Back to Journals » Journal of Hepatocellular Carcinoma » Volume 9
Preoperative Prognostic Nutritional Index May Be a Strong Predictor of Hepatocellular Carcinoma Recurrence Following Liver Transplantation
Authors Kornberg A , Kaschny L, Kornberg J, Friess H
Received 12 March 2022
Accepted for publication 23 June 2022
Published 27 July 2022 Volume 2022:9 Pages 649—660
DOI https://doi.org/10.2147/JHC.S366107
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmed Kaseb
Arno Kornberg, Linda Kaschny, Jennifer Kornberg, Helmut Friess
Technical University of Munich, School of Medicine, Klinikum rechts der Isar, Department of Surgery, Munich, Germany
Correspondence: Arno Kornberg, Technical University of Munich, Medical School, Klinikum rechts der Isar, Department of Surgery, Ismaningerstr. 22, Munich, D-81675, Germany, Tel +49 89 41405087, Fax +49 89 41404884, Email [email protected]
Purpose: Malnutrition is a major risk factor of immune dysfunction and poor outcome in cancer patients. The prognostic nutritional index (PNI), which is established by serum albumin level and peripheral lymphocyte count, was shown to correlate with prognosis of hepatocellular carcinoma (HCC) patients following liver resection and non-surgical interventions. The aim of this study was to analyze the predictive value of preoperative PNI in liver transplantation (LT) patients with HCC.
Patients and Methods: A total of 123 HCC patients that underwent LT were included in the analysis. The prognostic impact of preoperatively assessed clinical factors including the PNI on post-LT outcome was analyzed by uni- and multivariate analysis.
Results: Post-transplant tumor recurrence rates were 5.1% in high-PNI (> 42) and 55.6% in low-PNI (≤ 42) patients (p < 0.001). Preoperative high-PNI could be identified as a significant and independent promoter of both recurrence-free survival (hazard ratio [HR] = 10.12, 95% CI: 3.40– 30.10; p < 0.001) and overall survival (HR = 1.69, 95% CI: 1.02– 2.79; p = 0.004) following LT. Apart from that low-PNI proved to be a significant and independent predictor of microvascular tumor invasion (OR = 7.71, 95% CI: 3.17– 18.76; p < 0.001). In contrast, no tumor morphology features including the Milan criteria revealed an independent prognostic value.
Conclusion: Our data indicate that preoperative PNI correlates with biological tumor aggressiveness and outcome following LT in HCC patients and may therefore be useful for refining oncologic risk stratification.
Keywords: hepatocellular carcinoma, liver transplantation, prognostic nutritional index, malnutrition, tumor recurrence
Plain Language Summary
Malnutrition is associated with immune dysfunction and poor outcome in patients with malignant diseases. Nonetheless, the nutritional state is frequently not assessed and incorporated in individual decision making process. In recent years, the prognostic nutritional index (PNI), which is based on serum values of albumin and lymphocytes, has developed to a widely accepted biomarker of immunonutritional state and survival. There is growing evidence that the PNI correlates with risk of tumor recurrence and outcome in patients with hepatocellular carcinoma (HCC) that undergo liver resection or non-surgical interventions. However, the prognostic value of PNI in the liver transplantation (LT) setting has not been determined so far. In a series of 123 HCC patients, we identified pre-transplant high-PNI as a significant promoter of survival following LT. Assessment of high-PNI indicated favorable outcome even in patients with advanced HCC stages. Apart from that, low-PNI correlated significantly with presence of microvascular tumor invasion, which in turn is recognized as major oncologic risk factor in this clinical field. We therefore concluded that the PNI may be an easily available and promising serum biomarker to improve individual risk stratification and LT selection process in HCC patients.
Introduction
Despite significant advancements in targeted therapies and ablation techniques, surgical treatment still represents the best option for curation in patients with HCC. In comparison to liver resection, which may be inappropriate due to tumor multifocality and/or portal hypertension, LT provides superior oncological control, since it removes both the tumor and cancerogenic liver.1 Nonetheless, in order to reduce the risk of HCC relapse in the context of lifelong immunosuppressive treatment, a rigorous selection process remains necessary. In particular, the introduction of the Milan criteria (MC) in 1996 for defining an upper morphometric tumor burden limit became a milestone for implementing LT as standard treatment in non-resectable early HCC stages. From now on, post-LT long-term survival rates beyond 70% could be achieved, which indeed were not different from those in non-malignancy LT indications.2 Nowadays, in times of a worsening donor organ shortage, tumor size criteria are assessed obsolete, due to insufficient discriminatory capacities. Strict adherence to morphometric selection models, like the Milan or University of California San Francisco criteria, did not prove effective for persistently reducing the oncologic risk below 20% while, in turn, many patients with low-aggressive tumors exceeding standard tumor burden limits had been excluded from potentially curative LT option.3–5 It emerged clearly from numerous studies that biological behavior rather than tumor load determines the oncologic risk and post-LT outcome.5,6 In addition to intrinsic features of unfavorable tumor phenotype, like angioinvasiveness and poor differentiation, host systemic inflammatory response reaction is meanwhile accepted as another important trigger of tumor progression and metastasis.6 In recent years, the nutritional condition was identified as a major determinant of inflammatory state, immunocompetence and treatment response. Even though being a frequent complication in patients with cirrhosis and/or liver malignancy, malnourishment generally remains underdiagnosed and is not incorporated into risk assessment.7,8 Based on serum levels of albumin and lymphocytes, the prognostic nutritional index (PNI) represents an easily available parameter to describe the link between nutritional and immunologic state. Originally introduced to determine the perioperative risk in gastrointestinal surgery,9 it subsequently developed to an established biomarker in a wide range of different cancer entities, including gastric, esophageal, colorectal, and lung cancer.10–13 Recently, there is growing evidence in HCC patients that PNI correlates with prognosis following non-surgical treatment and liver resection.14–16 Its predictive significance in the LT setting remained, however, so far undetermined. Therefore, we conducted a retrospective study to explore the prognostic impact of preoperative PNI in HCC patients following LT.
Patients and Methods
Study Population and Waiting List Management
A total of 123 HCC patients who received a liver allograft between 1997 and 2014 were identified in a prospectively managed LT database. Transplant candidates gave informed consent that clinical data may be used for academic investigations. All organs were donated voluntarily with written informed consent, and this was conducted in accordance with German law and with the Declaration of Istanbul. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of the ethical committee of the Technical University Munich (Nr. 217/15). Diagnosis of HCC was based on radiographic imaging, without incorporating tumor biopsy. While elevated serum alpha-fetoprotein (AFP) level confirmed HCC diagnosis, negative AFP did not automatically exclude liver cancer. Waiting time on the LT list and Child Pugh classification were used for prioritization of organ allocation until November 2007, whereas the model of end-stage liver disease (MELD) score has been adopted thereafter, applying MELD exceptional points to patients with HCC meeting the MC.17 Dependent on functional liver capacity and tumor morphology and topography, transplant candidates underwent neoadjuvant locoregional tumor treatment by transarterial chemoembolization (TACE). Patients with tumor burden that was just not fulfilling the MC (one tumor nodule up to 5 cm, or up to 3 tumor nodules each not exceeding 3 cm in diameter, absence of macrovascular tumor invasion and extrahepatic tumor spread) were not generally excluded from LT. Based on an interdisciplinary LT board decision, they were listed without MELD priority and referred to a rescue liver allocation program. These patients underwent a very close clinical and radiographic re-evaluation minimum every 6 weeks. Rapid bilateral and/or multifocal tumor progression, macrovascular invasion of the tumor, extrahepatic HCC spread and tumor-related symptoms were contraindication for LT and resulted in patients’ delisting.
Surgical Procedure and Post-LT Follow-Up
Standard deceased donor LT without using veno-vevous bypass was performed in all study patients.1 The explanted tumor livers underwent histopathologic examination to describe tumor morphology and to identify features of tumor aggressiveness, including microvascular invasion (MVI), grading, lymphovascular infiltration (LVI) and rate of post-interventional tumor necrosis (> versus ≤ 50%). Post-LT immunosuppressive therapy consisted of a calcineurin inhibitor (CNI) based regimen by using cyclosporine A (CsA; intended trough level: 100–150 µg/L) or tacrolimus (Tac; intended trough level: 5–7 ng/dl), augmented by mycophenolate mofetil (2 x 500 mg/day). Corticosteroids were withdrawn latest after 3 months. Biopsy-proven allograft rejection (BPR) was treated by increase of CNI dose and additionally by steroid bolus when needed. The post-transplant surveillance protocol consisted of abdominal ultrasound and determination of serum AFP level every 3 months, and additionally by thoraco-abdominal tomographic imaging minimum twice a year.
PNI and Additional Outcome Variables
Based on preoperative serum analysis, PNI was calculated using the formula: 10 x albumin (g/dL) + 0.005 x lymphocyte count, as previously described.14–16 In order to comply with real-life decision-making process, we have primarily focused on pre-transplantation collected data. Therefore, in addition to PNI, the following clinical variables were used for our multivariable risk analysis: sex; age; etiology of liver cirrhosis; Child Pugh status; laboratory (lab)MELD score; Number and diameter of HCC; MC (Milan-in versus Milan-out); TACE; serum levels of AFP (normal value ≤ 6 ng/mL) and the inflammatory markers C-reactive protein (CRP; normal value < 0.5 mg/dl) and interleukin-6 (IL-6; normal value < 7 ng/L); and body mass index.
Statistical Analysis
The SPSS 25.0 software (IBM Inc., Munich, Germany) was used for all statistical analyses. Data are expressed as median and range and compared using Mann–Whitney U-test. Chi square test or Fisher’s test were used to analyse categorical variables. In order to establish the most appropriate PNI cut-off value for predicting tumor recurrence, receiver operating characteristic curves and the estimated area under the curve (AUC) were determined. Post-transplantation recurrence-free survival (RFS) and overall survival (OS) rates were calculated using the Kaplan-Meier method. For defining independent prognostic factors of RFS and OS, a Cox’s proportional hazard model was used. A logistic regression model was applied to identify independent predictors of MVI. A p-value < 0.05 was considered statistically significant for all investigations.
Results
Clinicopathologic Characteristics
Characteristics of the study group are demonstrated in Table 1. Seventy-four male and 49 female patients with a median age of 59 years at LT were enrolled in the analysis. Ethyltoxic disease (55.3%) and viral hepatitis (30.9%) were major causes of liver cirrhosis. Fifty-two patients demonstrated Child Pugh A cirrhosis (42.3%), whereas 71 patients were classified Child Pugh B or C (57.7%). Preoperative (lab)MELD score was ranging between 10 and 34. Eighty-three patients underwent TACE prior LT (67.5%); 41 of them have received more than one interventional application. At final pre-LT radiographic staging, tumor load was classified as meeting and exceeding the MC in 69 (56.1%) and 54 (43.9%) patients (Table 1).
 |
Table 1 Clinical Characteristics of the Study Group |
Overall Outcome
Median postoperative follow-up was 84 months (5–190 months). Five patients underwent liver re-transplantation (4.1%) due to organ non-functioning, and 19 patients had to be treated for BPR (15.4%). A total of 29 patients developed recurrent HCC (23.6%) after a median of 11 months (4–55), 9 of the Milan-in (13%) and 20 of the Milan-out cohort (37%; p = 0.002). Risk of HCC recurrence was comparable between patients treated (20.5%) and those not treated with neoadjuvant TACE (30%; p = 0.244). HCC recurrence rates were 4.9% and 36.4% (p < 0.001) in patients with post-TACE tumor necrosis ≤ versus > 50%. BPR with need of anti-rejection therapy did not significantly affect the oncologic risk (21.9% versus 27.8%; p = 0.650).
Predictive Value of PNI
Preoperative serum albumin level and peripheral lymphocyte count were 3.5 g/dL (2–4.9) and 1.9 x 109/L (0.55–3.40) respectively, calculated PNI was 46 (28–61) in median. Pre-transplant PNI was 37 (28–53) and 48 (29–61) in patients with and without HCC recurrence (p < 0.001). We established a PNI of 42 as threshold for predicting tumor recurrence (AUC 0.896, 95% CI: 0.831–0.961; p < 0.001); related positive and negative predictive values were 55.6% and 94.9%. Accordingly, we defined a low-PNI (PNI ≤ 42) and a high-PNI (PNI > 42) subset. Clinicopathologic differences between both PNI cohorts are demonstrated in Table 2. Low-PNI was associated with more severe liver dysfunction, when considering Child Pugh status and (lab)MELD score. In addition, low-PNI patients demonstrated a higher tumor diameter, increased body mass index and elevated serum levels of CRP and IL-6. Numbers of liver-re-transplants and BPR were comparable between both PNI subsets. With regard to histopathologic results, low-PNI patients were more likely to suffer from aggressive tumor phenotype (Table 2). Post-transplantation RFS and OS rates were significantly better in high-PNI compared to low-PNI patients (Figures 1 and 2). The implementation of PNI allowed for a significant oncologic risk stratification in both Milan-in and Milan-out patients (Figure 3). Tumor recurrence rate was only 6.5% in Milan-out patients with high-PNI, which was not different from high-PNI Milan-in patients (4.3%; p = 0.667), but significantly lower compared to low-PNI patients meeting the MC (31.8%; p = 0.015). The combination of Milan-out and low-PNI indicated an extraordinary risk of HCC recurrence (78.3%; p = 0.001).
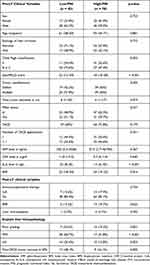 |
Table 2 Clinicopathologic Differences Between the Low-PNI and the High-PNI Group |
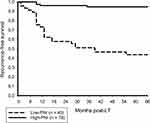 |
Figure 1 Post-LT 5-year RFS rates were 94.7% and 43.7% in high-PNI and low-PNI patients, respectively (p < 0.001). |
 |
Figure 2 Post-LT 5-year OS rate was 88.5% in high-PNI but only 46.4% in low-PNI (p < 0.001). |
Independent Predictors of Outcome
In addition to serum levels of AFP and CRP, high-PNI was identified as significant and independent predictor of post-LT RFS in multivariate analysis (HR = 10.12, 95% CI: 3.40–30.10) p < 0.001; (Table 3). Apart from that, PNI > 42 was shown to independently promote post-LT OS (HR = 1.69, 95% CI: 1.02–2.79; p = 0.004), along with recipients’ age, AFP level and CRP value (Table 4).
 |
Table 3 Uni- and Multivariate Analysis of Factors Promoting Post-Transplant RFS |
 |
Table 4 Uni- and Multivariate Analysis of Factors Promoting Post-Transplant OS |
Independent Predictors of MVI
On explant liver histopathologic examination, MVI could be detected in 17 high-PNI (21.8%) but in 30 low-PNI patients (66.7%; p < 0.001). Post-LT HCC recurrence rates were 6.6% in patients without, but 51.1% in those with evidence of MVI. When being incorporated in our multivariate analysis, absence of MVI was identified as another independent promoter of RFS (HR = 4.67, 95% CI: 1.66–13.11; p = 0.004), without however, PNI to lose its independent predictive value. In univariate analysis, Milan status, AFP level, CRP level, IL-6 value and PNI correlated significantly with MVI. Low-PNI was found to independently predict MVI on multivariate analysis (OR = 7.71, 95% CI: 3.17–18.76; p < 0.001), along with serum CRP value (Table 5).
 |
Table 5 Uni- and Multivariate Analysis of Predictors of MVI |
Discussion
It has been demonstrated in recent years that nonresolving inflammation is not only a trigger of hepatocarcinogenesis, but may also adversely affect treatment response and outcome.18 In addition to pro-inflammatory and immunosuppressive mechanisms induced by the tumor, the clinical state and functional performance determine host inflammatory response reaction and thereby prognosis.19,20 In this context, malnutrition and consecutive sarcopenia are particularly supposed to impair immunologic competence and survival.21,22 Thus, early assessment and consequent treatment of malnourishment may not only reduce perioperative morbidity and functional deterioration, but could also improve the oncologic risk profile.23
The PNI is a cost-effective serum-based parameter for immunonutritional evaluation. In recent years, it evolved to a widely accepted biomarker in many cancer entities, including HCC.10–16 Two meta-analyses reported on an independent prognostic value of PNI in patients following hepatectomy and non-surgical treatments, whereas predictive significance in the LT setting remained undetermined.24,25
To the best of our knowledge, we present the first study to define preoperative PNI as an independent prognostic factor in HCC patients receiving a liver transplant. In the context of other well-established tumor biological factors, such as AFP and CRP, low-PNI was identified as most powerful predictor of HCC relapse in our LT series (Table 2). In contrast, none of tumor morphometric variables, including the MC, demonstrated an independent prognostic value. Since we did not perform molecular-biological analyses in the framework of this study, we are currently not able to provide a clear pathomechanistic explanation for this notable observation. However, when considering defining components, a wide range of immuno-biological efficacies may be attributed to PNI. According to the respective formula, low-PNI results from hypoalbuminemia and/or lymphopenia.9,10 Lower serum albumin levels may be related to liver dysfunction, which in turn is discussed as tumor-promoting factor also in the LT setting.19 Hypoalbuminemia was also reported to correlate with unfavorable tumor characteristics.26 Another study suggested a direct cell-growth inhibiting effect of albumin.27 And moreover, as a negative acute-phase reactant, lower albumin could emphasize on systemic inflammatory response reaction as potentially cancerogenic factor.28 Lymphocytes are known to play a major role in anti-cancer immune defense, either by providing direct cytolytic capacity or by recruiting other immune cells via cytokine release.29 Peritumoral composition of CD4+, CD8+ and regulatory T cells was shown to correlate with tumor-specific survival,30,31 whilst B cells may suppress carcinogenesis by secretion of immunoglobulins with anti-HCC efficacies.32 Consequently, lymphopenia alone or integrated in composite inflammatory scores, such as neutrophil-to-lymphocyte ratio or platelet-to-lymphocyte ratio, was reported to act as an independent adverse predictor.6,33
In view of these tumor-immunologic mechanisms and correlations, we hypothesized that PNI could serve as tumor marker also in the LT setting. Noteworthy, we found a strong association between low-PNI and unfavorable histopathologic features, such as poor grading, MVI, LVI and post-interventional tumor necrosis rate (Table 2). Beyond that, PNI ≤ 42 emerged as strongest independent predictor of MVI in our analysis (Table 5), which was another important result of our study.
Clearly, the sole use of tumor size and number has in the past proven inappropriate for selecting eligible liver transplant recipients.1–5 Nowadays, vascular invasion instead of morphologic tumor burden is considered as most adverse prognostic factor.34 There is convincing evidence that LT of non-angioinvasive HCC patients is associated with low recurrence risk, regardless of meeting or exceeding the MC.35,36 While tumor infiltration into major hepatic vessels visualized on radiographic imaging generally excludes patients from LT, relevant data on MVI may be obtained only post-LT by explant liver histopathologic analysis. Thus, the incorporation of reliable surrogate markers indicating aggressive tumor phenotype is essential for an accurate oncologic risk stratification prior LT.4,5,35,36 Results of our study suggest that immunonutritional evaluation may significantly increase the discriminatory capacity of the MC, and thus, improve selection of eligible LT patients. Most importantly, high-PNI indicated excellent RFS in Milan-out patients (> 90%), which was in fact higher than in Milan-in recipients with low-PNI (68.2%; Figure 2). If we had realized a strict Milan-based selection process, about 25% of our study population would have been excluded from LT despite excellent survival probability. This noteworthy result emphasized on the predictive limitations of the MC and is another plea for expanding morphometric selection criteria by implementing surrogates of tumor biology. But also in case of a conservative allocation policy, it may be reasonable to implement data on immunonutritional condition. Even though meeting the MC, post-LT tumor relapse rate was exceeding 30% in our low-PNI subset (Figure 2), which could be a critical issue for transplant programs with a high waiting list pressure.
Pending validation in a larger study cohort, our findings may have useful implications not only for optimizing selection process, but also to improve perioperative treatment strategies. First, in view of an elevated oncologic risk profile, post-LT tumor surveillance should be performed more closely in low-PNI patients. In addition, it has to be clarified whether immunonutrition may improve PNI and thereby post-LT outcome.37 And furthermore, our results seem to implicate that well-established anti-tumor concepts should consequently be implemented in immunonutritive high-risk patients. In this context, feasibility and outcome of an intensified neoadjuvant tumor treatment have to be evaluated.38 As mentioned above, we did not identify a significant prognostic influence of pre-LT TACE versus no-TACE in our LT series, which may be related to selection bias and/or low case numbers. But we noted a significantly higher oncologic risk in case of post-interventional tumor necrosis rate ≤ versus > 50%, and notably, low-PNI patients were more likely to adequately respond to TACE (Table 2). The underlying mechanisms for a possible association between immunonutrional state and TACE eligibility and efficacy remained unclear. However, since degree of post-interventional tumor necrosis was reported to correlate with tumor biology and survival, this finding of our study at least seems to provide another indication for a close relationship between PNI and intrinsic HCC aggressiveness.39,40 Apart from that, low-PNI patients could probably benefit from a tailored immunosuppression, which, however, should not result in an aggravated risk of BPR, since anti-rejection treatment was identified as an important trigger of tumor recurrence.41–43 Immunosuppressive treatment including targeted CNI trough levels were standardized in our LT cohort. In addition, there was no significant difference with regard to applied CNI, rates of BPR and risk of liver re-transplant, which may point to a comparable immunologic state in both PNI subsets (Table 2). Nonetheless, it should be stressed that for an updated oncologic risk assessment and surveillance planning, not only preoperatively analyzed biomarkers, but also postoperatively determined features of tumor biology, including histopathologic data and factors of immune response, have to be considered.
Beside lack of molecular-biological investigations, the retrospective character was another limitation of our study, even though data had been prospectively collected. Moreover, our subgroups were rather small. And finally, we did not determine serial PNI values during listing period. Thus, PNI-related drop-out rates and intent-to-treat survival probabilities could not be calculated. This should be addressed in future studies. In contrast, our study was powered by a very close follow-up and a complete data collection. In addition, we were able to include a rather high number of advanced HCC patients.
Conclusion
In conclusion, our study identified preoperative PNI as a strong prognostic factor in liver transplant patients with HCC. Due to association with tumor biology and survival, it may be a useful outcome predictor that, following verification in a multicenter study cohort, should be incorporated for improving oncologic risk stratification, particularly when liberalizing tumor morphometric LT selection criteria.
Abbreviations
AFP, alpha-fetoprotein; AUC, area under the curve; BPR, biopsy-proven rejection; CsA, cyclosporine A; BMI, body mass index; HR, hazard ratio; HCC, hepatocellular carcinoma; LT, liver transplantation; LVI, lymphovascular invasion; MC, Milan criteria; MELD, model of end-stage liver disease; MVI, microvascular invasion; OR, odds ratio; OS, overall survival; PNI, prognostic nutritional index; RFS, recurrence-free survival; Tac, tacrolimus; TACE, transarterial chemoembolization.
Data Sharing Statement
Data related to the study can be provided by the corresponding author upon request.
Acknowledgments
The authors gratefully acknowledge the support of Dr. Ulrike Witt (TU Munich), Dr. Katharina Müller (FSU Jena) and Dr. Katharina Thrum (Helios Berlin) for critical review and editing of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol. 2017;14:203–217. doi:10.1038/nrgastro.2016.193
2. Mazzaferro V, Chun YS, Poon RT, et al. Liver transplantation for hepatocellular carcinoma. Ann Surg Oncol. 2008;15:1001–1007. doi:10.1245/s10434-007-9559-5
3. Bento de Sousa JH, Calil IL, Tustumi F, et al. Comparison between Milan and UCSF criteria for liver transplantation in patients with hepatocellular carcinoma: a systematic review and meta-analysis. Transl Gastroenterol Hepatol. 2021;11. doi:10.21037/tgh.2020.01.06
4. Piñero F, Anders M, Boin IF, et al. Liver transplantation for hepatocellular carcinoma: impact of expansion criteria in a multicenter cohort study from a high waitlist mortality region. Transpl Int. 2021;34:97–109. doi:10.1111/tri.13767
5. Halazun KJ, Sapisochin G, von Ahrens D, et al. Predictors of outcome after liver transplantation for hepatocellular carcinoma (HCC) beyond Milan criteria. Int J Surg. 2020;82:61–69. doi:10.1016/j.ijsu.2020.07.029
6. Sanghera C, The JJ, Pinato DJ. The systemic inflammatory response as a source of biomarkers and therapeutic targets in hepatocellular carcinoma. Liver Int. 2019;39:2008–2023. doi:10.1111/liv.14220
7. Schütte K, Schulz C, Malfertheiner P. Nutrition and hepatocellular cancer. Gastrointest Tumors. 2006;2:188–194. doi:10.1159/000441822
8. Huang TH, Hsieh CC, Kuo LM, et al. Malnutrition associated with an increased risk of postoperative complications following hepatectomy in patients with hepatocellular carcinoma. HPB. 2019;21:1150–1155.
9. Buzby GP, Mullen JL, Matthews DC, et al. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1998;139:160–167. doi:10.1016/0002-9610(80)90246-9
10. Kim KW, Lee K, Lee JB, et al. Preoperative nutritional risk index and postoperative one-year skeletal muscle loss can predict the prognosis of patients with gastric adenocarcinoma: a registry-based study. BMC Cancer. 2021;157. doi:10.1186/s12885-021-07885-7
11. Wang B, Jiang XW, Tian DL, et al. Combination of haemoglobin and prognostic nutritional index predicts the prognosis of postoperative radiotherapy for esophageal squamous cell carcinoma. Cancer Manag Res. 2020;12:8589–8597. doi:10.2147/CMAR.S266821
12. Lee SY, Lee SI, Min BW, et al. Prognostic implication of systemic inflammatory markers in young patients with resectable colorectal cancer. Ann Surg Treat Res. 2021;100:25–32.
13. Jiang AM, Zhao R, Liu N, et al. The prognostic value of pretreatment prognostic nutritional index in patients with small cell lung cancer and it’s influencing factors: a meta-analysis of observational studies. J Thorac Dis. 2020;12:5718–5728. doi:10.21037/jtd-20-1739
14. Rimini M, Yoo C, Lonardi S, et al. Role of the prognostic nutritional index in predicting survival in advanced hepatocellular carcinoma treated with regorafenib. Hepatol Res. 2021;51:796–802. doi:10.1111/hepr.13669
15. Imai D, Maeda T, Shimokawa M, et al. Prognostic nutritional index is superior as a predictor of prognosis among various inflammation-based prognostic scores in patients with hepatocellular carcinoma after curative resection. Hepatol Res. 2020;50:101–109. doi:10.1111/hepr.13431
16. Saito Y, Imura S, Morine Y, et al. Preoperative prognostic nutritional index predicts short- and long-term outcomes after liver resection in patients with hepatocellular carcinoma. Oncol Lett. 2021;21:153. doi:10.3892/ol.2020.12414
17. Pillai A, Couri T, Charlton M. Liver allocation policies in the USA: past, present, and the future. Dig Dis Sci. 2019;4:985–992. doi:10.1007/s10620-019-05549-y
18. Cescon M, Bertuzzo VR, Ercolani G, et al. Liver transplantation for hepatocellular carcinoma: role of inflammatory and immunologic state on recurrence and prognosis. World J Gastroenterol. 2013;19:9174–9182. doi:10.3748/wjg.v19.i48.9174
19. Kornberg A, Witt U, Schernhammer M, et al. The role of preoperative albumin-bilirubin grade for oncological risk stratification in liver transplant patients with hepatocellular carcinoma. J Surg Oncol. 2019;120:1126–1136. doi:10.1002/jso.25721
20. Marasco G, Serenari M, Renzulli M, et al. Clinical impact of sarcopenia assessment in patients with hepatocellular carcinoma undergoing treatments. J Gastroenterol. 2020;55:927–943. doi:10.1007/s00535-020-01711-w
21. Bourke CD, Berkley JA, Prendergast AJ. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol. 2016;37:386–398. doi:10.1016/j.it.2016.04.003
22. Schütte K, Tippelt B, Schulz C, et al. Malnutrition is a prognostic factor in patients with hepatocellular carcinoma (HCC). Clin Nutr. 2015;34:1122–1127. doi:10.1016/j.clnu.2014.11.007
23. Duong N, Sadowski B, Rangnekar AS. The impact of frailty, sarcopenia, and malnutrition on liver transplant outcomes. Clin Liver Dis. 2021;17:271–276. doi:10.1002/cld.1043
24. Man Z, Pang Q, Zhou L, et al. Prognostic significance of preoperative prognostic nutritional index in hepatocellular carcinoma: a meta-analysis. HPB (Oxford). 2018;20:888–895. doi:10.1016/j.hpb.2018.03.019
25. Fan X, Chen G, Li Y, et al. The preoperative prognostic nutritional index in hepatocellular carcinoma after curative hepatectomy: a retrospective cohort study and meta-analysis. J Invest Surg. 2019;34:826–833. doi:10.1080/08941939.2019.1698679
26. Carr BI, Guerra V. Serum albumin levels in relation to tumor parameters in hepatocellular carcinoma patients. Int J Biol Markers. 2017;32:391–396. doi:10.5301/ijbm.5000300
27. Bağırsakçı E, Şahin E, Atabey N, et al. Role of albumin in growth inhibition in hepatocellular carcinoma. Oncology. 2017;93:136–142. doi:10.1159/000471807
28. Artigas A, Wernerman J, Arroyo V, et al. Role of albumin in diseases associated with severe systemic inflammation: pathophysiologic and clinical evidence in sepsis and in decompensated cirrhosis. J Crit Care. 2016;33:62–70. doi:10.1016/j.jcrc.2015.12.019
29. Lu C, Rong D, Zhang B, et al. Current perspectives on the immunosuppressive tumor microenvironment in hepatocellular carcinoma: challenges and opportunities. Mol Cancer. 2019;18:130. doi:10.1186/s12943-019-1047-6
30. Tian MX, Liu WR, Wang H, et al. Tissue-infiltrating lymphocytes signature predicts survival in patients with early/intermediate stage hepatocellular carcinoma. BMC Med. 2019;17:106. doi:10.1186/s12916-019-1341-6
31. Unitt E, Marshall A, Gelson W, et al. Tumour lymphocytic infiltrate and recurrence of hepatocellular carcinoma. J Hepatol. 2006;45:246–253. doi:10.1016/j.jhep.2005.12.027
32. Brunner SM, Itzel T, Rubner C, et al. Tumor-infiltrating B cells producing antitumor active immunoglobulins in resected HCC prolong patient survival. Oncotarget. 2017;8:71002–71011. doi:10.18632/oncotarget.20238
33. Nagai S, Abouljoud MS, Kazimi M, et al. Peritransplant lymphopenia is a novel prognostic factor in recurrence of hepatocellular carcinoma after liver transplantation. Transplantation. 2014;97:694–701. doi:10.1097/01.TP.0000437426.15890.1d
34. Pommergaard HC, Rostved AA, Adam R, et al. European Liver and Intestine Transplant Association (ELITA). Vascular invasion and survival after liver transplantation for hepatocellular carcinoma: a study from the European Liver Transplant Registry. HPB (Oxford). 2018;20:768–775. doi:10.1016/j.hpb.2018.03.002
35. Mazzaferro V, Llovet JM, Miceli R, et al; Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi:10.1016/S1470-2045(08)70284-5
36. Sotiropoulos GC, Machairas N, Fouzas I, et al. Prediction of microvascular tumor invasion in liver transplant candidates with hepatocellular carcinoma: a feasible concept or a misleading illusion? Transplant Proc. 2019;51:421–423. doi:10.1016/j.transproceed.2019.01.016
37. Luo Y, Teng F, Fu H, Ding G-S. Immunotherapy in liver transplantation for hepatocellular carcinoma: pros and cons. World J Gastrointest Oncol. 2022;14:163–180. doi:10.4251/wjgo.v14.i1.163
38. Lai Q, Vitale A, Lesari S, et al; European Hepatocellular Cancer Liver Transplant Study Group. The intention-to-treat effect of bridging treatments in the setting of Milan criteria-in patients waiting for liver transplantation. Liver Transpl. 2019;25:1023–1033. doi:10.1002/lt.25492
39. Ho MH, Yu CY, Chung KP, et al. Locoregional therapy-induced tumor necrosis as a predictor of recurrence after liver transplant in patients with hepatocellular carcinoma. Ann Surg Oncol. 2011;18:3632–3639. doi:10.1245/s10434-011-1803-3
40. Allard M-A, Sebagh M, Ruiz A, et al. Does pathological response after transarterial chemoembolization for hepatocellular carcinoma in cirrhotic patients with cirrhosis predict outcome after liver resection or transplantation? J Hepatol. 2015;63:83–92. doi:10.1016/j.jhep.2015.01.023
41. Vivarelli M, Bellusci R, Cucchetti A, et al. Low recurrence rate of hepatocellular carcinoma after liver transplantation: better patient selection or lower immunosuppression? Transplantation. 2002;74:1746–1751. doi:10.1097/00007890-200212270-00017
42. Gül-Klein S, Kästner A, Haber PK, et al. Recurrence of hepatocellular carcinoma after liver transplantation is associated with episodes of acute rejections. J Hepatocell Carcinoma. 2021;8:133–143.
43. Lai Q, Iesari S, Finkenstedt A, et al. Hepatocellular carcinoma recurrence after acute liver allograft rejection treatment: a multicenter European experience. Hepatobiliary Pancreat Dis Int. 2019;18:517–524. doi:10.1016/j.hbpd.2019.05.006
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

