Back to Journals » International Journal of General Medicine » Volume 16
Preoperative Inflammation-Associated Blood Cell Markers in Patients with Non-Metastatic Clear Cell Renal Cell Carcinoma: A Retrospective Study
Received 19 May 2023
Accepted for publication 7 July 2023
Published 19 July 2023 Volume 2023:16 Pages 3067—3080
DOI https://doi.org/10.2147/IJGM.S417948
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Yuling Cheng,* Wei Kou,* Yu Zhu
Department of Urology, Shanghai Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yu Zhu, Department of Urology, Shanghai Ruijin Hospital, Ruijin 2nd Road, Huangpu District, Shanghai, 200025, People’s Republic of China, Tel +86 21-64370045, Email [email protected]
Purpose: This study aimed to investigate the association between preoperative inflammation-associated blood cell markers and the prognosis of patients with non-metastatic clear cell renal cell carcinoma (ccRCC) who underwent nephrectomy.
Patients and Methods: We retrospectively analyzed data from our single-center cohort of patients who underwent radical or partial nephrectomy for non-metastatic ccRCC. The optimal cutoff values for red blood cell distribution width (RDW), platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), and lymphocyte-to-monocyte ratio (LMR) were determined using X-tile software. We evaluated recurrence-free survival (RFS), cancer-specific survival (CSS), and overall survival (OS) using the Kaplann-Meier method. Cox proportional-hazards regression models were utilized to assess predictors of RFS, CSS, and OS. The predictive accuracy was evaluated using Harrell’s Concordance Index (C-index).
Results: A total of 444 patients who underwent nephrectomy were included in the study. The optimal cutoff values for RDW, PLR, NLR, and LMR were determined as 13.1, 157.3, 3.4, and 2.7, respectively. On univariate Cox regression analysis, NLR, PLR, and LMR were significant predictors for RFS, CSS, and OS. After adjusting for important prognostic factors, only NLR remained a significant prognostic marker for both CSS and OS. When NLR was added to the stage, size, grade, and necrosis (SSIGN) model, the C-index increased from 0.777 to 0.826 for CSS and from 0.703 to 0.734 for OS. Similarly, when NLR was added to the University of California, Los Angeles, Integrated Staging System (UISS), the C-index increased from 0.796 to 0.811 for CSS and from 0.735 to 0.745 for OS.
Conclusion: NLR is a reliable prognostic biomarker for patients with non-metastatic ccRCC. The prognostic capabilities of UISS and SSIGN models could be improved by adding NLR to UISS and SSIGN models.
Keywords: clear cell renal cell carcinoma, neutrophil-to-lymphocyte ratio, prognosis, recurrence
Introduction
Renal cell carcinoma (RCC) is the most common type of kidney tumor, accounting for 2–3% of all malignancies worldwide.1,2 The predominant subtype of RCC is clear cell renal cell carcinoma, which makes up approximately 80–90% of all RCC cases.3 Although ccRCC is not the most aggressive histological type, it is associated with lower survival rates compared to other subtypes such as chromophobe and papillary RCCs.4 For ccRCC patients without metastasis, surgery is the standard and most effective treatment, unless the patients are unable to tolerate the risks associated with surgery.4 The prognosis of patients with non-metastatic clear cell renal cell carcinoma (ccRCC) is influenced by many factors such as the pathological stage and Fuhrman grade. Recent studies have indicated that the five-year survival rate for non-metastatic patients ranges from 60% to 90%.5 However, it is important to note that localized ccRCC patients (cT1–3N0M0) have a relapse probability of 20% to 30% after nephrectomy. For advanced ccRCC patients (cT4N0M0), the recurrence or metastasis rate is estimated to be around 30% to 40%.6 To guide treatment planning and follow-up for RCC patients, it is crucial to accurately predict the individual risk of recurrence and progression following surgery.
Several pathological prognostic factors, including nuclear grade and Tumor, Node, Metastasis (TNM) staging, are commonly employed to predict the prognosis of postoperative RCC patients. University of California, Los Angeles (UCLA) developed a prognostic model, known as the UISS, to predict survival of patients with localized and metastatic RCC after surgery. The model classified patients into three risk groups: low-risk, intermediate-risk, and high-risk according to three factors (grade, stage, and Eastern Cooperative Oncology Group performance status).7 A large multicenter study involving 4202 patients validated and tested this model. For patients with localized RCC, the 5-year survival rates were 92% for the low-risk group, 67% for the intermediate-risk group, and 44% for the high-risk group.8 For patients with metastatic RCC, the 3-year survival rates were 37% for the low-risk group, 23% for the intermediate-risk group, and 12% for the high-risk group.8 Another study conducted by Frank et al focused on predicting cancer-specific survival in patients with clear cell RCC who underwent nephrectomy. They developed a risk model called SSIGN, which differs from UISS as it incorporates only four pathologic parameters: TNM stage, tumor size, nuclear grade, and histologic tumor necrosis.9 According to the study, the 5-year cancer-specific survival rates varied based on the SSIGN score.9 Patients with a score of 0 to 2 had a 100% survival rate, those with a score of 3 to 4 had a 90.5% survival rate, those with a score of 5 to 6 had a 63.6% survival rate, those with a score of 7 to 9 had a 46.8% survival rate, and those with a score of 10 or more had a 0% survival rate.10 However, accurate prediction of individual prognosis remains difficult.11 Thus, it is necessary to find novel preoperative prognostic markers to give prognostic information on non-metastatic RCC patients.
Increasing evidence supports the role of tumor-associated inflammation in the development and progression of tumors. Several factors, including NLR, PLR, LMR, and RDW, have been identified as prognostic predictors in patients with localized ccRCC (cT1–3N0M0) and metastatic ccRCC (cT1–4N0–1M1).12–17 However, there is limited research on all these markers in ccRCC patients, particularly those with non-metastatic disease. In this study, we aimed to evaluate the impact of NLR, PLR, LMR, and RDW on recurrence and survival in non-metastatic ccRCC patients. Additionally, we investigated the effect of incorporating these markers into the SSIGN and UISS models.
Materials and Methods
This study was approved by the ethics committee and institution review board of our hospital. The medical records of non-metastatic RCC patients, who underwent radical or partial nephrectomy at Shanghai Ruijin Hospital from January 2015 to December 2017, were reviewed. Of this dataset, we excluded cases with non-clear cell histology and restricted our analyses to clear cell RCC only. Other exclusion criteria were as follows: (1) Concurrent infections, immune disorders, or hematological conditions; (2) Presence of other tumors; (3) Incomplete clinical and pathological data.
For each patient, the following clinicopathological information were gathered: age at surgery, gender, smoking history, alcohol history, hypertension, diabetes, body mass index (BMI), surgical method, pathologic tumor stage (T stage), pathologic node stage (N stage), tumor size, Eastern Cooperative Oncology Group performance status (ECOG-PS), Charlson Comorbidity Index (CCI), as well as nuclear grade and tumor necrosis. The pathology report recorded tumor size as the largest diameter (in centimeters). Tumor necrosis was categorized as either present or absent, without quantitative assessment. If necrosis was not mentioned, it was regarded as absent. Alcohol history was defined as patients who had been or are currently consuming alcohol more than three times a week, with each instance involving more than 100mL. Smoking history was defined as patients reporting a smoking habit and smoking more than 10 cigarettes per week. Nuclear grade was determined according to Fuhrman criteria.18 Tumor TNM staging was performed according to the American Joint Committee on Cancer (AJCC) TNM classification system. UISS was determined by TNM stage, Fuhrman grade and ECOG-PS. Patients were divided into low-, intermediate-, and high-risk UISS groups.7 SSIGN score was determined by TNM stage, tumor size, nuclear grade, and histologic tumor necrosis (SSIGN score= stage, size, grade, and necrosis). Patients were divided into low- (0–2), intermediate- (3–5), and high-risk (≥6) SSIGN groups.9
Laboratory data, such as hemoglobin, neutrophil count, platelet count, white blood cell count, lymphocyte count, monocyte count, and red blood cell distribution width, were obtained from pre-surgery blood screening tests. If multiple preoperative blood tests were available for a patient, the value closest to the date of nephrectomy was used. The NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count. The PLR was calculated by dividing the absolute platelet count by the absolute lymphocyte count. The LMR was calculated by dividing the absolute lymphocyte count by the absolute monocyte count. Recurrence free survival was defined as the interval from the date of surgery to the date of recurrence, distant metastasis or last follow-up. Cancer-specific survival (CSS) was defined as the interval from the data of surgery to mortality for a consequence of RCC. Overall survival (OS) was defined as the time from the date of surgery to mortality of any cause or last follow-up.
The statistical analysis was performed using the SPSS 26.0 the R software 4.2.1. Demographic and pathological parameters are described with frequency and percentage for categorical variables and median and interquartile range for continuous variables. X-tile software was used to determine the optimal cutoff for each inflammation-associated blood cell marker. X-tile presents a new tool for the assessment of biological relationships between a marker (NLR/ PLR/ LMR/ RDW) and outcome (RFS/ CSS/ OS) and discover the population cut-points based on maker values.19 Pearson χ2-test or Fisher’s exact test was used to compare categorical variables. Kaplan-Meier curves were plotted, and any differences between the survival curves were assessed using the Log rank test. We utilized univariate Cox proportional-hazards models to identify factors that could predict the risk of recurrence-free survival (RFS), cancer-specific survival (CSS), and overall survival (OS). This involved estimating hazard ratios (HRs) and calculating 95% confidence intervals (CIs). For variables that showed statistical significance in the univariate analysis, a multivariate Cox proportional hazards regression was conducted. All significant risk factors were incorporated into the final multivariate model. To evaluate the predictive accuracy of the model, Harrell’s concordance index (C-index) was utilized. C-index varies from 0.5 (no predictive capacity) to 1 (perfect prediction).20 All statistical tests were two-sided and a P<0.05 was considered statistically significant.
Results
Baseline Characteristics and Cut-Off Values for Inflammatory-Related Blood Marker
Between January 2015 and December 2017, 444 patients who underwent nephrectomy and whose postoperative pathology revealed clear cell RCC were identified. Table 1 shows the baseline characteristics of the patients. For this cohort, the mean (IQR) values of inflammatory-related blood markers were as follows: NLR, 2.0 (1.6–2.6); PLR, 112.9 (87.1–143.9); LMR, 3.8 (3.0–5.0); 13.2 (12.9–13.7). The optimal cutoff values for RDW, PLR, NLR, and LMR were 13.1, 157.3, 3.4, and 2.7, respectively (by the X-tile software). Based on the cutoff values of RDW, PLR, NLR, and LMR, the patients were divided into low groups and high groups.
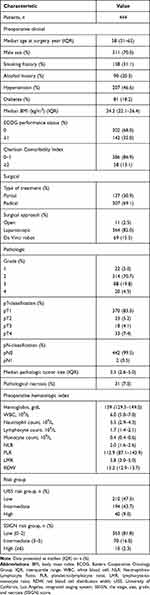 |
Table 1 Clinicopathologic Features of the Cohort |
The Relationship Between Inflammatory-Related Blood Markers and Patients’ Characteristics
The association of NLR, PLR, LMR and RDW with clinicopathological characteristics are listed in Table 2. As depicted, NLR, PLR and LMR were all significantly associated with Fuhrman grade, age, and SSIGN risk group. In addition, significant correlations were found between gender and LMR, BMI and PLR, T stage and LMR. RDW was only associated with pathologic tumor size.
 |
Table 2 The Clinical and Pathological Characteristics of Different Groups |
Preoperative Inflammation-Related Blood Markers and Survival Outcomes
The median follow-up time after nephrectomy was 70 months. During this period, 38 (8.6%) patients died, 29 patients (6.5%) of whom died from ccRCC and 9 (2.0%) died from other causes. 58 patients (13.1%) experienced recurrence. Figure 1 shows the Kaplan-Meier curves for patients’ RFS, CSS, and OS. Figure 1 reveals that high NLR, high PLR, and low LMR seems to be a significant factor for poor prognosis in patients with ccRCC (Log rank test P < 0.05 for all 3 tested end points). On univariate Cox regression analysis, PLR, NLR, and LMR were all prognostic predictors for RFS, CSS and OS (Table 3). After, adjusting for other important prognostic factors, only NLR remained an independent prognostic factor for both CSS (hazard ratio [HR], 5.40; 95% confidence interval [CI], 2.03–14.36; P = 0.001) and OS (HR, 4.42; 95% CI, 1.88–10.40; P = 0.001). No evidence showed an association between RDW and prognosis of ccRCC patients in our study.
 |
Table 3 Predictors of Recurrence Free, Cancer-Specific and Overall Survival |
NLR and Prognostic Risk Models for ccRCC
On univariate Cox regression analysis, NLR, UISS, and SSIGN were significant predictors for CSS and OS (Table 4). After, adjusting for UISS, NLR remained an independent factor for both CSS (HR,6.72; 95% CI, 3.16–14.28; P < 0.001) and OS (HR, 5.09; 95% CI,2.58–10.03; P < 0.001). Moreover, after adjusting for SSIGN, NLR was still an important maker for CSS (HR,5.08; 95% CI,2.40–10.78; P < 0.001) and OS (HR,4.17; 95% CI,2.11–8.25; P < 0.001). When NLR was combined with the SSIGN model, the c-index increased from 0.777 to 0.826 for CSS and from 0.703 to 0.734 for OS. When NLR was combined with UISS, the c-index increased from 0.796 to 0.811 for CSS and from 0.735 to 0.745 for OS.
 |
Table 4 Univariate and Multivariate Cox Regression Models Incorporating Prognostic Risk Models and NLR in Prediction of Cancer-Specific Survival, and Overall Survival |
Discussion
In this study, we investigated on the relationship between the preoperative inflammation-related blood markers and survival outcomes of 444 non-metastatic ccRCC patients. Our findings revealed that the Neutrophil-to-Lymphocyte Ratio (NLR) could be served as an independent prognostic indicator for Cancer-Specific Survival (CSS) and Overall Survival (OS), even after adjusting for other important prognostic factors. However, none of the inflammatory markers examined in our study showed prognostic significance for Recurrence-Free Survival (RFS).
Several laboratory indicators are associated with systemic inflammation, such as elevated CRP levels, low albumin levels, elevated cytokine levels, and an increase in neutrophils. The inflammatory response triggers the production and release of neutrophils from the bone marrow, which can suppress the cytolytic functions of immune cells like lymphocytes, natural killer cells, and activated T cells.21 The combination of elevated neutrophil levels and decreased lymphocyte levels can result in an increased NLR ratio. An elevated preoperative NLR has been previously demonstrated as a poor prognosis factor for different human cancer types, including gastrointestinal, nasopharyngeal, and lung cancer.21 Numerous studies have shown that the NLR can predict outcomes for both localized (T1–3N0M0) and metastatic (T1–4N0–1M1) ccRCC. Cumulating evidence in metastatic RCC indicates that an elevated NLR could potentially serve as an independent negative prognostic indicator in interferon-treated,17 interleukin-2 treated,16 as well as in sunitinib treated patients.15 However, only a few investigations on non-metastatic ccRCC have been published, and the reported findings are contradictory. In a cohort of 192 non-metastatic RCC patients, Ohno et al found that an elevated NLR was an independent predictor for recurrence-free survival.22 Another study with 678 patients demonstrated that an elevated NLR was an independent negative indicator for overall survival, but not for cancer-specific survival. Kim et al conducted a study with 309 patients and found that preoperative NLR served as an independent marker for recurrence-free survival in non-metastatic ccRCC.23 However, it is worth noting that some studies have reported contradictory findings, stating that NLR is not a prognostic factor for non-metastatic ccRCC.24,25
In our study, we found that NLR was an independent prognostic predictor for CSS and OS after adjusting for other prognostic factors. The exact mechanism behind the connection between an increased NLR and unfavorable cancer prognosis is still not fully understood. However, it has been observed that elevated levels of neutrophils can release significant quantities of reactive oxygen species. This release of reactive oxygen species has the potential to cause damage to the DNA of cells and lead to genetic instability. Consequently, this creates a favorable environment for tumor invasion and the spread of cancer to other parts of the body. Moreover, circulating neutrophils have the ability to secrete cytokines such as IL-1 and tumor necrosis factor, as well as produce a proangiogenic factor called vascular endothelial growth factor (VEGF).15,26 VEGF contributed to tumor angiogenesis and metastasis.27 The reduction of lymphocyte count is an important factor in the body’s inflammatory response to tumor development. Studies have shown that a decrease in CD4+ T lymphocytes and an increase in CD8+ T lymphocytes can contribute to an immune response against cancer.28 Therefore, the rise in NLR may be explained by the inflammatory state induced by the tumor (an increase in neutrophils) and the suppression of the body’s immune response (a decrease in lymphocytes).21
Kim et al had found that preoperative PLR was an independent factor for RFS in patients with non-metastatic ccRCC.23 As for LMR, Hutterer et al have found that a decreased LMR presents an independent prognostic factor. Adding the LMR to well-established model (Leibovich prognostic score), the c-index increased from 0.83 to 0.86.14 RDW is a commonly used index to quantify the degree of erythrocyte anisocytosis and has been shown to correlate with inflammation, nutritional status, renal function, and other serum markers such as C-reactive protein and albumin.29–31 Meta-analyses have also demonstrated an association between an elevated RDW and poor outcomes for patients with solid tumors such as esophageal cancer, lung cancer, and breast cancer.29–31 A recent study has shown that RDW was an independent predictor of CSS in patients with RCC.32 However, in our study, the prognostic effect of PLR, LMR, and RDW have not been discovered in patients with non-metastatic ccRCC.
The UCLA Integrated Staging System (UISS) is a prognostic tool created at UCLA to forecast the survival rate of patients with localized and metastatic renal cell carcinoma (RCC) following nephrectomy. The UISS model has been validated in a multicenter study, demonstrating a c-index ranging from 0.76 to 0.86 for patients with localized disease and 0.58 to 0.77 for patients with metastatic disease.8 Another predictive model, known as the Mayo Clinic SSIGN score, was developed by Frank et al to estimate the cancer-specific survival of clear cell RCC.9 The SSIGN model has also been validated, showing a c-index of 0.88.10 Despite the availability of these two prognostic models for RCC, accurately predicting an individual’s prognosis remains challenging. In our study, we have demonstrated that NLR could improve the prognostic ability of established models (UISS and SSIGN).
Preoperative full blood tests can easily provide all 4 inflammatory marker values included in our study. Those markers can serve as cost-effective and convenient prognostic markers. Most studies on blood markers have focused on a single marker and have not assessed their usefulness in established prognostic scoring systems. In our study, we have investigated the impact of all markers and integrated an independent prognostic marker (NLR) with established prognostic models, making our study unique and novel. However, our study has certain limitations. Firstly, it is retrospective and non-randomized in nature. Patients with incomplete medical records were excluded, which may introduce selection bias. Additionally, our study only included patients who underwent nephrectomy between January 2015 and December 2017, which may limit the generalizability of the results to other time periods. Furthermore, we were unable to account for concurrent comorbidities or ongoing infections in the patients, which could potentially influence the inflammation-related blood markers. Moreover, the precise mechanism underlying the relationship between poor prognosis and elevated NLR has not been identified.
Conclusion
In conclusion, the preoperative neutrophil-to-lymphocyte ratio appears to be a prognostic biomarker for non-metastatic clear cell renal cell carcinomas. Additionally, it has the potential to enhance the predictive capabilities of the UISS and SSIGN models.
Ethics Approval and Informed Consent
This study was conducted in accordance with the principles of the Helsinki Declaration and received approval from the Shanghai Ruijin Hospital, Shanghai Jiao Tong University School of Medicine (KY2021-096-B). Due to the retrospective nature of the study, the requirement for informed consent was waived. To ensure patient confidentiality, all identifying information was removed.
Acknowledgments
We would like to thank all members in our group.
Funding
This work was supported by the grants from the National Science Foundation of Shanghai (NO.21ZR1440700).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi:10.3322/caac.21166
2. Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54(1):8–29. doi:10.3322/canjclin.54.1.8
3. Moch H, Cubilla AL, Humphrey PA, et al. The 2016 WHO classification of tumours of the urinary system and male genital organs-part a: renal, penile, and testicular tumours. Eur Urol. 2016;70(1):93–105. doi:10.1016/j.eururo.2016.02.029
4. Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European association of urology guidelines on renal cell carcinoma: the 2019 update. Eur Urol. 2019;75(5):799–810. doi:10.1016/j.eururo.2019.02.011
5. Jonasch E, Gao J, Rathmell WK. Renal cell carcinoma. BMJ. 2014;349(nov10 11):g4797. doi:10.1136/bmj.g4797
6. Stewart SB, Thompson RH, Psutka SP, et al. Evaluation of the national comprehensive cancer network and American Urological Association renal cell carcinoma surveillance guidelines. J Clin Oncol. 2014;32(36):4059–4065. doi:10.1200/JCO.2014.56.5416
7. Zisman A, Pantuck AJ, Wieder J, et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol. 2002;20(23):4559–4566. doi:10.1200/JCO.2002.05.111
8. Patard -J-J, Kim HL, Lam JS, et al. Use of the University of California Los Angeles integrated staging system to predict survival in renal cell carcinoma: an international multicenter study. J Clin Oncol. 2004;22(16):3316–3322. doi:10.1200/JCO.2004.09.104
9. Frank I, Blute ML, Cheville JC, et al. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168(6):2395–2400. doi:10.1016/S0022-5347(05)64153-5
10. Ficarra V, Martignoni G, Lohse C, et al. External validation of the mayo clinic stage, size, grade and necrosis (SSIGN) score to predict cancer specific survival using a European series of conventional renal cell carcinoma. J Urol. 2006;175(4):1235–1239. doi:10.1016/S0022-5347(05)00684-1
11. Shuch BM, Lam JS, Belldegrun AS, et al. Prognostic factors in renal cell carcinoma. Semin Oncol. 2006;33(5):563–575. doi:10.1053/j.seminoncol.2006.06.006
12. Hu H, Yao X, Xie X, et al. Prognostic value of preoperative NLR, dNLR, PLR and CRP in surgical renal cell carcinoma patients. World J Urol. 2017;35(2):261–270. doi:10.1007/s00345-016-1864-9
13. de Martino M, Pantuck AJ, Hofbauer S, et al. Prognostic impact of preoperative neutrophil-to-lymphocyte ratio in localized nonclear cell renal cell carcinoma. J Urol. 2013;190(6):1999–2004. doi:10.1016/j.juro.2013.06.082
14. Hutterer GC, Stoeckigt C, Stojakovic T, et al. Low preoperative lymphocyte-monocyte ratio (LMR) represents a potentially poor prognostic factor in nonmetastatic clear cell renal cell carcinoma. Urol Oncol. 2014;32(7):1041–1048. doi:10.1016/j.urolonc.2014.04.001
15. Keizman D, Ish-Shalom M, Huang P, et al. The association of pre-treatment neutrophil to lymphocyte ratio with response rate, progression free survival and overall survival of patients treated with sunitinib for metastatic renal cell carcinoma. Eur J Cancer. 2012;48(2):202–208. doi:10.1016/j.ejca.2011.09.001
16. Donskov F, von der Maase H. Impact of immune parameters on long-term survival in metastatic renal cell carcinoma. J Clin Oncol. 2006;24(13):1997–2005. doi:10.1200/JCO.2005.03.9594
17. Atzpodien J, Royston P, Wandert T, et al. Metastatic renal carcinoma comprehensive prognostic system. Br J Cancer. 2003;88(3):348–353. doi:10.1038/sj.bjc.6600768
18. Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6(7):1235–1239. doi:10.1097/00000478-198210000-00007
19. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. doi:10.1158/1078-0432.CCR-04-0713
20. Harrell FE Jr, Califf RM, Pryor DB, et al. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. doi:10.1001/jama.1982.03320430047030
21. Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi:10.1093/jnci/dju124
22. Ohno Y, Nakashima J, Ohori M, et al. Pretreatment neutrophil-to-lymphocyte ratio as an independent predictor of recurrence in patients with nonmetastatic renal cell carcinoma. J Urol. 2010;184(3):1041–1048. doi:10.1016/j.juro.2010.05.028
23. Kim TW, Lee JH, Shim KH, et al. Prognostic significance of preoperative and follow-up neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with non-metastatic clear cell renal cell carcinoma. Invest Clin Urol. 2019;60(1):14–20. doi:10.4111/icu.2019.60.1.14
24. Lee A, Lee HJ, Huang HH, et al. Prognostic significance of inflammation-associated blood cell markers in nonmetastatic clear cell renal cell carcinoma. Clin Genitourin Cancer. 2020;18(4):304–313. doi:10.1016/j.clgc.2019.11.013
25. Attawettayanon W, Choorit T, Chalieopanyarwong V, et al. Significance of preoperative hematologic scoring in predicting death among patients with non-metastatic renal cell carcinoma undergoing nephrectomy. Asian J Surg. 2021;44(7):952–956. doi:10.1016/j.asjsur.2021.01.029
26. Soehnlein O. An elegant defense: how neutrophils shape the immune response. Trends Immunol. 2009;30(11):511–512. doi:10.1016/j.it.2009.07.002
27. Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A. 2006;103(33):12493–12498. doi:10.1073/pnas.0601807103
28. Gooden MJ, de Bock GH, Leffers N, et al. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105(1):93–103. doi:10.1038/bjc.2011.189
29. Forhecz Z, Gombos T, Borgulya G, et al. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J. 2009;158(4):659–666. doi:10.1016/j.ahj.2009.07.024
30. Montagnana M, Danese E. Red cell distribution width and cancer. Ann Transl Med. 2016;4(20):399. doi:10.21037/atm.2016.10.50
31. Lippi G, Targher G, Montagnana M, et al. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009;133(4):628–632. doi:10.5858/133.4.628
32. Zyczkowski M, Rajwa P, Gabrys E, et al. The relationship between red cell distribution width and cancer-specific survival in patients with renal cell carcinoma treated with partial and radical nephrectomy. Clin Genitourin Cancer. 2018;16(3):e677–e683. doi:10.1016/j.clgc.2017.12.003
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

