Back to Journals » International Journal of General Medicine » Volume 16
Preoperative Immature Neutrophils Predict Clinical Outcomes in Patients with Uncomplicated Type-B Aortic Dissection After Thoracic Endovascular Aortic Repair
Authors Abu Bokha A, Li CH, Song MY, Wei X, Li R
Received 12 April 2023
Accepted for publication 4 July 2023
Published 21 August 2023 Volume 2023:16 Pages 3637—3644
DOI https://doi.org/10.2147/IJGM.S414567
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Anas Abu Bokha, Chen-He Li, Ming-Yang Song, Xiang Wei, Rui Li
Division of Cardiothoracic and Vascular Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030, People’s Republic of China
Correspondence: Rui Li, Division of Cardiothoracic and Vascular Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030, People’s Republic of China, Email [email protected]
Purpose: Inflammation is a hallmark of the initial development and progression of aortic dissection. This study aimed to investigate the predictive value of preoperative neutrophils in aorta-related adverse events (AAEs) after thoracic endovascular aortic repair (TEVAR) for type B aortic dissection (TBAD).
Patients and Methods: A total of 80 patients with TBAD undergoing TEVAR were enrolled in our hospital. Preoperative inflammatory markers, including white blood cells (WBCs), neutrophils, neutrophil-to-lymphocyte ratio (NLR) and plasma high-sensitivity C-reactive protein (hs-CRP), were measured. Circulating neutrophil subpopulation was determined by flow cytometry. Kaplan–Meier curve was performed to determine whether neutrophil subsets independently predicted aorta-related adverse events (AAEs) after TEVAR.
Results: Compared with control group, the prevalence of hypertension and the levels of inflammatory indicators including WBCs, total neutrophils, NLR, immature neutrophils and hs-CRP were significantly higher in TBAD patients. Receiver operating characteristic (ROC) curve showed that NLR, absolute number of total neutrophils and percent CD10− immature neutrophils had excellent area under curves. During the 18-month follow-up, 16 (20.0%) were reported to occur AAEs, while 4 deaths (5.0%) were documented. Percent immature neutrophil was markedly higher in TBAD patients experiencing AAEs as compared with those without AAEs. Kaplan–Meier curve and Cox regression analysis demonstrated that percent immature neutrophil was the only predictor correlated with the occurrence of AAEs (hazard ratio 7.66, 95% CI: 2.91, 20.17, P = 0.018).
Conclusion: Increased CD10− immature neutrophils could act as a potential biomarker related to long-term adverse outcomes in TBAD patients following TEVAR.
Keywords: type B aortic dissection, thoracic endovascular aortic repair, aorta-related adverse events, neutrophil subsets
Introduction
Aortic dissection (AD) is a life-threatening vascular disease that can involve almost any part of the aorta.1 Pathological features of AD are always described as medial degeneration with intimal tear and crossing of blood into the artery wall, which ensuing causes the formation of a false lumen. Recently, thoracic endovascular aortic repair (TEVAR) becomes the optical treatment strategy for type B AD (TBAD).2–4 Although TEVAR achieves superior aortic remodeling and is proven to be better than optical medical therapy in long-term follow-up, early prediction and prevention of aorta-related adverse events (AAEs) after TEVAR warrants extensive attention.5
Apart from genetic variants, hypertension, atherosclerosis and inherited connective tissue diseases, clinical and experimental data support a role for sterile inflammation innate immunity in the pathogenesis of AD.6,7 Accelerating infiltration of macrophages, neutrophils and lymphocytes marks the acute phase of AD.8 Accumulating epidemiological and clinical studies have revealed that the increased number of circulating neutrophils was independently associated with the occurrence of TBAD.7 However, whether circulating neutrophils could independently predict the post-TEVAR outcome in TBAD patients remain controversial.
Within the immune compartment, it is recognized that neutrophils possess pleiotropic effects, including release of inflammatory cytokines, phagocytosis of pathogens and necrotic cells, formation of neutrophil extracellular traps and oxidative stress.9 Recent advances in single-cell transcriptomics have uncovered the heterogeneity and developmental states of neutrophils in both circulation and tissue.10 By contrast to CD10+ mature neutrophils, CD10− immature neutrophils exert immunostimulatory function through priming for IFN-γ production in activated CD4+ T lymphocytes.11 It is reported that CD10− immature neutrophils expanded under pathological conditions such as cancer, sepsis and sterile inflammation. Several studies also indicated that CD10− immature neutrophils were significantly associated with the incidence of myocardial infarction and atherosclerotic plaque vulnerability.12,13
In light of the aforementioned issues, the aim of our study was to determine the predictive value of neutrophil subsets for the outcomes after TEVAR in TBAD patients.
Methods
Study Population
Between January 2019 and March 2021, a total of 80 TBAD patients who underwent TEVAR in our hospital were included in the study. Seventy-one age- and gender-matched healthy individuals without coronary artery disease (CAD), cerebrovascular disease (CVD) and chronic kidney disease (CKD) from Physical Examination Center of our hospital were recruited. Baseline demographics, laboratory examinations, operative and follow-up data were collected. The study was approved by Ethics Committee of our institution, and all individuals provided written informed concept before participation.
The determination and morphological characteristics of TBAD were confirmed by computed tomography angiography (CTA) scans. Using the images, the initial measurements mainly indicated the number of false lumens as the basis of lesion classification. True lumen compression was defined as the true lumen area smaller than the false lumen, the spiral dissection was characterized by angles of at least 90 degrees between the false lumen and the true lumen. The branch artery consisted of the subclavian, mesenteric, renal, iliac, and femoral arteries. The diameter of the entry tear and the length to the left subclavian artery (LSA) were calculated in the coronal and sagittal images, respectively. Patients were grouped according to the proximal entry site. As described in the previous report, Zone 3 indicated that the proximal landing zone ≤2 cm of the LSA without covering it, while those over 2 cm distal to the LSA could be described as Zone ≥4.14 Whether stent-graft extended to abdominal aorta was documented. Perpendicular to the center lumen line, the maximum descending aortic diameter measurements were performed.
The inclusion criteria were as follows: (1) TBAD patients which has a primary tear and blood flowing in the false lumen; (2) uncomplicated TBAD without complications, such as malperfusion and refractory hypertension; (3) TBAD with an onset-to-TEVAR time less than 90 days. The exclusion criteria were as follows: (1) complicated TBAD; (2) patients who did not undergo TEVAR due to anatomic reasons or refusing surgery; (3) Marfan syndrome; (4) the onset-to-TEVAR times over 90 days; (5) history of malignant tumors.
Thoracic Endovascular Aortic Repair Procedure
Device selection and stent implantation were performed at the surgeon’s discretion after a comprehensive assessment using CT and aortic angiography. After the stent release, repeat angiography was performed to check for the stent position and rupture. The procedure was completed if the stent was in a suitable position and the rupture was well sealed. A second stent was implanted if necessary.
Measurement of Neutrophil Subsets
Approximately 5 mL whole blood samples were taken at admission before TEVAR surgery. Peripheral leukocytes, whole neutrophils, monocytes and lymphocytes were evaluated by Reflotron Plus Hematology Analyzer (Roche, Swiss). Ethylenediaminetetraacetic acid (EDTA)-anticoagulated blood samples from enrollments were processed for flow cytometry to determine the number of neutrophil subsets as previously described. After incubated with Fc-block (BioLegend, USA), cells were stained with CD66b (clone G10F5, BD Bioscience, USA), CD11b (clone ICRF44, BD Bioscience, USA), CD14 (clone M5E2, BD Bioscience, USA), CD101 (clone BB27, BD Bioscience, USA) and CD10 (clone HI10a, BD Bioscience, USA) for 15 minutes at room temperature. FSC/SSC gate was positioned to exclude cell debris, then total neutrophils were identified by CD11b, CD66b and CD14. Human circulating neutrophils could be categorized into three subpopulations: a numerically dominant CD10+CD101+ mature neutrophils, CD10−CD101+ immature neutrophils and CD101− pre-neutrophils. FlowJo software (Version 10, USA) was conducted to analyze the data generated by flow cytometry.
Follow-Up
Blood samples for high-sensitivity cardiac troponin T (hs-cTnT) were obtained 24 hours after TEVAR. All patients were followed up by a telephone inquisition and clinical visit. Postoperative aortic-related adverse events (AAEs) were evaluated using follow-up CTA images, which were taken routinely at 1-, 3-, 6-, and 12-month intervals after primary TEVAR procedure, and yearly thereafter. During the post-TEVAR follow-up, death and the aortic/stent-related complications which required re-interventions including type I endoleak, retrograde type A aortic dissection (RTAD) and distal stent-induced new entry (dSINE) were documented as AAEs. Type I endoleak refers to the leak at graft attachment site above, below, or between graft components.
Statistical Analysis
Continuous variables with normal distribution were expressed as mean ± standard deviation, while those with non-normal distribution were expressed as median and interquartile range. Categorical variables were presented as numbers and percentages. Data for categorical variables were tested using the χ2 test. Difference between continuous variables were analyzed using Student t-test or Mann–Whitney U-test if they were non-normal distributed. Of note, given the small sample size of TBAD patients occurring AAEs, we applied Mann–Whitney U-test to compare the differences between TBAD patients with and without AAEs. A Kaplan–Meier curve with the Log rank test was conducted to perform survival analysis and investigate the differences in the time-to-event endpoints. A P < 0.05 was considered statistical significance.
Results
Baseline Characteristics of TBAD Patients in Our Study
The baseline characteristics of enrollments are summarized in Table 1. The mean age of the included TBAD patients was 66.33 ± 8.50 years, and 57 were men (71.3%). The prevalence of hypertension was higher in TBAD group than in control group (57.5% vs 37.4%, P < 0.01). Both groups showed similar incident rate of diabetes and smoking. TBAD patients had higher levels of D-dimer, hs-CRP, WBC, total neutrophils and neutrophil-to-lymphocyte ratio (NLR) compared with healthy controls.
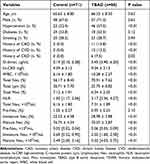 |
Table 1 Baseline Characteristics of TBAD Patients with TEVAR |
Immature Neutrophils are Associated with the Incidence of TBAD
Compared with healthy controls, percentage and absolute number of immature neutrophils were significantly higher, while a substantial decrease in mature neutrophils was observed in TBAD patients (Table 1). ROC analysis was performed to evaluate the diagnostic implication of total neutrophils, NLR and percent immature neutrophils in TBAD group. The area under the curve (AUC) was 0.871 of NLR, 0.971 of total neutrophils and 0.908 of percent immature neutrophils (Figure 1). The cut-off value of percent immature neutrophils for predicting TBAD was 24.55.
Association Between Neutrophil Subsets and AAEs During the Follow-Up
The mean follow-up was 18.1 months. Among the 80 TBAD patients who received TEVAR, 16 (20.0%) were reported to occur AAEs during the follow-up. Among them, 4 death (5.0%) due to distal dissection rupture. Of other TBAD patients experiencing AAEs, 6 (7.5%) were documented as type I endoleaks, 2 (2.5%) as RTAD and 4 (5.0%) as dSINE. The serum levels of hs-cTnT at 24-hour after TEVAR were moderately higher in TBAD patients who experienced AAEs than those without AAEs (non-AAEs 0.013 [0.008, 0.020] vs AAEs 0.017 [0.010, 0.028], P = 0.21). TBAD patients with proximal endograft attachment site at the Zone 3 had a significantly higher incidence of AAEs than those at the Zone ≥4 (P = 0.04, Table 2). There was no significant difference in the proportion of stent extension to abdominal aorta between TBAD patients with and without AAEs after TEVAR (P = 0.52, Table 2).
 |
Table 2 Association of Operative Parameters and Postoperative Cardiac Troponin with the Incidence of AAEs |
Compared with TBAD patients without postoperative AAEs, the percentage of immature neutrophils was remarkably increased in the AAE group (non-AAEs 28.54 [25.75, 30.95] vs AAEs 30.82 [29.39, 33.84], P = 0.017), as accompanied with a moderate decline in the percentage of mature neutrophils (non-AAEs 70.07 [67.92, 73.22] vs AAEs 69.14 [66.47, 73.15], P = 0.452). There was no difference in the NLR, absolute number of WBC and total neutrophils between non-AAE and AAE groups during the post-TEVAR follow-up as shown in Figure 2.
To further explore the correlation between the proportion of immature neutrophils and AAEs during the post-TEVAR follow-up, we then performed Kaplan–Meier curve and Cox regression analysis (Figure 3). According to the cut-off value of percent immature neutrophils calculated by ROC curve, TBAD patients with higher percentage of immature neutrophils had a higher AAE rate than those with lower percent immature neutrophils (hazard ratio 7.66, 95% CI: 2.91, 20.17, P = 0.018).
Discussion
To our best knowledge, our study was the first analysis to assess the association of specific neutrophil subpopulation with the outcomes of TBAD patients following TEVAR. According to the above findings, we demonstrated that higher percentage of immature neutrophils was significantly associated with post-TEVAR mortality and complication rates, but did not independently predict the incidence of TBAD.
Given the aggressive development and high mortality of TBAD, early warning and detection of TBAD is still required.6 Previous study suggested that D dimer was a potential index for AD, with excellent sensitivity but poor specificity diagnostic performance.15 Furthermore, since the initiation of TBAD is always followed by a cascade of sterile inflammatory response, several inflammation markers have been studied for the diagnosis of AD.16,17 Among these known biomarkers, NLR may be a potent surrogate for the determination of TBAD as reported by other study and our findings.18 Nonetheless, our study did not conclude that NLR was an independent risk factor for TBAD. On the other hand, elevated NLR was also closely associated with acute myocardial infarction, hypertension and pulmonary embolism. Therefore, NLR could not be considered as an ideal biomarker for predicting the occurrence of TBAD.
Compared to surgical repair and optimal medical treatment comprising anti-hypertensive agents, it is widely accepted that TEVAR is a preferred treatment strategy for patients with complicated and uncomplicated (initially stable) TBAD.19,20 With sophisticated operator skills and high-end technology, recent clinical trials corroborated long-term beneficial outcomes of TEVAR in subacute and acute TBAD, whereas suitable anatomy and systematic evaluation of disease states remains warranted for improved survive and delayed disease progression.21 Accumulating evidence revealed that elevated NLR and systemic immune inflammation index were highly associated with poor outcomes after TEVAR surgery, implying that inflammatory factors could predict the post-TEVAR AAEs.17,22 In this regard, we explored the prognostic ability of specific neutrophil subpopulation, finding that immature neutrophil could be an independent indicator for adverse events following TEVAR in TBAD patients.
To data, the exact mechanism underlying the interaction between specific neutrophil subsets and the prognosis of TBAD after TEVAR surgery remains unclear. In contrast to steady state, emergency granulopoiesis is initiated and accelerates the release of immature and mature neutrophils into the peripheral blood and pathologic tissue upon acute inflammation and stress-associated protein activation.23 On basis of cell marker expression, nuclear morphology, differentiation properties and multifaceted roles, circulating neutrophils could be divided into minor CD101− pre-neutrophils, intermediate CD10− immature neutrophils and predominant CD10+ mature neutrophils.24 Recent findings indicated that immature neutrophils exerted immunostimulatory function through promoting T lymphocyte expansion and orchestrated breast cancer metastasis opposite to those of mature neutrophils.11,25 Likewise, previous studies reported that percent immature neutrophils could act as a powerful indicator for the incidence of myocardial infarction and the vulnerability of atherosclerotic plaques.12,13 To our best knowledge, the role of preoperative neutrophil subpopulation in AD is still lack of relevant reports. Our data delineated that an increased proportion of CD10− immature neutrophil is independently correlated with poor outcomes after TEVAR surgery. It could be explained by the fact that upregulated immature neutrophils facilitate a prominent Th1 response, monocyte recruitment, IL-1β and IL-6 production, all of which ensuing exacerbate AD progression.26–28 Thus, future studies are recommended to explore the mechanism underlying specific neutrophil subpopulation mediating AD development.
Our study has several limitations. First, the relatively small sample size limits the generalizability of our data. It is required to expand sample size and enroll complicate TBAD patients in future studies. Second, we applied whole blood samples to analyze the neutrophil subpopulation, which did not allow us to distinguish whether immature subsets originated from low-density neutrophils of mononuclear cell fractions or normal-density neutrophils of granulocyte fractions. However, it should be noted that low-density neutrophils of mononuclear cell fractions were extremely low in both steady state and upon acute inflammatory response.11,12
Conclusions
Higher levels of circulating CD10− immature neutrophils could predict the long-term adverse outcomes in TBAD patients following TEVAR.
Data Sharing Statement
The data associated with this manuscript are available from the corresponding author upon reasonable request.
Ethical Approval
The study was approved by Ethics Committee of Tongji Hospital, Tongji Medical College Huazhong University of Science and Technology, and all individuals provided written informed concept before participation. This study was conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was supported by National Natural Science Foundation of China (No. 82000440).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gouveia EMR, Mourao M, Caldeira D, et al. A systematic review and meta-analysis of the incidence of acute aortic dissections in population-based studies. J Vasc Surg. 2022;75(2):709–720. doi:10.1016/j.jvs.2021.08.080
2. Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(41):2873–2926. doi:10.1093/eurheartj/ehu281
3. Czerny M, Pacini D, Aboyans V, et al. Current options and recommendations for the use of thoracic endovascular aortic repair in acute and chronic thoracic aortic disease: an expert consensus document of the European Society for Cardiology (ESC) Working Group of Cardiovascular Surgery, the ESC Working Group on Aorta and Peripheral Vascular Diseases, the European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the ESC and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg. 2021;59(1):65–73. doi:10.1093/ejcts/ezaa268
4. Wu J, Wu Y, Li F, et al. Natural history of isolated abdominal aortic dissection: a prospective cohort study. Front Cardiovasc Med. 2023;10:1002832. doi:10.3389/fcvm.2023.1002832
5. Evangelista A, Isselbacher EM, Bossone E, et al. Insights from the International Registry of Acute Aortic Dissection: a 20-year experience of collaborative clinical research. Circulation. 2018;137(17):1846–1860. doi:10.1161/CIRCULATIONAHA.117.031264
6. Golledge J, Eagle KA. Acute aortic dissection. Lancet. 2008;372(9632):55–66. doi:10.1016/S0140-6736(08)60994-0
7. Gao J, Cao H, Hu G, et al. The mechanism and therapy of aortic aneurysms. Signal Transduct Target Ther. 2023;8(1):55. doi:10.1038/s41392-023-01325-7
8. Wortmann M, Peters AS, Erhart P, Korfer D, Bockler D, Dihlmann S. Inflammasomes in the pathophysiology of aortic disease. Cells. 2021;10(9):2433. doi:10.3390/cells10092433
9. Quail DF, Amulic B, Aziz M, et al. Neutrophil phenotypes and functions in cancer: a consensus statement. J Exp Med. 2022;219(6):e20220011.
10. Montaldo E, Lusito E, Bianchessi V, et al. Cellular and transcriptional dynamics of human neutrophils at steady state and upon stress. Nat Immunol. 2022;23(10):1470–1483. doi:10.1038/s41590-022-01311-1
11. Marini O, Costa S, Bevilacqua D, et al. Mature CD10(+) and immature CD10(-) neutrophils present in G-CSF-treated donors display opposite effects on T cells. Blood. 2017;129(10):1343–1356. doi:10.1182/blood-2016-04-713206
12. Fraccarollo D, Neuser J, Moller J, Riehle C, Galuppo P, Bauersachs J. Expansion of CD10(neg) neutrophils and CD14(+)HLA-DR(neg/low) monocytes driving proinflammatory responses in patients with acute myocardial infarction. Elife. 2021;10:e66808. doi:10.7554/eLife.66808
13. Wan M, Lu Y, Mao B, et al. Immature neutrophil is associated with coronary plaque vulnerability based on optical coherence tomography analysis. Int J Cardiol. 2023;374:89–93. doi:10.1016/j.ijcard.2023.01.004
14. Fillinger M, Greenberg R, McKinsey J, Chaikof E. Society for vascular surgery Ad Hoc Committee on TEVAR reporting standards: reporting standards for thoracic endovascular aortic repair (TEVAR). J Vasc Surg. 2010;52(4):1022–1033. doi:10.1016/j.jvs.2010.07.008
15. Xu Y, Liang S, Liang Z, et al. Admission D-dimer to lymphocyte counts ratio as a novel biomarker for predicting the in-hospital mortality in patients with acute aortic dissection. BMC Cardiovasc Disord. 2023;23(1):69. doi:10.1186/s12872-023-03098-x
16. Jia Y, Li D, Yu J, et al. Prognostic value of interleukin-33, sST2, myeloperoxidase, and matrix metalloproteinase-9 in acute aortic dissection. Front Cardiovasc Med. 2022;9:1084321. doi:10.3389/fcvm.2022.1084321
17. Zhao Y, Jiang J, Yuan Y, et al. Prognostic value of the systemic immune inflammation index after thoracic endovascular aortic repair in patients with type B aortic dissection. Dis Markers. 2023;2023:2126882. doi:10.1155/2023/2126882
18. Zhao Y, Hong X, Xie X, et al. Preoperative systemic inflammatory response index predicts long-term outcomes in type B aortic dissection after endovascular repair. Front Immunol. 2022;13:992463. doi:10.3389/fimmu.2022.992463
19. Wilson-Smith AR, Muston B, Kamalanathan H, et al. Endovascular repair of acute complicated type B aortic dissection-systematic review and meta-analysis of long-term survival and reintervention. Ann Cardiothorac Surg. 2021;10(6):723–730. doi:10.21037/acs-2021-taes-17
20. Huang YH, Chiu KL, Shen CW, Bair MJ, Chen CY. Evaluating prescription pattern and effectiveness of antihypertensive drugs in non-operated aortic dissection patients. J Clin Med. 2023;12(5):1962. doi:10.3390/jcm12051962
21. Nienaber CA, Kische S, Rousseau H, et al. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv. 2013;6(4):407–416. doi:10.1161/CIRCINTERVENTIONS.113.000463
22. Yang F, Liu J, Chen L, et al. Impact of lymphocyte-related blood parameters on short- and long-term outcomes of patients undergoing thoracic endovascular aortic repair. Angiology. 2021;72(10):953–960. doi:10.1177/00033197211012514
23. Chen S, Zhang Q, Lu L, et al. Heterogeneity of neutrophils in cancer: one size does not fit all. Cancer Biol Med. 2022;19(12):1629–1648. doi:10.20892/j.issn.2095-3941.2022.0426
24. Evrard M, Kwok IWH, Chong SZ, et al. Developmental analysis of bone marrow neutrophils reveals populations specialized in expansion, trafficking, and effector functions. Immunity. 2018;48(2):364–379 e368. doi:10.1016/j.immuni.2018.02.002
25. Hsu BE, Tabaries S, Johnson RM, et al. Immature low-density neutrophils exhibit metabolic flexibility that facilitates breast cancer liver metastasis. Cell Rep. 2019;27(13):3902–3915 e3906. doi:10.1016/j.celrep.2019.05.091
26. Tsilimigras DI, Sigala F, Karaolanis G, et al. Cytokines as biomarkers of inflammatory response after open versus endovascular repair of abdominal aortic aneurysms: a systematic review. Acta Pharmacol Sin. 2018;39(7):1164–1175. doi:10.1038/aps.2017.212
27. Stilo F, Catanese V, Nenna A, et al. Biomarkers in EndoVascular Aneurysm Repair (EVAR) and abdominal aortic aneurysm: pathophysiology and clinical implications. Diagnostics. 2022;12(1):183. doi:10.3390/diagnostics12010183
28. Blazkova J, Gupta S, Liu Y, et al. Multicenter systems analysis of human blood reveals immature neutrophils in males and during pregnancy. J Immunol. 2017;198(6):2479–2488. doi:10.4049/jimmunol.1601855
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



