Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Preliminary Findings on the Potential Use of Magnetic Resonance Elastography to Diagnose Lacunar Infarction
Authors Wang L, Ke J , Hu X, Zhu M, Yu Y
Received 2 May 2022
Accepted for publication 27 July 2022
Published 1 August 2022 Volume 2022:18 Pages 1583—1591
DOI https://doi.org/10.2147/NDT.S371404
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Lingjie Wang,* Jun Ke,* Xiaoyin Hu, Mo Zhu, Yixing Yu
Department of Radiology, The First Affiliated Hospital of Soochow University, Soochow, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mo Zhu; Yixing Yu, Department of Radiology, The First Affiliated Hospital of Soochow University, No. 188 Shizi Road, Soochow, 215000, People’s Republic of China, Tel +86-512-67973009, Fax +86-512-67973061, Email [email protected]; [email protected]
Purpose: Lacunar infarction is usually diagnosed by conventional technologies, such as CT and diffusion weighted imaging (DWI). To improve the accuracy of diagnosis, neurocognitive screening is still needed. Therefore, additional imaging methods that can assist and provide more accurate and rapid diagnostics are urgently needed. As an initial step towards potentially using MR elastography (MRE) for such diagnostic purposes, we tested the hypothesis that the mechanical properties of tissue in the vicinity of cerebral vasculature change following lacunar infarction in a way that can be quantified using MRE.
Patients and Methods: MRE and MR angiography (MRA) images from 51 patients diagnosed with lacunar infarction and 54 healthy volunteers were acquired on a 3T scanner. All diagnoses were confirmed by matching neurocognitive test results to locations of flow obstruction in MRA. ROIs of the cerebral vessels segmented on the MRA images were mapped to the MRE images. Interpolation-based inversion was applied to estimate the regional biomechanical properties of ROIs that included cerebral vessels. The effects of lacunar infarction, sex, and age were analyzed using analysis of covariance (ANOCOVA).
Results: Shear moduli over vessel ROIs were significantly lower for the lacunar infarction group than those of the healthy control group. A positive correlation between modulus over vessel ROIs and age was observed. However, no significant correlation was found between sex and the regional biomechanical properties of the vessel ROIs.
Conclusion: Results supported the hypothesis and suggest that biomechanical properties may be of utility in diagnosis of lacunar infarction.
Keywords: magnetic resonance elastography, MRE, cerebral vasculature, lacunar infarction, biomechanical properties
Introduction
Lacunar infarction is a small and non-cortical infarct that accounts for a quarter of ischemic stroke.1 In pathophysiology, it mainly shows media thickening, transparent degeneration, and fibrinoid degeneration of small perforating arteries.2 Infarct may result from the degeneration of tiny arteries in the deep white matter of the brain and the occlusion of perforating arteries, leading to local cerebral ischemia, followed by necrosis and liquefaction under the action of macrophages.
In most cases, lacunar infarction does not produce clinical symptoms, such as focal neurological signs, aphasia, hemiplegia and paresthesia, because the blood supply area of the perforating artery is small and there are few affected brain tissue is present. Half of the patients with a first-ever lacunar ischemic stroke have mild cognitive impairment.3 However, another extreme situation is that the function of the brain tissue involved in it is very important, and the surrounding brain tissue cannot be replaced after necrosis. Clinical symptoms will be relatively obvious, similar to acute cerebral infarction, and clinical symptoms are severe. Different from the other intracranial lesions, the lesions are mostly found in the deep white matter, basal ganglia and brain stem, showing an irregular round, oval shape, with a diameter of less than 15mm, mostly 3 to 4mm.
Clinical diagnosis of lacunar infarction is usually based on CT, MRA, or DWI images. Although studies have shown that while DWI could provide better accuracy in diagnosis, compared with other imaging techniques, the precision is still technique dependent.1,4 Clinical observation of neurocognitive behavior is usually needed for accurate diagnosis. Therefore, additional imaging methods that can help improve the accuracy and efficiency of the diagnosis of lacunar infarction are in great desire.
Magnetic resonance elastography (MRE), a new noninvasive imaging method that visualizes and quantifies tissue elasticity, can measure soft tissue properties, including cerebrovascular, and for the diagnosis of neurological and degenerative disease.5–7 In recent years, non-invasive methods of the mechanical properties of the brain have been visualized and measured by MRE, which is a particularly sensitive medical imaging technology that may increase the potential for early diagnosis.8 Studies of cardiac-gated homeostatic MRE suggest that MRE may be useful in detecting cerebrovascular disease.9
Early studies showed ultrasound could provide a qualitative estimate of vessel stiffness.10 The relative change of the lumen cross-sectional area for a given blood pressure could be used for measurement.11 However, technical constraints limited its clinical applications. Other techniques such as CT angiography (CTA) could not directly estimate the tissue properties.12 MRE is a non-invasive technique for measuring the biomechanical properties of soft tissues including brain.13 By synchronizing the motion encoding gradient with the externally applied vibration, MRE can record the harmonic motion of soft tissues, and biomechanical properties of the brain tissues could be estimated from the acquired displacement field.5–7,14–19 A recent study using cardiac-gated, steady-state MRE with spiral readout showed that the brain tissue softened during cerebral systole.20 This was the first study showing that MRE could be potentially useful for detection of cerebral vascular diseases. The same group has also shown that perfusion affects the viscoelasticity of brain tissue.21 Although the biomechanical properties of the whole brain may be related to cerebral vascular disease, specific investigation on lacunar infarction is still desired.
It is well known that the biomechanical properties of blood vessels are closely related to development, aging, and vessel disease.22 Studies have shown that intracranial vessels lose their elasticity as the ratio of elastin to collagen decreases, which increases the chance of brain stroke or aneurysm.23 However, it is challenging to measure the biomechanical properties of the blood vessels directly due to the small cross-sectional area of the vessels. Therefore, many studies have used a region-of-interest (ROI) that covers both the vessel and the lumen area for analysis. In this study, we also investigate the ROI covering the vessel and lumenarea as a preliminary step.
In this study, to test the hypothesis that biomechanical properties of ROIs encompassing the cerebral vessels change after lacunar infarction, we used MRE and MRA images to quantify the shear modulus of the ROIs. The vessel ROIs were segmented from MR angiography (MRA) images. An interpolation-based inversion was applied to estimate the shear modulus of the vessel ROIs after registration of MRA and MRE images. Correlations between the biomechanical properties of the vessel ROIs and the presence of lacunar infarction, sex, and age were investigated.
Materials and Methods
Participants and Image Acquisition
Fifty-one patients and 54 healthy volunteers were recruited in 2019. The patients, all diagnosed with lacunar infarction, included 25 males and 26 females with an age range from 30 to 88 years (mean=65, SD=11). Among patients, 45 cases (88%) were chronic; 49 cases (96%) had lacunar infarcts less than 0.5 cm in size; 4 patients (8%) had atherosclerosis; and 42 patients (82%) presented with multiple lacunar infarcts. In addition, 43 patients presented with lacunar infarcts in the right hemisphere, and 49 in the left hemisphere. The healthy volunteers included 27 males and 27 females, with ages ranging from 18 to 77 (mean=45, SD=13). Inclusion criteria were as follows: (1) Consistent with the clinical diagnosis of lacunar infarction. (2) The first diagnosis. (3) Non-large-scale cerebral infarction, infarct focus diameter is not greater than 5mm, single or multiple, unilateral or bilateral. (4) Intracranial vascular MRA showed normal, without congenital variation. (5) No metabolic diseases, no history of long-term medication use. (6) Blood pressure was in the normal range. (7) A cerebrovascular ultrasound examination showed normal blood flow from the bilateral middle cerebral arteries. Exclusion criteria were as follows: (1) large area of cerebral infarction, diameter >5mm. (2) Cerebral vascular stenosis. (3) Inconscious, unable to cooperate. (4) A history of cardiovascular and cerebrovascular atherosclerosis. (5) Hyperlipidemia and hyperglycemia. (6) Hypertension. (7) Take cardiovascular and cerebrovascular treatment drugs. Sample numbers were obtained at study time screening based on inclusion criteria and clinical experience.
For each patient or volunteer, MRA and MRE images were acquired using a 3T human MRI scanner (Skyra, Siemens, Erlangen, Germany). MRA images were obtained using a 3D time-of-flight (TOF) MRA sequence (TR/TE=21/3.4ms; in-plane resolution=0.3mm×0.3mm; slice thickness=0.7mm). A pneumatic actuator with a soft pillow driver (Resoundant; Rochester, MN, USA) was used to generate shear waves in the brain. The actuation frequency was 50 Hz and 8 temporal points were sampled for each wave cycle. MRE images were obtained using an echoplanar imaging MRE sequence24 (TR/TE=6720/65ms; field-of-view=240mm; slice thickness=3mm; voxel size=3×3×3mm3). This study was approved by the Institutional Review board of Soochow University and with the Declaration of Helsinki. All participants provided written, informed consent.
ROI Segmentation and Image Registration
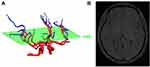
We used a 3D region growing algorithm25 to segment ROIs encompassing the anterior and middle cerebral arteries upward from the communicating segment (C7) from MRA images (Figure 1). To register the segmented ROIs from MRA to MRE data, masks of the ROIs and magnitude images of MRE were interpolated to the same in-plane resolution with the same voxel size of 0.75 mm × 0.75 mm × 3 mm. Then, the 3D ventricle regions were segmented based on the resized MRA images and magnitude images of MRE (Figure 2). The registration was achieved by minimizing the cross correlation of the 3D ventricle regions of the MRA and MRE images. An analysis of registration accuracy is presented in Supplement I (Figure S1). Considering the resolution of MRE and the registration accuracy, we removed vessels with diameters less than 1 mm.
 |
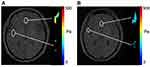
Figure 2 Example ROIs of the ventricle regions in (A) MRA images and the corresponding (B) magnitude images of MRE. The image slices including the ventricle regions were used for registration. |
Analysis of Vessel ROIs
A two-stage procedure was adopted to estimate the viscoelastic properties of cerebral vessels. 4D MRE phase images were unwrapped and a curl operation was applied before estimation of shear moduli (Figure 3). The regional absolute shear modulus of the vessel ROIs was estimated from these data at a resolution determined by the displacement map acquired at a voxel size of 0.75 mm×0.75mm×3 mm. Complex shear moduli (G*) were estimated using a 3D direct inversion algorithm with an isotropic kernel size of three voxels.26,27 The mean absolute shear modulus over vessel ROIs was estimated by applying the registered masks of the vascular ROIs to the whole brain modulus maps. Validation of this interpolation-based modulus estimation is presented in the Supplemental Document (Supplement II, Figures S2–S4).
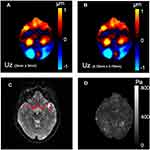 |
Figure 3 (A) The displacement field (through plane component) acquired from MRE. (B) The displacement field (through plane component) after 4 times interpolation.(C) The magnitude images of MRE and the vessel ROIs. (D) The corresponding maps of the modulus over vessel ROIs estimated by a simulation validated, interpolation-based inversion (Supplements I, II). |
Statistical Analysis
Relationships between shear moduli and the presence of infarction, sex, and age were analyzed. Taking the status of infarction and sex as categorical factors and age as continuous factors, MATLAB function ANOVAN was used to perform ANOCOVA, with a confidence level of 95%.
Results
For patients with diagnosed lacunar infarcts, the shear modulus of the vessel ROIs was lower than that of a healthy volunteer (Figure 4). Table 1 presents the outcome of the ANOCOVA used to analyze the effects of sex, age and the presence of infarction (Table 1). In cases of luminal infarction, sensorimotor syndrome occurs more frequently and atypical lacunar syndrome occurs less frequently. Table 2 presents the frequency of the different clinical lacunar syndromes in the study sample (Table 2). Table 3 presents the baseline parameters for patient group and control group (Table 3), and the comparative results of sex, age, and elasticity values. Table 4 presents the clinical features for female and male patient groups (Table 4), including the location, size, acute and chronic, single bilateral, single multiple, and other clinical manifestations of lacunar infarction.
 |
Table 1 Results of Three-Way Analysis of Covariance |
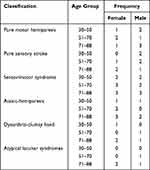 |
Table 2 The Frequency of the Different Clinical Lacunar Syndromes in the Study Sample |
 |
Table 3 Baseline Parameters for Patient Group and Control Group |
 |
Table 4 Clinical Features for Female and Male Patient Groups |
The shear modulus of ROIs containing vessels in patients with lacunar infarcts (143±14 Pa, mean±SD) was significantly lower than that for healthy patients (151±20 Pa, p < 0.003) (Figure 5). With respect to sex, the mean shear moduli of the vessel ROIs of males and females were 147±18Pa and 147±18Pa, respectively, and were not statistically distinguishable (p=0.83). There was a significant negative correlation between the mean shear modulus of the vessel ROIs and luminal infarction (r=−0.27, p=0.005). However, the results showed that the effect of age on modulus over vessel ROIs was significant (p<0.05). A linear fitting showed that the modulus increased with age (Figure 5).
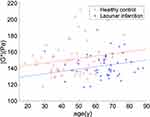 |
Figure 5 A linear fitting of the modulus over vessel ROIs and age. Separate lines were fitted to the healthy control and lacunar infarction groups, and the lines were constrained to be parallel. |
Discussion
MRE is an imaging technique for measuring viscoelastic tissue properties that has proved to be a sensitive indicator of neural tissue integrity. Assessing differences in MRE parameters between mild and healthy older adults suggests that these data support the use of MRE as a novel imaging biomarker.17 In cardiovascular studies, stiffness may be an important biomarker, and MRE is a phase-contrast MR imaging technique that noninvasively measures of vascular stiffness, namely the biomechanical property.28 MRE provides healthy participants with the biomechanical properties of the brain gray matter and white matter, as potential baseline values that will be used for clinical research.7
Shear modulus of ROIs encompassing the cerebral vessels from lacunar infarction patients as measured by MRE was lower than that for healthy patients. This motivated the exploration of shear modulus as a potential biomarker of lacunar infarction.
The way that we envision shear modulus measurements being of clinical utility is through comparison of ROIs throughout the neurovasculature of a single patient. Indeed, in individual patients, the ROIs associated with lacunar infarct had significantly lower stiffness (p<0.05). However, it should be noted that a larger sample study is needed to demonstrate its clinical usefulness.
Conventional MRE image acquisition methods were used in this study, which facilitates the clinical application of the current MRE technique. This measurement is different from that of Schrank et al, who used a cardiac-gated steady-state MRE during cerebral systole. Rather than the vessel ROIs, they found a decreased |G*| value of the brain tissue during the cerebral arterial pulsation. Since collagen fibers, especially collagen type I, contributed to the tissue stiffness,29 it is possible that the architecture of collagen type I that regulated ECM contributed to the decrease in stiffness after the infarction. In addition, the blood–brain barrier is disrupted after infarction; therefore, the smooth muscle cell (SMC) and structure of the extracellular matrix (ECM) that contribute to the stiffness of the vessels could be disrupted,22 ultimately resulting in a decreased modulus.
In lacunar infarction, women differ from men in the distribution of risk factors and stroke subtype, stroke severity, and outcome.30 Our results showed no statistical differences in the shear modulus of brains with male and female patients, consistent with the observations of others.31 Sex differences in biomechanical properties have, however, been reported in the occipital and temporal lobes.6 Although whole brain stiffness has been reported to decrease with age6,17,31 and Alzheimer’s disease,5 we observed a slight increase in shear modulus over vessel ROIs with age. ANOCOVA results showed the significance of age was marginal, compared with that of the infarction.
The proposed method has several limitations that bear mention. Blood vessels are smaller than the resolution of current MRE, which makes it impossible to estimate the properties of the vessels themselves. Accurate segmentation and registration were necessary, but complicated the measurements, and may currently pose obstacles to clinical applications. Although simulations validated the method of displacement field interpolation, improvements in imaging resolution of MRE would further improve accuracy.32 The vascular ROIs included not only the cerebral vessels but also the fluid inside, which reduces the accuracy of modulus estimates.
Thus, the measurements presented are not a direct measurement of the properties of the target vessels. However, the evaluation of these ROIs still appears to provide important information. We cannot be certain that lacunar infarct caused the observed modulus differences, or whether people with lower modulus are more susceptible to infarct; this is an important topic for future investigation.
Conclusions
By registering images acquired from MRA and MRE, we were able to delineate ROIs containing segmented vessel in the brain. Simulations showed that after interpolation of the displacement field, improved contrast to distinguish small features was obtained from an estimated modulus map. This provided a direct estimate of the shear modulus of vessel ROIs. Results from a clinical investigation of 105 cases support the current hypothesis that the biomechanical properties of regions incorporating cerebral vessels are closely related to lacunar infarction. There was also a slight positive correlation between age and modulus over vessel ROIs, while no significant differences were observed across sex groups. By combining MRE and MRA, mechanical properties of cerebral vessel ROIs have the potential to contribute to the diagnosis of lacunar infarction.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Norrving B. Long-term prognosis after lacunar infarction. Lancet Neurol. 2003;2(4):238–245. doi:10.1016/S1474-4422(03)00352-1
2. Rudilosso S, Rodríguez-Vázquez A, Urra X, Arboix A. The potential impact of neuroimaging and translational research on the clinical management of lacunar stroke. Int J Mol Sci. 2022;23(3):1497. PMID: 35163423; PMCID: PMC8835925. doi:10.3390/ijms23031497
3. Blanco-Rojas L, Arboix A, Canovas D, Grau-Olivares M, Oliva Morera JC, Parra O. Cognitive profile in patients with a first-ever lacunar infarct with and without silent lacunes: a comparative study. BMC Neurol. 2013;13:203. PMID: 24341857; PMCID: PMC3866944. doi:10.1186/1471-2377-13-203
4. Sacco S, Marini C, Totaro R, Russo T, Cerone D, Carolei A. A population-based study of the incidence and prognosis of lacunar stroke. Neurology. 1335–1338;66(9). doi:10.1212/01.wnl.0000210457.89798.0e
5. Murphy MC, Jones DT, Jack CR, et al. Regional brain stiffness changes across the Alzheimer’s disease spectrum. Neuroimage Clin. 2015;10:283–290. doi:10.1016/j.nicl.2015.12.007
6. Arani A, Murphy MC, Glaser KJ, et al. Measuring the effects of aging and sex on regional brain stiffness with MR elastography in healthy older adults. Neuroimage. 2015;111:59–64. doi:10.1016/j.neuroimage.2015.02.016
7. Schwarb H, Johnson CL, Daugherty AM, et al. Aerobic fitness, hippocampal viscoelasticity, and relational memory performance. Neuroimage. 2017;153:179–188. doi:10.1016/j.neuroimage.2017.03.061
8. Hiscox LV, Johnson CL, Barnhill E, et al. Magnetic resonance elastography (MRE) of the human brain: technique, findings and clinical applications. Phys Med Biol. 2016;61(24):R401–R437. PMID: 27845941. doi:10.1088/0031-9155/61/24/R401
9. Schrank F, Warmuth C, Tzschätzsch H, et al. Cardiac-gated steady-state multifrequency magnetic resonance elastography of the brain: effect of cerebral arterial pulsation on brain viscoelasticity[J]. J Cereb Blood Flow Metab. 2020;40(5):991–1001. doi:10.1177/0271678X19850936
10. Wang Y, Zhang Y, Wang WQ. Wavelet feature extraction of Doppler blood flow waveforms.
11. Safar ME, Lacolley P. Disturbance of macro- and microcirculation: relations with pulse pressure and cardiac organ damage. Am J Physiol Heart Circ. 2007;293(1):H1–H7. doi:10.1152/ajpheart.00063.2007
12. Nishida T, Kinoshita M, Tanaka H, Fujinaka T, Yoshimine T. Quantification of cerebral artery motion during the cardiac cycle,” (in English). Am J Neuroradiol. 2011;32(11):E206–E208. doi:10.3174/ajnr.A2354
13. Venkatesh SK, Ehman RL. Magnetic resonance elastography of liver. Magn Reson Imaging Clin N Am. 2014;22(3):433–446. PMID: 25086938; PMCID: PMC8525633. doi:10.1016/j.mric.2014.05.001
14. ElSheikh M, Arani A, Perry A, et al. MR elastography demonstrates unique regional brain stiffness patterns in dementias. Am J Roentgenol. 2017;209(2):403–408. doi:10.2214/AJR.16.17455
15. Hiscox LV, Johnson CL, McGarry MD, et al. Mechanical property alterations across the cerebral cortex due to Alzheimer’s disease. Brain commun. 2019;2(1). doi:10.1093/braincomms/fcz049
16. Sack I, Beierbach B, Wuerfel J, et al. The impact of aging and gender on brain viscoelasticity. NeuroImage. 2009;46(3):652–657. doi:10.1016/j.neuroimage.2009.02.040
17. Hiscox LV, Johnson CL, McGarry MDJ, et al. High-resolution magnetic resonance elastography reveals differences in subcortical gray matter viscoelasticity between young and healthy older adults. Neurobiol Aging. 2018;65:158–167. doi:10.1016/j.neurobiolaging.2018.01.010
18. Bunevicius A, Schregel K, Sinkus R, Golby A, Patz S. REVIEW: MR elastography of brain tumors. NeuroImage. 2020;25:102109. doi:10.1016/j.nicl.2019.102109
19. Johnson CL, Schwarb H, Horecka KM, et al. Double dissociation of structure-function relationships in memory and fluid intelligence observed with magnetic resonance elastography. NeuroImage. 2018;171:99–106. doi:10.1016/j.neuroimage.2018.01.007
20. Schrank F, Warmuth C, Tzschätzsch H, et al. Cardiac-gated steady-state multifrequency magnetic resonance elastography of the brain: effect of cerebral arterial pulsation on brain viscoelasticity. J Cereb Blood Flow Metab. 2019:0271678X19850936. doi:10.1177/0271678X19850936
21. Hetzer S, Birr P, Fehlner A, et al. Perfusion alters stiffness of deep gray matter. J Cereb Blood Flow Metab. 2018;38(1):116–125. doi:10.1177/0271678X17691530
22. Lacolley P, Regnault V, Segers P, Laurent S. Vascular smooth muscle cells and arterial stiffening: relevance in development, aging, and disease. Physiol Rev. 2017;97(4):1555–1617. doi:10.1152/physrev.00003.2017
23. Lasheras JC. The biomechanics of arterial aneurysms. Annu Rev Fluid Mech. 2007;39(1):293–319. doi:10.1146/annurev.fluid.39.050905.110128
24. Chaze CA, McIlvain G, Smith DR, et al. Altered brain tissue viscoelasticity in pediatric cerebral palsy measured by magnetic resonance elastography. NeuroImage. 2019;22:101750. doi:10.1016/j.nicl.2019.101750
25. Gonzalez RC, Woods RE. Digital Image Processing.
26. Feng Y, Clayton EH, Okamoto RJ, Engelbach J, Bayly PV, Garbow JR. A longitudinal magnetic resonance elastography study of murine brain tumors following radiation therapy. Phys Med Biol. 2016;61(16):6121–6131. doi:10.1088/0031-9155/61/16/6121
27. Bayly PV, Clayton EH, Genin GM. Quantitative imaging methods for the development and validation of brain biomechanics models. Annu Rev Biomed Eng. 2012;14:369–396. doi:10.1146/annurev-bioeng-071811-150032
28. Khan S, Fakhouri F, Majeed W, Kolipaka A. Cardiovascular magnetic resonance elastography: a review. NMR Biomed. 2018;31(10):e3853. PMID: 29193358; PMCID: PMC5975119. doi:10.1002/nbm.3853
29. Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15(12):802–812. doi:10.1038/nrm3896
30. Arboix A, Blanco-Rojas L, Oliveres M, García-Eroles L, Comes E, Massons J. Clinical characteristics of acute lacunar stroke in women: emphasis on gender differences. Acta Neurol Belg. 2014;114(2):107–112. PMID: 24194419. doi:10.1007/s13760-013-0257-8
31. Sack I, Streitberger K-J, Krefting D, Paul F, Braun J. The influence of physiological aging and atrophy on brain viscoelastic properties in humans. PLoS One. 2011;6(9):e23451. doi:10.1371/journal.pone.0023451
32. Johnson CL, Holtrop JL, McGarry MDJ, et al. 3D multislab, multishot acquisition for fast, whole-brain MR elastography with high SNR efficiency. Magn Reson Med. 2014;71(2):477–485. doi:10.1002/mrm.25065
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.