Back to Journals » International Journal of General Medicine » Volume 14
Preoperative Glucose-to-Lymphocyte Ratio is an Independent Predictor for Acute Kidney Injury After Cardiac Surgery in Patients in Intensive Care Unit
Received 24 August 2021
Accepted for publication 23 September 2021
Published 8 October 2021 Volume 2021:14 Pages 6529—6537
DOI https://doi.org/10.2147/IJGM.S335896
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Lu Li,1,* Gaorui Zou,2,* Jie Liu1
1Department of Nephrology, The First People’s Hospital of Jiangxia District, Wuhan, 430299, People’s Republic of China; 2Department of Anesthesiology, Wuhan No. 1 Hospital, Wuhan, 430022, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jie Liu
Department of Nephrology, The First People’s Hospital of Jiangxia District, No. 1 of Cultural Avenue, Wuhan, 430299, People’s Republic of China
Tel +86 27-87958740
Email [email protected]
Background: We aimed to investigate the association between preoperative glucose-to-lymphocyte ratio (GLR) and cardiac surgery associated with acute kidney injury (CSA-AKI) in patients in the intensive care unit (ICU).
Methods: The Medical Information Mart for Intensive Care IV (MIMIC-IV version 1.0) database was used to identify adults’ patients who performed cardiac surgery during ICU stay. The primary outcome was the development of AKI based on the KDIGO criteria. Multivariable logistic regression was applied to investigate the association between GLR and clinical outcomes, and propensity score matching (PSM) and inverse probability of treatment weighting (IPTW) were also used to validate our findings.
Results: The optimal cut-off value for GLR was 1.28. Among the 7181 patients who conducted cardiac surgery, 2072 high-GLR group (≥ 1.28) patients and 2072 low-GLR group (< 1.28) patients, had similar propensity scores were included in this study. After matching, the high-GLR group had a significantly higher incidence of AKI (odds ratio, OR, 3.28, 95% confidence index, 95% CI, 2.81– 3.84, P < 0.001) even after adjustment for confounding factors. Moreover, the performance of GLR was superior to that of SOFA scores and GLR plus clinical model could add more net benefit for CSA-AKI than clinical model alone.
Conclusion: Preoperative GLR could serve as a good predictor for CSA-AKI in patients in ICU.
Keywords: glucose to lymphocyte, cardiac surgery, acute kidney injury, Medical Information Mart for Intensive Care, propensity score matching
Introduction
As one of the most common major complications with limited therapy options, acute kidney injury (AKI) is still one of the most frustrating disease worldwide, especially for patients in intensive care units (ICU) in the last decades.1–4 Among them, cardiac surgery-associated AKI (CSA-AKI), as the second most common cause of AKI for ICU patients, is independently associated with increased morbidity and mortality, prolonged length of hospital stays and increased healthcare costs.5–8 It is currently acknowledged that timely identifying patients at high risk of AKI and then providing appropriate interventions at an early stage could improve the prognosis of AKI patients.9–11 Hence, a novel biomarker with high prediction accuracy and easily accessible is essential for clinicians in immediate and appropriate decision-making regarding treatment options.
The glucose-to-lymphocyte ratio (GLR), which is calculated from the serum glucose concentration and total lymphocyte count in the peripheral blood, is an index that reflects glucose metabolism and systemic inflammatory status and has been demonstrated to be a good prognostic marker for patients with different cancers.12–14 However, to the best of our knowledge, no study was conducted to investigate the association between GLR and AKI, considering that inflammation involves the initiation, development and progression of AKI. Thus, in the current study, we conducted a retrospective study to verify the predictive value of the preoperative GLR for the development of postoperative AKI after cardiac surgery in patients in ICU using a large public database.
Materials and Methods
Data Source
The data were collected from a large US-based critical care database called Medical Information Mart for Intensive Care IV version 1.0 (MIMIC IV v1.0).15 It is a publicly and freely accessible database containing information of more than 50,000 patients admitted to the ICUs of Beth Israel Deaconess Medical Center in Boston, MA, from 2008 to 2019. The database has been approved by the Institutional Review Board (IRB) of the Massachusetts Institute of Technology (MIT). After successfully accomplishing the National Institutes of Health’s (NIH) online training course and the Protection of Human Research Participants Examination, we had the access to extract data from MIMIC-IV database. Considering that all patients in this database were de-identified, informed consent was waived, and data were extracted using Structured Query Language with PostgreSQL 9.6.
Selection of Participants
Patients who performed cardiac surgery based on the ninth or tenth revision of the International Classification of Diseases (ICD-9/10) code during their admissions were included in this study. AKI was defined based on the KDIGO guideline of seven days. For patients readmitted to the ICU, only the first ICU admissions were included in this study. We excluded patients who were younger than 18 years old or who spent less than 48 hours in the ICU. Patients with end-stage kidney disease (ESKD), missing values of glucose or lymphocyte, and developed AKI before surgery were also excluded in this study (Figure 1).
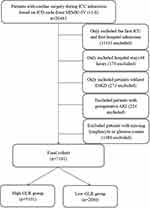 |
Figure 1 Study flow diagram in the present study. |
Variable Extraction
Baseline characteristics and admission information: age, gender, admission type, surgery type, body mass index (BMI), and severity score measured by the sequential organ failure assessment (SOFA) score, the oxford acute severity of illness score (OASIS), acute physiology score III (APSIII), the simplified acute physiology score II (SAPSII) and the Charlson comorbidity score were calculated as described in previous studies.16–20 Commodities including hypertension, diabetes, chronic kidney disease (CKD), congestive heart failure (CHF), myocardial infarct and liver disease were also collected for analysis based on the (ICD-9/10) codes in the MIMIC-III database. Use of mechanical ventilation (MV), vasopressors and renal replacement therapy before the diagnosis of AKI were also recorded in this study. Moreover, initial vital signs and laboratory results were also extracted using Structured Query Language with PostgreSQL 9.6.
The GLR was calculated using preoperative serum blood glucose (mmol/L)/lymphocyte count (× 109/L).
The primary outcome of this study was the incidence of AKI after cardiac surgery, which was stratified based on its severity, according to the KDIGO Clinical Practice Guidelines for AKI.
Statistical Analysis
Continuous variables were expressed as mean (standard deviation), categorical covariates were reported as number (percentage). The receiver operating characteristic (ROC) curve was used to determine the optimal cut-off GLR based on the Youden index. Propensity score matching (PSM) and propensity score-based inverse probability of treatment weighting (IPTW) were also applied to adjust the imbalance of the covariates between the two groups to ensure the robustness of our results. One-to-one nearest neighbor matching with a caliper width of 0.2 was used in the current study. The standardized mean differences (SMDs) were calculated to evaluate the effectiveness of the PSM and IPTW models. We also calculated Spearman correlation coefficients for the relationship between GLR and the severity of AKI stage in the original cohort as well as in the matched cohort. Multivariate logistic regression and adjusted odds ratios (ORs) were also conducted in the original cohort, matched cohort and weighted cohort, respectively, to investigate the association between GLR and the incidence of AKI. The added value of GLR in the risk prediction model was assessed using the likelihood ratio test of nested models. Discrimination was also assessed by the integrated discrimination index (IDI). Improvement in clinical risk stratification was assessed by calculating net reclassification improvement (NRI). Finally, the decision curve analysis (DCA) was also performed to evaluate the potential clinical usefulness and benefits of the GLR, clinical model, and GLR + clinical model. All analyses were performed using R (version 4.1.0) and p < 0.05 was considered statistically significant.
Results
Patients Characteristics
A total of 7181 patients who performed cardiac surgery during their ICU admission were included in this study and the flow chart of the included population is shown in Figure 1. Of the 7181 patients, CSA-AKI occurred in 5167 (72.0%) patients. According to the KDIGO criteria, 1756 (24.5%) patients were AKI stage I, 2895 (40.3%) patients were AKI stage II and 516 (7.2%) patients were AKI stage III. The included patients were divided into two groups according to the optimal cut-off value of GLR, 5101 patients in the high-GLR group (≥1.28), and 2080 patients in the low-LMR group (<1.28). As described in Table 1, before PSM, 14/33 covariates (BMI, admission type, mechanical ventilation or vasopressors usage, SOFA score, OASIS score, SAPSII score, APSIII score, hemoglobin, platelet, albumin, bicarbonate, blood urea nitrogen, potassium) were imbalanced between high-GLR group and low-GLR group. Based on the estimated propensity scores, PSM and IPTW were applied to standardize the differences between the two groups. As shown in Table 1 and Figure 2, the imbalance between the high-GLR group and low-GLR group was significantly decreased, and all covariates were comparable between the two groups.
 |
Table 1 Comparisons of Baseline Characteristics Between the Original Cohort, Matched Cohort and Weighted Cohort |
 |
Figure 2 Standardized mean difference (SMD) of variables before and after propensity score matching and weighting. |
GLR as a Predictor for the Primary End Point
As described in Table 1, compared with patients in low-GLR group, high-GLR group had a relative higher incidence of AKI (79.3% versus 54.0%, P < 0.001). The Spearman correlation coefficients showed that GLR was significantly positively correlated with AKI stage in the original cohort (r=0.280, P<0.001) as well as in the matched cohort (r=0.383, P<0.001).
The univariate logistic regression analysis indicated that high GLR group patients were associated with increased incidence of AKI, with the crude odds ratio (OR) was 3.26 (95% confidence index, 95% CI, 2.92–3.64, P<0.001) and the association remained robust after PSM (OR, 2.43, 95% CI 2.13–2.77, P<0.001) and IPTW (OR, 2.48, 95% CI 2.21–2.78, P<0.001) (Table 2). These findings were further confirmed by the results of the multivariate analyses. High GLR group was still an independent predictor for AKI in original cohort (OR=3.24, 95% CI 2.85–3.70, P<0.001), in matched cohort (OR=3.28, 95% CI 2.81–3.84, P<0.001) and in weighted cohort (OR=3.16, 95% CI 2.77–3.61, P<0.001) after adjustment for age, gender, BMI, admission type, surgery type, comorbidities, score system, interventions, vital signs and laboratory results (Table 2).
 |
Table 2 Summary of Results of Primary Outcome |
Effect of GLR on Risk Reclassification of AKI
To determine whether GLR materially improved risk reclassification of AKI, NRI and IDI were used in the original cohort and in the matched cohort. As described in Table 3, the addition of GLR importantly improved the risk reclassification (as measured using NRI and IDI) of AKI compared to the SOFA score and clinical model alone.
 |
Table 3 NRI and IDI Analyses for Risk Reclassification of AKI in Original Cohort and Matched Cohort |
Clinical Usefulness of GLR
A DCA curve was introduced to evaluate the clinical use of GLR for AKI. According to the DCA, when the threshold probability for a patient was within the range of 0–100%, the GLR added more net benefit than the “treat all” or “treat none” strategies both in the original cohort and in the matched cohort. Moreover, GLR could also add more net benefits for clinical model than clinical model alone (Figure 3A and B). Hence, these results indicated that GLR could be of clinical usefulness.
 |
Figure 3 Decision curve analysis for preoperative GLR and clinical model to detect its clinical usefulness in the original cohort (A) and in the matched cohort (B). |
Discussion
In the current study, we retrospectively enrolled 7181 ICU patients with cardiac surgery and found that high GLR group patients had increased incidence of CSA-AKI and demonstrated that preoperative GLR was an independent predictor for AKI after adjusting confounding factors. In addition, the performance of GLR was superior to that of SOFA scores and GLR plus clinical model could add more net benefit for CSA-AKI than clinical model alone. Hence, those results suggested that the GLR might be a good predictor for identifying patients at high risk of CSA-AKI in patients in ICU.
CSA-AKI is the second most common cause of AKI for critically ill patients (followed by sepsis) and is significantly associated with increased mortality, prolonged length of hospital stays and increased health-care cost.21–23 In the current study, the rate of postoperative AKI in ICU patients with cardiac surgery is 72.0%, which is higher than previous studies for hospitalized patients in general ward.24,25 Hence, a novel biomarker that could identify patients at high risk of AKI after cardiac surgery earlier might lead to better clinical outcomes and lower hospital expenses.
The association between GLR and clinical outcomes had been demonstrated in previous studies in patients with different cancers. Zhang et al measured the levels of blood glucose and lymphocyte and concluded that preoperative GLR is an independent predictor to predict the overall survival of pancreatic ductal adenocarcinoma patients who underwent curative resection.14 Similar results had also been found in patients with pancreatic cancer as well as resected pT2 gallbladder cancer.13 Nevertheless, to the best of our knowledge, this is the first study to investigate the association between preoperative GLR and CSA-AKI in patients in ICU, and we concluded that GLR could be an independent predictor for CSA-AKI with good discrimination and clinical usefulness.
As we all known that inflammation has been associated with the initiation, development and prognosis of AKI and AKI in some way was associated with intrarenal and systemic inflammation.26 Moreover, a high GLR represents a high glucose content and a low lymphocyte content. As we all know, diabetes is reported as a risk factor for AKI considering that diabetes patients have significantly higher rate of AKI and nearly half of diabetes patients develop AKI and this remains true for patients with preexisting CKD.27,28 Furthermore, high levels of blood glucose could indicate states of exacerbation of the neuroendocrine-metabolic response to cardiac surgery. Higher preoperative blood glucose levels are associated with higher morbidity of complications in the postoperative period of pediatric cardiac surgery.29 As one of the most common components of the systemic inflammatory response, lymphocytes are well-known inflammatory markers involved in AKI. Increasing studies have taken the lymphocyte-based serum inflammatory biomarkers into consideration for the development of AKI and prognosis of patients in different populations, such as neutrophil-to-lymphocyte ratio,8,30,31 platelet-to-lymphocyte ratio,32,33 prognostic nutritional index34,35 and so on. However, limited data have focused on the combination of inflammatory factors and glucose metabolic factor. In the current study, we retrospectively enrolled 7181 patients who were admitted to ICU after cardiac surgery using a large public database and found a positive correlation between GLR and the development of AKI. Moreover, this association became robust even after PSM and IPTW were applied to eliminate the imbalance of the covariates between high-GLR and low-GLR groups.
Several limitations should be considered in this study. First, this study is based on a single-center public database, which may lead to may lead to some inherent and selection biases even after a relatively larger sample size. Secondly, we only calculated the GLR before cardiac surgery but did not assess changes in GLR during ICU admission. Thirdly, due to the limited database, some important intraoperative and postoperative risk factors, such as cardiopulmonary bypass (CPB) time, hemodilution, embolism, nephrotoxins and so on, were missing in this study. Finally, this is a retrospective single-center study, and further prospective multicenter studies are needed to validate our findings.
Conclusions
In this study, we firstly demonstrated that preoperative GLR could serve as an independent predictor for postoperative CSA-AKI in patients in ICU, with good discrimination and clinical usefulness. Preoperative GLR, which is an easily accessible and cost-effective parameter, provides a helpful index for clinicians to stratify the risk of AKI and to plan treatment strategies.
Statement of Ethics
Access to the MIMIC-IV database for research was approved by the Institutional Review Boards of the Massachusetts Institute of Technology (Cambridge, MA, USA) and the Beth Israel Deaconess Medical Center and was granted a waiver of informed consent. Due to the HIPAA-compliant deidentification in the MIMIC-IV database, our institutional IRB requirement was waived. In summary, the study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Moreover, this study was also approved by the Ethics Committee of the first people’s hospital of Jiangxia district of Wuhan city, and informed consent was waived.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval for the version to be published; and agreed to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declared that there is no conflict of interest.
References
1. Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394(10212):1949–1964. doi:10.1016/S0140-6736(19)32563-2
2. Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41(8):1411–1423. doi:10.1007/s00134-015-3934-7
3. Hoste E, Kellum JA, Selby NM, et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. 2018;14(10):607–625. doi:10.1038/s41581-018-0052-0
4. Hu Y, Liu H, Du L, et al. Serum cystatin C predicts AKI and the prognosis of patients in coronary care unit: a prospective, observational study. Kidney Blood Press Res. 2017;42(6):961–973. doi:10.1159/000485341
5. Wang Y, Bellomo R. Cardiac surgery-associated acute kidney injury: risk factors, pathophysiology and treatment. Nat Rev Nephrol. 2017;13(11):697–711. doi:10.1038/nrneph.2017.119
6. Nakamura T, Mikamo A, Matsuno Y, et al. Impact of acute kidney injury on prognosis of chronic kidney disease after aortic arch surgery. Interact Cardiovasc Thorac Surg. 2020;30:273–279.
7. Hu Y, Zhou J, Cao Q, et al. Utilization of echocardiography after acute kidney injury was associated with improved outcomes in patients in intensive care unit. Int J Gen Med. 2021;14:2205–2213. doi:10.2147/IJGM.S310445
8. Manuel V, Miana LA, Turquetto A, et al. The role of the neutrophil-lymphocyte ratio for pre-operative risk stratification of acute kidney injury after tetralogy of Fallot repair. Cardiol Young. 2021;31(6):1009–1014. doi:10.1017/S1047951121001943
9. Hobson C, Ozrazgat-Baslanti T, Kuxhausen A, et al. Cost and mortality associated with postoperative acute kidney injury. Ann Surg. 2015;261(6):1207–1214. doi:10.1097/SLA.0000000000000732
10. Collister D, Pannu N, Ye F, et al. Health care costs associated with AKI. Clin J Am Soc Nephrol. 2017;12(11):1733–1743. doi:10.2215/CJN.00950117
11. Hu Y, Liu H, Fu S, et al. Red blood cell distribution width is an independent predictor of AKI and mortality in patients in the coronary care unit. Kidney Blood Press Res. 2017;42(6):1193–1204. doi:10.1159/000485866
12. Zhong A, Cheng C-S, Kai J, et al. Clinical significance of Glucose to Lymphocyte Ratio (GLR) as a prognostic marker for patients with pancreatic cancer. Front Oncol. 2020;10:520330. doi:10.3389/fonc.2020.520330
13. Navarro J, Kang I, Hwang HK, et al. Glucose to lymphocyte ratio as a prognostic marker in patients with resected pT2 gallbladder cancer. J Surg Res. 2019;240:17–29. doi:10.1016/j.jss.2019.02.043
14. Zhang Y, Xu Y, Wang D, et al. Prognostic value of preoperative glucose to lymphocyte ratio in patients with resected pancreatic cancer. Int J Clin Oncol. 2021;26(1):135–144. doi:10.1007/s10147-020-01782-y
15. Zhou S, Zeng Z, Wei H, et al. Early combination of albumin with crystalloids administration might be beneficial for the survival of septic patients: a retrospective analysis from MIMIC-IV database. Ann Intensive Care. 2021;11(1):42. doi:10.1186/s13613-021-00830-8
16. Vincent JL, de Mendonca A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European society of intensive care medicine. Crit Care Med. 1998;26(11):1793–1800. doi:10.1097/00003246-199811000-00016
17. Chen Q, Zhang L, Ge S, et al. Prognosis predictive value of the Oxford acute severity of illness score for sepsis: a retrospective cohort study. Peer J. 2019;7:e7083. doi:10.7717/peerj.7083
18. Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636. doi:10.1378/chest.100.6.1619
19. Godino M, Tommasino N, Mizraji R, et al. Prediction of the evolution to brain death in the neurocritical patient: SPN model showed better performance than simplified acute physiology score II and acute physiology and chronic health evaluation II. Transplant Proc. 2020;52(4):1066–1069. doi:10.1016/j.transproceed.2020.02.069
20. Christensen DM, Strange JE, Gislason G, et al. Charlson comorbidity index score and risk of severe outcome and death in Danish COVID-19 patients. J Gen Intern Med. 2020;35(9):2801–2803. doi:10.1007/s11606-020-05991-z
21. Yuan SM. Acute kidney injury after cardiac surgery: risk factors and novel biomarkers. Braz J Cardiovasc Surg. 2019;34(3):352–360. doi:10.21470/1678-9741-2018-0212
22. Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119(18):2444–2453. doi:10.1161/CIRCULATIONAHA.108.800011
23. Leballo G, Chakane PM. Cardiac surgery-associated acute kidney injury: pathophysiology and diagnostic modalities and management. Cardiovasc J Afr. 2020;31(4):205–212. doi:10.5830/CVJA-2019-069
24. Grieshaber P, Möller S, Arneth B, et al. Predicting cardiac surgery-associated acute kidney injury using a combination of clinical risk scores and urinary biomarkers. Thorac Cardiovasc Surg. 2020;68(05):389–400. doi:10.1055/s-0039-1678565
25. Tseng PY, Chen YT, Wang CH, et al. Prediction of the development of acute kidney injury following cardiac surgery by machine learning. Crit Care. 2020;24(1):478. doi:10.1186/s13054-020-03179-9
26. Rabb H, Griffin MD, McKay DB, et al. Inflammation in AKI: current understanding, key questions, and knowledge gaps. J Am Soc Nephrol. 2016;27(2):371–379. doi:10.1681/ASN.2015030261
27. Hapca S, Siddiqui MK, Kwan RSY, et al. The relationship between AKI and CKD in patients with type 2 diabetes: an observational cohort study. J Am Soc Nephrol. 2021;32(1):138–150. doi:10.1681/ASN.2020030323
28. Wang Y, Wei L, Guan Y, et al. Diabetes is a risk factor for high-dose methotrexate-associated AKI in lymphoma patients. Ren Fail. 2020;42(1):1111–1117. doi:10.1080/0886022X.2020.1838926
29. Alves RL, Cerqueira MP, Kraychete NC, et al. Perioperative blood glucose level and postoperative complications in pediatric cardiac surgery. Arq Bras Cardiol. 2011;97(5):372–379. doi:10.1590/S0066-782X2011005000097
30. Bi JB, Zhang J, Ren YF, et al. Neutrophil-to-lymphocyte ratio predicts acute kidney injury occurrence after gastrointestinal and hepatobiliary surgery. World J Gastrointest Surg. 2020;12(7):326–335. doi:10.4240/wjgs.v12.i7.326
31. Zhu J, Zeng C, Zhang L, et al. Red blood cell distribution width and neutrophil-to-lymphocyte ratio in predicting adverse outcomes of acute kidney injury in hospitalized patients. Kidney Dis. 2020;6(5):371–381. doi:10.1159/000507859
32. Hudzik B, Szkodziński J, Korzonek-Szlacheta I, et al. Platelet-to-lymphocyte ratio predicts contrast-induced acute kidney injury in diabetic patients with ST-elevation myocardial infarction. Biomark Med. 2017;11(10):847–856. doi:10.2217/bmm-2017-0120
33. Velibey Y, Oz A, Tanik O, et al. Platelet-to-lymphocyte ratio predicts contrast-induced acute kidney injury in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Angiology. 2017;68(5):419–427. doi:10.1177/0003319716660244
34. Hu Y, Cao Q, Wang H, et al. Prognostic nutritional index predicts acute kidney injury and mortality of patients in the coronary care unit. Exp Ther Med. 2021;21(2):123. doi:10.3892/etm.2020.9555
35. Dong X, Wang B, Chen S, et al. Association between prognostic nutritional index and contrast-associated acute kidney injury in patients complicated with chronic kidney disease and coronary artery disease. J Interv Cardiol. 2021;2021:2274430. doi:10.1155/2021/2274430
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
