Gleason System. The total prostate volume (T) was calculated according to the preoperative B-ultrasonography using the following formula: total prostate volume(m3)= (π/6) × width × length × height. The scoring system has the maximal score of 15 with a minimum of 0. According to this scoring system, a score of 15 signifies the highest risk of BCR after RP while a score of 0 means the lowest risk after RP. In the light of the total scoring, the patients were classified into the low-risk group (0–4), the moderate-risk group (5–9) and the high-risk group (10–15).
  | Table 1 The PROSTATE scoring system |
Ethics approval and informed consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and National Research Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Patient samples
We retrospectively analyzed the clinical records of 340 patients who were preoperatively biopsy-proven with clinically localized PCa and underwent laparoscopic radical prostatectomy in the Urology Department of Peking University First Hospital between November 2007 and March 2016, excluding 26 patients (7.1%) who failed to follow-up. The neurovascular bundles preservation operation was not performed on these patients. Patients, who were treated with neoadjuvant therapy or received adjuvant therapy soon after operation with undetectable tPSA were excluded. The preoperative variables were incorporated into the PROSTATE scoring system. Additionally, the patients’ previous smoking status, which had reported contradictory results in the risk of PCa, was recorded.14–16 The RP specimens were cut transversely at regular intervals from the apex of the prostate to the tips of the seminal vesicles and the entire tumor tissue was submitted for microscopic examination by experienced urological pathologists. Postoperative pathological factors such as lymph node involvement, postoperative score of Gleason, perineural and vessels invasion, and positive surgical margin were collected and grouped. The data update of the patients’ postoperative follow-up, including total prostate specific antigen, was completed by the end of March 2017. The maximum follow-up time was 10 years and the minimum was 1 year. All patients were informed in detail, and approved for being recruited as subjects for scientific purposes during postoperative telephone follow-up. All patients provided oral informed consent over phone for being part of the retrospective study. The study was accepted and approved by the Biomedical Research Ethics Committee of Peking University First Hospital.
BCR after RP was considered as a rise in the level of tPSA ≥0.2 ng/mL in two consecutive tPSA level blood tests, while the patients after RP without BCR (BCR-free) were patients whose tPSA level <0.2 ng/mL at the time of the latest follow-up. When the tPSA reached the level of BCR for the first time after RP, the number of months between RP and BCR development was defined as time to BCR. We defined the patient as a never-smoker if he had smoked less than 100 cigarettes during his lifetime, while the others were defined as former or current smokers.17
Statistics
Basic statistical analysis for continuous variables was done using the Student’s t-test and the categorical variables were compared using the chi-squared test. The performance of PROSTATE score to predict BCR after RP was analyzed using ROC curves. According to the total PROSTATE score as defined above among low, moderate and high risk groups, the probability of BCR-free was compared using Kaplan–Meier analysis, and the log-rank test was applied to compare the BCR-free distributions between the two of them. Cox proportional hazards regression was used to analyze the performance of the different risk groups to predict BCR after RP. All statistical analysis was performed using SPSS, version 22.0, and a 2-sided P <0.05 was considered statistically significant.
Results
Patient characteristics
From November 2007 to March 2016, 340 male patients who underwent laparoscopic radical prostatectomy in the Urology Department of Peking University First Hospital were enrolled in the study. Table 2 lists the clinical and pathological characteristics of the 340 study patients. The mean ± SD age and body mass index were 66.1±6.91 years and 24.3±2.77 kg/m2. There were 81 (23.8%) patients who were former or current smokers. The mean ± SD preoperative PSA was 14.12±11.31 ng/mL and their total prostate volume was 42.8±24.4 mL. The T stage by preoperative MRI scan were as follows: 121 patients (35.6%) in stage T2a or lower, 147 patients (43.2%) in stage T2b and 72 (21.2%) in stage T2c or higher. According to the postoperative pathological results, 117 patients’ PCa (34.4%) were found with perineural and vessels invasion and 137 patients (40.3%) with PSM.
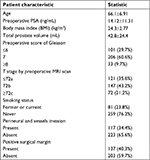  | Table 2 Clinical and pathological characteristics of 340 patients who underwent RP in the Urology Department at Peking University First Hospital |
Association between PROSTATE scores and the risk of BCR after RP
As identified by the exact postoperative follow-up data, 91 cases (26.8%) developed BCR out of the 340 patients who were included. Table 3 shows the detailed comparison of the variables of PROSTATE scores between BCR-developed and BCR-free groups. As shown, the PSA level (P<0.001), the ratio of positive biopsy needles (P<0.001), score of Gleason (P<0.001), the T stage by preoperative MRI scan (P=0.009) and age (P=0.041) were independent preoperative predictors for the risk of BCR after RP. Using logistic regression analysis, the PROSTATE scores were statistically different between BCR-developed and BCR-free groups (P<0.001) (OR =1.375, 95% CI 1.144–1.653). According to the ROC curve analysis, the PROSTATE scores had an accuracy of 70.7% (95% CI 0.643–0.771) (P<0.001).
  | Table 3 Comparison of the variables of PROSTATE scores between BCR-developed and BCR-free patients Abbreviation: BCR, biochemical recurrence. |
Association between different risk groups and BCR-free probability after RP
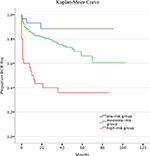
The distribution of PROSTATE scores among the patients included is shown in Table 4, classified based on three different risk groups. The percentage of BCR-developed patients in the high-risk group, moderate-risk group and low-risk group, was 63.3%, 24.6% and 10.3% (P<0.001), respectively. Figure 1 shows that the high-risk group had an increased likelihood of BCR compared to the moderate-risk group (P<0.001) and the low-risk group (P<0.001) with Kaplan–Meier analysis, and the low-risk group was less likely to become BCR-developed than the moderate-risk group (P<0.001).
  | Table 4 Distribution of PROSTATE scores among the 340 patients included and the percentage of BCR on the basis of different risk groups Abbreviation: BCR, biochemical recurrence. |
Multivariate analysis for predictors of BCR after RP
Four of the established postoperative pathological factors and smoking status are compared in Table 5, between patients who developed BCR and those without BCR. Postoperative score of Gleason, perineural and vessels invasion and PSM are statistically different between BCR-developed and BCR-free patients (P<0.001), while the smoking status and lymph node involvement do not have statistical significance.
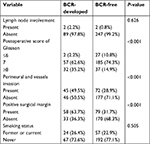  | Table 5 Comparison of the postoperative pathological factors and smoking status between BCR-developed and BCR-free patients Abbreviation: BCR, biochemical recurrence. |
Adjusting for confounding variables, including smoking status, postoperative pathological factors such as lymph node involvement, postoperative score of Gleason, perineural and vessels invasion and PSM, the Cox proportional hazards regression analysis revealed that the grouped PROSTATE score with low, moderate and high risks was an independent predictor of BCR after RP (HR=2.002; 95% CI 1.222–3.280) (P=0.006), as shown in Table 6.
  | Table 6 Multivariate analysis of predictors of BCR after RP |
Discussion
When PCa patients after RP develop BCR, the subsequent management is important for these patients. Patients after RP are always anxious about whether or not they are faced with BCR, as about one third of PCa patients develop BCR after RP.18 For them, BCR after RP means the return of PCa, which they had hoped of being cured of. It is also a dilemma for both surgeons and patients to deal with BCR. Moreover, the time from operation to BCR has been associated with disease progression and prostate cancer-specific mortality.19 In short, the practical BCR-risk assessment model is greatly needed to classify different risk groups and identify them early for secondary therapy.
We performed a MEDLINE review of the English language articles from 2000 to 2017 to identify variables that could predict the risk of BCR after RP. We discovered that many researchers used, postoperatively, variables such as PSM after RP. We did not find relevant literature using preoperative PROSTATE scores to predict the risk of BCR after RP. The PROSTATE scoring system uses a combination of reproducible and easily standardized parameters, obtained from preoperative characteristics of PCa patients and has been proven to be anovel tool for PSM predicting of RP.13 The UCSF-CAPRA score and the CAPRA-S score was proposed in 2005 and in 2011 to assess the risk of PCa. There are other tools in current applications such as Partin tables and Memorial Sloan Kettering Cancer Center nomograms for PCa risk stratification.20 It was considered that preoperative prediction tools could be inaccurate in regard to true PCa grade and extent, so the CAPRA-S score is developed on the basis of the CAPRA score.21 The CAPRA-S score was first proposed by Cooperberg et al,11 using the variables of preoperative PSA, pathologic Gleason score, surgical margins, extracapsular extension, seminal vesicle invasion and lymph node invasion. But the UCSF-CAPRA score has also been externally validated in many studies in America and Europe, yet relying on preoperative PSA, Gleason score, clinical T stage, biopsy results and patient ages.22,23
In the present study, the PROSTATE scoring system has exhibited its performance in predicting BCR after RP. The patients who underwent RP with higher PROSTATE score are more likely to suffer BCR. Making use of the PROSTATE scoring system to predict BCR after RP has a projected accuracy of 70.7%, which is acceptable in clinical application. In the light of the different risk classifications of PROSTATE scores, the high-risk group with a score of 10–15 may have more than six times of risk of developing BCR than the low-risk group with a score of 0–4. Last but not the least, the grouped PROSTATE scores with three grades can perform their prediction of BCR independently, with the evidence shown in multivariate analysis, even though the postoperative pathological factors are thought to be of much stronger clinical evidence in predicting BCR. In considering these results of the predictive scoring system, significant observations support that the PROSTATE scores can be applied in preoperative patient counseling.
The PROSTATE scoring system is also a preoperative predicting tool without using postoperative pathological parameters. The obvious advantage of the preoperative PROSTATE scoring system is that, it can assist in operative decision-making. For patients in the high-risk group classified by the PROSTATE scoring system, more careful operative procedures, and more precise operating technology are required to avoid the attached likely factors of increasing the risk of BCR after RP. It is reported that bilateral nerve-sparing procedures during RP are associated with the increased risk of PSM, which increases the risk of BCR after RP.24 It is recommended that the nerve-sparing procedures should be used on appropriately selected patients but not to increase the probability of PSM or BCR after RP.25 As a result, the nerve-sparing procedures should be more carefully evaluated preoperatively considering the risk of PSM and BCR after RP when the result of the PROSTATE score is at high risk, including the surgeon’s surgical experience and actual operation situation.
Combining the former conclusion that the PROSTATE scoring system can predict PSM of RP and current results that the scoring system can predict the risk of BCR after RP, the scoring system is effectively needed. This scoring system can help divide PCa patients into different risk groups preoperatively and provide the surgeon with the risks of PSM and BCR after RP, so that the decision-making regarding the operation can be more scientific and realistic before the patients lie in the operation room. If the PROSTATE score is 10–15 (high-risk group), a suitable, wide resection during RP should be considered. Overall, the PROSTATE scoring system helps to select, preoperatively, the right patients and the appropriate operating approach.
Besides, for patients with high scores (10–15), except for careful and thorough intraoperative operation, the regular check of PSA after RP should also be extremely stressed. Moreover, radiotherapy or androgen deprivation therapy may be recommended for patients with PROSTATE scores near 15.
There are some limitations in the present study. First, the data is still limited especially for low-risk and high-risk groups, which may affect statistical analysis. Second, the follow-up time is not sufficient to analysis metastasis and cancer specific mortality. Third, the comparison between different tools for predicting BCR after RP, which may lead to a more objective evaluation to the PROSTATE scoring system, is not included in our study. In addition, the patients who did not undergo lymph node dissection during RP were regarded as lymph node negative and most of the patients studied did not undergo lymph node dissection, but the result involved in the present study was qualitative same on subanalysis without this variable “lymph node involvement”. Last but not the least, more independent validation studies of the PROSTATE scoring system using databases from different institutions are required before the scoring system can be widely used in the clinical practice. Despite these limitations and lack of validation in more studies, the present study applied the PROSTATE scoring system in BCR prediction and statistically proved its performance.
Conclusion
The presented findings suggest that the PROSTATE scoring system can predict the risk of BCR after RP. Furthermore, the PROSTATE scoring system can assist in decision-making about operation for the high-risk patients. For patients in the high-risk group, regular check of PSA after RP should be emphasized especially for the high risk of developing BCR. The PROSTATE scoring system may be used to help choose the best operative time for patients whose scores are exactly at ends of the range, but more evidence is still needed. It should be externally validated in future research before the PROSTATE scoring system can be used widely.
Abbreviations
BCR, biochemical recurrence; RP, radical prostatectomy; PCa, prostate cancer; ROC curve, receiver operating characteristic curve; PSM, positive surgical margin; PSA, prostate specific antigen; CAPRA-S, Cancer of the Prostate Risk Assessment Postsurgical Score; UCSF, University of California, San Francisco.
Acknowledgment
Special thanks to Jihong Wei (Department of Applied Linguistics, Peking University, People’s Republic of China) for the contribution to the language of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. | ||
Veeratterapillay R, Goonewardene SS, Barclay J, Persad R, Bach C. Radical prostatectomy for locally advanced and metastatic prostate cancer. Ann R Coll Surg Engl. 2017;99(4):259–264. | ||
Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003;169(2):517–523. | ||
Lou DY, Fong L. Neoadjuvant therapy for localized prostate cancer: Examining mechanism of action and efficacy within the tumor. Urol Oncol. 2016;34(4):182–192. | ||
Hong MK, Namdarian B, Corcoran NM, et al. Prostate tumour volume is an independent predictor of early biochemical recurrence in a high risk radical prostatectomy subgroup. Pathology. 2011;43(2):138–142. | ||
Meng Y, Liao YB, Xu P, Wei WR, Wang J. Perineural invasion is an independent predictor of biochemical recurrence of prostate cancer after local treatment: a meta-analysis. Int J Clin Exp Med. 2015;8(8):13267–13274. | ||
Tanimoto R, Fashola Y, Scotland KB, et al. Risk factors for biochemical recurrence after robotic assisted radical prostatectomy: a single surgeon experience. BMC Urol. 2015;15:27. | ||
Zhang GM, Zhu Y, Ma XC, et al. Pretreatment Neutrophil-to-Lymphocyte Ratio: A Predictor of Advanced Prostate Cancer and Biochemical Recurrence in Patients Receiving Radical Prostatectomy. Medicine. 2015;94(41):e1473. | ||
Waldert M, Schatzl G, Swietek N, Rom M, Klatte T. Sex hormone-binding globulin is an independent predictor of biochemical recurrence after radical prostatectomy. J Urol. 2012;188(3):792–797. | ||
Masuda H, Fukushima H, Kawakami S, et al. Impact of advanced age on biochemical recurrence after radical prostatectomy in Japanese men according to pathological stage. Jpn J Clin Oncol. 2013;43(4):410–416. | ||
Cooperberg MR, Hilton JF, Carroll PR. The CAPRA-S score: A straightforward tool for improved prediction of outcomes after radical prostatectomy. Cancer. 2011;117(22):5039–5046. | ||
Cooperberg MR, Pasta DJ, Elkin EP, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005;173(6):1938–1942. | ||
Xu B, Luo C, Zhang Q, Jin J. Preoperative characteristics of the P.R.O.S.T.A.T.E. scores: a novel predictive tool for the risk of positive surgical margin after radical prostatectomy. J Cancer Res Clin Oncol. 2017;143(4):687–692. | ||
Murphy AB, Akereyeni F, Nyame YA, et al. Smoking and prostate cancer in a multi-ethnic cohort. Prostate. 2013;73(14):1518–1528. | ||
Ho T, Howard LE, Vidal AC, et al. Smoking and risk of low- and high-grade prostate cancer: results from the REDUCE study. Clinical Cancer Research. 2014;20(20):5331–5338. | ||
Bae JM, Li ZM, Shin MH, Kim DH, Lee MS, Ahn YO. Cigarette smoking and prostate cancer risk: negative results of the Seoul Male Cancer Cohort Study. Asian Pac J Cancer Prev. 2013;14(8):4667–4669. | ||
Rieken M, Shariat SF, Kluth LA, et al. Association of Cigarette Smoking and Smoking Cessation with Biochemical Recurrence of Prostate Cancer in Patients Treated with Radical Prostatectomy. Eur Urol. 2015;68(6):949–956. | ||
Fakhrejahani F, Madan RA, Dahut WL. Management Options for Biochemically Recurrent Prostate Cancer. Curr Treat Options Oncol. 2017;18(5):26. | ||
Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294(4):433–439. | ||
Partin AW, Mangold LA, Lamm DM, Walsh PC, Epstein JI, Pearson JD. Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology. 2001;58(6):843–848. | ||
Tilki D, Mandel P, Schlomm T, et al. External validation of the CAPRA-S score to predict biochemical recurrence, metastasis and mortality after radical prostatectomy in a European cohort. J Urol. 2015;193(6):1970–1975. | ||
Zhao KH, Hernandez DJ, Han M, Humphreys EB, Mangold LA, Partin AW. External validation of University of California, San Francisco, Cancer of the Prostate Risk Assessment score. Urology. 2008;72(2):396–400. | ||
Lughezzani G, Budäus L, Isbarn H, et al. Head-to-head comparison of the three most commonly used preoperative models for prediction of biochemical recurrence after radical prostatectomy. Eur Urol. 2010;57(4):562–568. | ||
Preston MA, Breau RH, Lantz AG, et al. The association between nerve sparing and a positive surgical margin during radical prostatectomy. Urol Oncol. 2015;33(1):18.e1–1818. | ||
Nelles JL, Freedland SJ, Presti JC, et al. Impact of nerve sparing on surgical margins and biochemical recurrence: results from the SEARCH database. Prostate Cancer Prostatic Dis. 2009;12(2):172–176. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.