Back to Journals » Cancer Management and Research » Volume 12
Preoperative Bilirubin-Adjusted Carbohydrate Antigen 19-9 as a Prognostic Factor for Extrahepatic Cholangiocarcinoma Patients at a Single Center
Authors Li J , Chen Q , Jin B, Shi Y, Wu X, Xu H, Zheng Y, Wang Y , Du S , Lu X, Mao Y , Sang X
Received 30 August 2019
Accepted for publication 17 December 2019
Published 20 January 2020 Volume 2020:12 Pages 411—417
DOI https://doi.org/10.2147/CMAR.S229329
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Jiayi Li, 1,* Qiao Chen, 1,* Bao Jin, 1 Yue Shi, 1 Xiangan Wu, 1 Haifeng Xu, 1 Yongchang Zheng, 1 Yingyi Wang, 2 Shunda Du, 1 Xin Lu, 1 Yilei Mao, 1 Xinting Sang 1
1Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, People’s Republic of China; 2Department of Oncology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shunda Du
Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, 1# Shuai-Fu-Yuan, Wang-Fu-Jing, Beijing 100730, People’s Republic of China
Tel +86-139 1183 2900
Fax +86-10-69156043
Email [email protected]
Purpose: The aims of our study were to investigate the prognostic impact of the rate of preoperative serum carbohydrate antigen 19-9/bilirubin (CA19-9/BR) on patients with extrahepatic bile duct cancer.
Patients and Methods: We collected clinical data from 89 patients who underwent surgery for extrahepatic cholangiocarcinoma (ECC) at Peking Union Medical College Hospital between January 2012 and December 2017. The Kaplan–Meier analysis for univariate analysis and the Cox proportional hazards models for multivariate analysis were used to determine possible independent prognostic factors.
Results: CA19-9/BR was classified as elevated compared with normal based on the upper serum normal values of CA19-9 (37 U/mL) and bilirubin (1.5 mg/dL), which gives a cut-off at 25 U/mL/mg/dL. Univariate analysis showed that the overall survival of patients with a high CA19-9/BR ratio was significantly worse compared with patients with a low CA19-9/BR ratio (Hazard Ratio [HR] 2.149; 95% Confidence Interval [95% CI] 1.027– 4.495; P=0.042). Multivariate analysis revealed that a high CA19-9/BR ratio (HR 3.250; 95% CI 1.165– 9.067; P=0.024), low differentiation (HR 3.551; 95% CI 1.231– 10.244; P=0.019), and positive margin (HR 2.555; 95% CI 1.111– 5.875; P=0.027) remained independent prognostic factors after adjusting for age at diagnosis, maximal diameters, and other possible factors.
Conclusion: The preoperative CA19-9/BR ratio is a good prognostic factor in predicting survival in ECC patients and closer follow-up is recommended in patients with a higher CA19-9/BR ratio before surgery.
Keywords: prognosis, preoperative bilirubin-adjusted carbohydrate antigen 19-9, extrahepatic cholangiocarcinoma
Introduction
Cholangiocarcinoma is a common malignant tumor, with an incidence that has continued to rise over the past 40 years.1,2 China has a high incidence of cholangiocarcinoma, which is far higher than that in Europe and the United States.3 Cholangiocarcinoma is divided into intrahepatic cholangiocarcinoma and extrahepatic cholangiocarcinoma (ECC). Surgical resection is the first consideration for the treatment of both types of cholangiocarcinoma.4 The surgical procedure for ECC can be grouped based on the location,5 as follows: the different conditions of the patients, radiotherapy, chemotherapy, target therapy, and immunotherapy for treatment. However, the prognosis for ECC patients may still be different, and the factor that determines the prognoses of patients with cholangiocarcinoma is of concern to researchers. TNM staging is the most common prognostic evaluation system.6 However, in practice, clinicians often find that there is a difference in the prognosis of patients with the same stage. The reason for this may be that in addition to tumor size, lymph node metastasis, and distant metastasis, there are more factors affecting the patient’s prognosis. Finding independent factors that have predictive value for the prognosis of cancer patients has generated research interest.
The increase in tumor markers can indicate the occurrence of tumors. Among many tumor markers in past studies, carbohydrate antigen 19–9 (CA19-9) is considered to have a strong correlation with cholangiocarcinoma.7 However, the simple increase in tumor indicators is not enough to diagnose cholangiocarcinoma.8 For example, the increase of CA19-9 can also be seen in conditions such as pancreatic cancer,9 chronic pancreatitis, cholelithiasis,10 and cholangitis.11 The rise in CA19-9 in these diseases is a result of obstruction causing the CA19-9 secreted by the normal bile duct, or pancreatic epithelium, flow into the bloodstream.10 Therefore, researchers used the total bilirubin level to calibrate CA19-9, which can rule out the effect of obstructive biliary disease on the increase of CA19-9. Bilirubin-adjusted CA 19–9 was considered to be an emerging tumor prognostic indicator in pancreatic cancer.12,13
However, there are few studies about bilirubin-adjusted CA 19–9 in patients with ECC. Therefore, the aim of this study was to estimate whether the factor CA19-9 that was calibrated using total bilirubin is a good predictor of ECC patient prognosis.
Materials and Methods
Patients
We retrospectively reviewed data from patients who were diagnosed with ECC after surgery at Peking Union Medical College Hospital (PUMCH) between 2012 and 2017. The inclusion criteria were as follows: 1) Open cholangiocarcinoma radical surgery excision; 2) postoperative pathology-confirmed cholangiocarcinoma; and 3) cholangiocarcinoma location is extrahepatic. Exclusion criteria were as follows: 1) Postoperative pathological type miscellaneous; 2) other malignant tumors; and/or 3) death resulting from postoperative complications of other serious diseases. There were 109 patients enrolled under these criteria, but only 89 of them had a serum CA19-9 test before surgery. Patients were followed-up every 3 months in the first year after surgery and every 6 months after the first year as outpatients. Telephone calls were performed in April 2019 for all patients.
Measurement
Measurements made in this study are described below. Basic information included age at diagnosis, sex, symptoms before operation (fever), and presence of diabetes mellitus. Laboratory indicators were preoperative CA19-9 and total bilirubin before percutaneous transhepatic cholangial drainage. These laboratory investigations were performed using an automation assembly line (Beckmancoulter, Brea, Florida, USA). Pathological information was as follows: tumor size, tumor node metastasis (TNM) stage in accordance with the 7th TNM classification system of the American Joint Committee on Cancer, vascular invasion, neurological invasion, and margin (negative or positive).
Follow-up information was the overall survival (OS) duration, which was defined as the time from the date of primary surgery until that of death or the last follow-up. Additionally, the following calculated indicator was determined: CA19-9/BR was classified as elevated (>25 U/mL/mg/dL) or normal (<25 U/mL/mg/dL) based on the upper serum normal values of CA19-9 (37 U/mL) and bilirubin (1.5 mg/dL), resulting in a cut-off value of 25 U/mL/mg/dL.
Statistical Analysis
Categorical variables were compared using the χ2 test or Fisher’s exact test. Continuous variables were compared using the t-test or Mann–Whitney U-test for variables with an abnormal distribution. The unadjusted associations of variables with OS were assessed using Kaplan–Meier analysis and log-rank test. The adjusted associations between variables and OS were assessed using the Cox proportional hazard regression models. Covariates meeting the significance level of P≤0.10 or with clinical significance were included in multivariable models, and a backward elimination strategy was used to exclude uncorrelated or redundant predictors (P>0.05). Statistical analysis was performed using SPSS 22.0 (IBM, Amok, New York, USA) and GraphPad Prism version 7.00 (GraphPad Software, San Diego, California, USA). P<0.05 was considered to be significant.
Results
Patients’ Clinicopathological Characteristics
Among the 89 patients (61 male and 28 females), the mean age at diagnosis was 60.6±7.8 years between 2012 to 2019. All of the patients underwent open cholangiocarcinoma radical surgery excision and extrahepatic bile ductal adenocarcinoma was confirmed by pathological results. Patients with a high CA 19-9/bilirubin (CA19-9/BR) ratio and a low CA19-9/BR ratio did not show significant differences in age, gender, fever before operation, diabetes mellitus, and pathological results such as TNM stage, maximal tumor diameter, surgical margins, presence of lymph node metastasis, nerve invasion, and vascular invasion. None of the patients had metastatic disease (Table 1). There were 72 (80.9%) patients with elevated CA19-9 and 68 (76.4%) patients with elevated bilirubin. CA 19–9 was significantly correlated with OS (r=0.246, P=0.036).
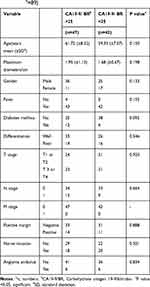 |
Table 1 Demographic and Patient Characteristic in the Entire Cohort (Na=89) |
The median survival time was 18 months for all patients and 21 and 16.5 months for patients with a low and a high CA19-9/BR ratio, respectively. The cumulative 1-year survival rates were 81.1% and 73.0% in these two groups.
Influence of CA19-9/BR Ratio on Survival
Univariate analysis based on the Kaplan–Meier curves was shown in Figure 1. OS of the patients with high CA19-9/BR ratio was significantly worse compared with patients with low CA19-9/BR ratio (Hazard Ratio [HR] 2.149; 95% Confidence Interval [95% CI] 1.027–4.495; P=0.042). Male sex (HR 3.822; 95% CI 1.470–9.934; P=0.006), low differentiation (HR 2.652; 95% CI 1.318–5.336; P=0.006), and positive margin (HR 2.686; 95% CI 1.332–5.415; P=0.006) also showed significant value for prognosis in OS for our patients. Other variables are shown in Table 2.
 |
Table 2 Univariable Analysis of Cox Regression Analysis of Clinicopathological Factors Associated with Survival |
Multivariate analysis for the predictors of survival revealed that a high CA19-9/BR ratio (HR 3.250; 95% CI 1.165–9.067; P=0.024), low differentiation (HR 3.551; 95% CI 1.231–10.244; P=0.019), and positive margin (HR 2.555; 95% CI 1.111–5.875; P=0.027) remained independent prognostic factors after adjusting for age at diagnosis, maximal diameters, and other possible factors. Male sex lost its independence (Table 3).
 |
Table 3 Multivariable Analysis of Cox Regression Analysis Results for Clinicopathological Factors That are Associated with Survival |
Discussion
ECC is a malignant tumor with a poor prognosis, and the associated 5-year OS is currently limited to 13–54%.14–16 In addition to prognostic factors that are associated with ECC that were shown in several studies,17,18 some studies identified CA19-9 as a predicting prognosis factor for cancer.
CA19-9 was initially widely studied as having prognostic value in tumors, such as in pancreatic cancer.19 Recently, an increasing number of studies showed the association between CA19-9 and cholangiocarcinoma. Kondo et al identified preoperative CA19-9 ≥200 IU/mL as an independent prognostic factor for poor OS in resectable cholangiocarcinoma.20 Kato et al and Yamashita et al found that non-normalization of CA19-9 after biliary tract cancer surgery was associated with poor OS.21,22 Wang et al investigated the outcome of resectable hilar cholangiocarcinoma patients and showed the predictive value of serum CA19-9.23 Bolm et al identified preoperative bilirubin-adjusted CA19-9 as a good predictive factor for distal cholangiocarcinoma in Western patients.13 Most of their samples were mixed ECC with intrahepatic cholangiocarcinoma and gallbladder cancer. Moreover, most of them evaluated the prognostic value of serum CA19-9 instead of bilirubin-adjusted CA19-9. The identification of adjusted preoperative markers might be more precise to establish and optimize therapeutic strategies for ECC.
Thus, in the present study, we retrospectively analyzed 89 patients with ECC and investigated the influence of bilirubin-adjusted CA19-9 on the prognosis of patients with ECC. CA19-9 is a tumor-associated antigen which is produced by bile duct epithelium. Thus, biliary obstruction has been suggested to stimulate CA19-9 secretion from biliary ductal epithelial cells.24 Bile acid has also been suggested to induce cyclooxygenase-2 (COX-2) expression, which might induce cholangiocarcinoma genesis and progression.25,26 Considering the influence of tumor cells and cholestasis on CA19-9, the CA19-9/BR ratio has been suggested to increase the specificity of serum CA19-9.12 Herein, the preoperative CA19-9/BR ratio could independently predict the survival of ECC patients and serve as a predictive marker. In our study, a high preoperative serum CA19-9/BR ratio was a negative independent prognostic factor for ECC. Thus, we found a preoperative predictive factor that can predict the long-term survival in ECC patients. It is recommended that patients with a high CA19-9/BR ratio should follow-up at clinics more frequently after surgery and pay more attention to their health.
Our results also demonstrated the prognostic effect of the positive margin. At the microscopic level, ECC often shows extensive spreading, which makes it difficult to achieve a negative surgical margin in ECC patients. Negative margin resection rates range from 46 to 100%.15 A negative surgical margin is known to be necessary for carcinoma because a positive margin might indicate the postoperative growth and spread of a tumor.27,28 The present study showed that the tumor-free surgical margin will improve the prognosis in ECC patients, and it also reminds us to pay more attention to the tumor margin in ECC patients during surgery.
Sex was another prognostic factor in our study although it lost its independence in the multivariate analysis. In a population-based study, Mosadeghi observed a higher incidence of ECC among men than among women.29 Similarly, sex has been identified as a prognostic factor in many malignant tumors, such as papillary thyroid cancer, lung cancer, oropharyngeal and non-oropharyngeal head and neck squamous cell cancer, and gastrointestinal cancer.30–33 The reasons for sex disparities in survival outcomes are unknown. Men often smoke, drink alcohol, and eat more than women do, and these different lifestyle choices may result in different cancer risks.33 The prognostic value of sex in ECC patients requires more research.
We found that the degree of tumor differentiation in patients with ECC is an independent risk factor. Generally, a tumor with a low degree of differentiation means that the difference between tumor tissue and normal tissue is larger, and the tumor cells have higher growth abilities.34,35 In our patients, there was no evidence of postoperative follow-up evidence of tumor enlargement and tumor metastasis. Studies have shown that for patients with low degree of differentiation after surgery, we should remind patients to undergo postoperative radiotherapy and chemotherapy to prolong the survival cycle.36 Additionally, patients are reminded to attend the hospital for regular examinations. It is also important to measure computed tomography (CT) or positron emission tomography (PET) imaging examinations and serum tumor indicators.
Our study investigated the prognostic value of CA19-9/BR ratio in a single center in China and verified a predictive factor that might predict the survival before surgery and help formulate therapeutic strategy. Adjusting CA19-9 using bilirubin made it more believable. This is the first study in China that investigated the CA19-9/BR in ECC patients and we showed its predictive value. We hope that it can be applied clinically and it may be able to address patients’ doubts about their outcomes and prognosis in the future.
There are several limitations to this study. It was a retrospective study design and it was conducted at a single center. Thus, care should be taken when generalizing the results and conclusions of our research to the whole country. However, the single-center study made our patients more generous in peri-operative care and operative level. In future, a multicenter study should be conducted to verify our results.
Conclusion
A high CA19-9/BR ratio, low differentiation, and positive margin remained independent prognostic factors for ECC after adjusting for age at diagnosis, maximal diameters, and other possible factors. Thus, we retrospectively found that the preoperative CA19-9/BR ratio is a good prognostic factor for predicting survival in ECC patients and closer follow-up is recommended in patients with a higher CA19-9/BR ratio before surgery.
Ethics Approval and Informed Consent
This study was approved by the Institutional Review Board of Peking Union Medical College Hospital. All participants provided written informed consent.
Data Sharing Statement
All findings of this study are based on diagnostic examinations performed during patient hospitalization. The publication of these data was authorized by Peking Union Medical College Hospital. Data sharing is applicable when required.
Acknowledgments
The authors thank the participants, doctors and nurses participating in this study. The authors also thank Jodi Smith, PhD, from Liwen Bianji, Edanz Editing China, for editing the English text of a draft of this manuscript.
Author Contributions
Shunda Du, Jiayi Li, and Qiao Chen contributed to conception and design. Jiayi Li and Qiao Chen contributed to data acquisition, interpretation, and drafting the article. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Chinese Academy of Medical Sciences Initiative for Innovative Medicine (CAMS-2017-I2M-4-002) and the National Natural Science Foundation of China (81972698). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. Oncologist. 2016;21(5):594–599. doi:10.1634/theoncologist.2015-0446
2. Gupta A, Dixon E. Epidemiology and risk factors: intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2017;6(2):101–104. doi:10.21037/hbsn
3. Banales JM, Cardinale V, Carpino G, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13(5):261–280.
4. Oliveira IS, Kilcoyne A, Everett JM, Mino-Kenudson M, Harisinghani MG, Ganesan K. Cholangiocarcinoma: classification, diagnosis, staging, imaging features, and management. Abdominal Radiol. 2017;42(6):1637–1649. doi:10.1007/s00261-017-1094-7
5. Radtke A, Konigsrainer A. Surgical therapy of cholangiocarcinoma. Visceral Med. 2016;32(6):422–426. doi:10.1159/000452921
6. Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366(9493):1303–1314. doi:10.1016/S0140-6736(05)67530-7
7. Hatzaras I, Schmidt C, Muscarella P, Melvin WS, Ellison EC, Bloomston M. Elevated CA 19-9 portends poor prognosis in patients undergoing resection of biliary malignancies. HPB. 2010;12(2):134–138. doi:10.1111/j.1477-2574.2009.00149.x
8. Ali Tüzün I, Kemal YLZ, Birol B, et al. Roles of serum and biliary CEA, CA19-9, VEGFR3, and TAC in differentiating between malignant and benign biliary obstructions. Turk J Gastroenterol. 2014;25(2):162–169. doi:10.5152/tjg.2014.6056
9. Chen Y, Gao SG, Chen JM, et al. Serum CA242, CA19-9, CA125, CEA, and TSGF are biomarkers for the efficacy and prognosis of cryoablation in pancreatic cancer patients. Cell Biochem Biophys. 2015;71(3):1287–1291. doi:10.1007/s12013-014-0345-2
10. Liu W, Liu Q, Wang W, et al. Differential diagnostic roles of the serum CA19-9, total bilirubin (TBIL) and the ratio of CA19-9 to TBIL for benign and malignant. J Cancer. 2018;9(10):1804–1812. doi:10.7150/jca.25093
11. Mei Y, Chen L, Peng CJ, et al. Diagnostic value of elevated serum carbohydrate antigen 199 level in acute cholangitis secondary to choledocholithiasis. World J Clin Cases. 2018;6(11):40–45. doi:10.12998/wjcc.v6.i11.441
12. Dumitra S, Jamal MH, Aboukhalil J, et al. Pancreatic cancer and predictors of survival: comparing the CA 19-9/bilirubin ratio with the McGill Brisbane symptom score. HPB (Oxford). 2013;15(12):1002–1009. doi:10.1111/hpb.12085
13. Bolm L, Petrova E, Weitz J, et al. Prognostic relevance of preoperative bilirubin-adjusted serum carbohydrate antigen 19-9 in a multicenter subset analysis of 179 patients with distal cholangiocarcinoma. HPB (Oxford). 2019;21:1513–1519. doi:10.1016/j.hpb.2019.03.363
14. Mukkamalla SKR, Naseri HM, Kim BM, Katz SC, Armenio VA. Trends in incidence and factors affecting survival of patients with cholangiocarcinoma in the United States. J Natl Compr Canc Netw. 2018;16(4):370–376. doi:10.6004/jnccn.2017.7056
15. Zhou Y, Liu S, Wu L, Wan T. Survival after surgical resection of distal cholangiocarcinoma: a systematic review and meta-analysis of prognostic factors. Asian J Surg. 2017;40(2):129–138. doi:10.1016/j.asjsur.2015.07.002
16. Wellner UF, Shen Y, Keck T, Jin W, Xu Z. The survival outcome and prognostic factors for distal cholangiocarcinoma following surgical resection: a meta-analysis for the 5-year survival. Surg Today. 2017;47(3):271–279. doi:10.1007/s00595-016-1362-0
17. Chang JS, Tsai CR, Chen LT. Medical risk factors associated with cholangiocarcinoma in Taiwan: a population-based case-control study. PLoS One. 2013;8(7):e69981. doi:10.1371/journal.pone.0069981
18. Tao LY, He XD, Qu Q, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a case-control study in China. Liver Int. 2010;30(2):215–221. doi:10.1111/j.1478-3231.2009.02149.x
19. Imaoka H, Shimizu Y, Senda Y, et al. Post-adjuvant chemotherapy CA19-9 levels predict prognosis in patients with pancreatic ductal adenocarcinoma: a retrospective cohort study. Pancreatology. 2016;16(4):658–664. doi:10.1016/j.pan.2016.04.007
20. Kondo N, Murakami Y, Uemura K, et al. Elevated perioperative serum CA 19-9 levels are independent predictors of poor survival in patients with resectable cholangiocarcinoma. J Surg Oncol. 2014;110(4):422–429. doi:10.1002/jso.23666
21. Kato Y, Takahashi S, Gotohda N, Konishi M. Prognostic impact of the initial postoperative CA19-9 level in patients with extrahepatic bile duct cancer. J Gastrointest Surg. 2016;20(8):1435–1443. doi:10.1007/s11605-016-3180-5
22. Yamashita S, Passot G, Aloia TA, et al. Prognostic value of carbohydrate antigen 19-9 in patients undergoing resection of biliary tract cancer. Br J Surg. 2017;104(3):267–277. doi:10.1002/bjs.10415
23. Wang JK, Hu HJ, Shrestha A, et al. Can preoperative and postoperative CA19-9 levels predict survival and early recurrence in patients with resectable hilar cholangiocarcinoma? Oncotarget. 2017;8(28):45335–45344. doi:10.18632/oncotarget.17336
24. Marrelli D, Caruso S, Pedrazzani C, et al. CA19-9 serum levels in obstructive jaundice: clinical value in benign and malignant conditions. Am J Surg. 2009;198(3):333–339. doi:10.1016/j.amjsurg.2008.12.031
25. Yoon JH, Higuchi H, Werneburg NW, Kaufmann SH, Gores GJ. Bile acids induce cyclooxygenase-2 expression via the epidermal growth factor receptor in a human cholangiocarcinoma cell line. Gastroenterology. 2002;122(4):985–993. doi:10.1053/gast.2002.32410
26. Sirica AE, Lai GH, Zhang Z. Biliary cancer growth factor pathways, cyclo-oxygenase-2 and potential therapeutic strategies. J Gastroenterol Hepatol. 2001;16(4):363–372. doi:10.1046/j.1440-1746.2001.02438.x
27. Sasaki R, Takeda Y, Funato O, et al. Significance of ductal margin status in patients undergoing surgical resection for extrahepatic cholangiocarcinoma. World J Surg. 2007;31(9):1788–1796. doi:10.1007/s00268-007-9102-7
28. Chua TC, Mittal A, Arena J, Sheen A, Gill AJ, Samra JS. Resection margin influences survival after pancreatoduodenectomy for distal cholangiocarcinoma. Am J Surg. 2017;213(6):1072–1076. doi:10.1016/j.amjsurg.2016.09.049
29. Mosadeghi S, Liu B, Bhuket T, Wong RJ. Sex-specific and race/ethnicity-specific disparities in cholangiocarcinoma incidence and prevalence in the USA: an updated analysis of the 2000–2011 surveillance, epidemiology and end results registry. Hepatol Res. 2016;46(7):669–677. doi:10.1111/hepr.12605
30. Yorke E, Melck A, Wiseman SM. Impact of sex on the clinicopathological characteristics and prognosis of papillary thyroid cancer. Can J Surg. 2016;59(4):287–288. doi:10.1503/cjs
31. Wainer Z, Wright GM, Gough K, et al. Impact of sex on prognostic host factors in surgical patients with lung cancer. ANZ J Surg. 2017;87(12):1015–1020. doi:10.1111/ans.2017.87.issue-12
32. Wan JF, Yang LF, Shen YZ, et al. Sex, race, and age disparities in the improvement of survival for gastrointestinal cancer over time. Sci Rep. 2016;6:29655. doi:10.1038/srep29655
33. Fakhry C, Westra WH, Wang SJ, et al. The prognostic role of sex, race, and human papillomavirus in oropharyngeal and nonoropharyngeal head and neck squamous cell cancer. Cancer. 2017;123(9):1566–1575. doi:10.1002/cncr.v123.9
34. Ryu HS, Chung JH, Lee K, et al. Overexpression of epithelial-mesenchymal transition-related markers according to cell dedifferentiation: clinical implications as an independent predictor of poor prognosis in cholangiocarcinoma. Hum Pathol. 2012;43(12):2360–2370. doi:10.1016/j.humpath.2012.07.004
35. Navaneethan U, Lourdusamy V, Gk Venkatesh P, Willard B, Sanaka MR, Parsi MA. Bile proteomics for differentiation of malignant from benign biliary strictures: a pilot study. Gastroenterol Rep. 2015;3(2):136–143. doi:10.1093/gastro/gou066
36. Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma – evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15(2):95–111. doi:10.1038/nrclinonc.2017.157
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

