Back to Journals » International Journal of Women's Health » Volume 15
Predisposing Factors for Granulomatous Lobular Mastitis: A Case-Control Study
Authors Zeng Y, Zhang D, Zhao W, Fu N, Huang Q, Li S, Gao C, Yu J
Received 24 March 2023
Accepted for publication 11 July 2023
Published 18 July 2023 Volume 2023:15 Pages 1063—1075
DOI https://doi.org/10.2147/IJWH.S414054
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Elie Al-Chaer
Yifei Zeng,1,2 Dongxiao Zhang,1 Wenjie Zhao,1 Na Fu,1 Qiao Huang,1 Shuqi Li,2 Chang Gao,2 Jiale Yu2
1Department of Galactophore, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing, People’s Republic of China; 2Graduate School, Beijing University of Chinese Medicine, Beijing, People’s Republic of China
Correspondence: Dongxiao Zhang, Department of Galactophore, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, No. 23, Art Museum Back Street, Dongcheng District, Beijing, People’s Republic of China, Tel +86 13811077684, Email [email protected]
Background: Despite the rising incidence rate of granulomatous lobular mastitis (GLM), uncertainties persist about its etiologic and predisposing factors to guide clinical treatment and early prevention. The objective of this study is to explore the predisposing factors for GLM.
Patients and methods: This case-control study was conducted from 2018 to 2021 at Beijing Hospital of Traditional Chinese Medicine, Capital Medical University. Patients with GLM (cases) were matched with healthy examinees (controls) in a 1:1 ratio according to gender and living area. We analyzed their demographic features and investigated 75 factors that may be relevant to GLM using a standard questionnaire. Univariate and multivariable binary conditional logistic regression analyses were used to compare the differences between the two groups and evaluate the predisposing factors that may induce GLM.
Results: There were 594 female GLM patients and 594 matched controls included in the study. The average age of the cases was 32.78 years (mainly 20 to 40). The incidence was high within five years after childbirth, and lesions were mainly in the unilateral breast. Univariate and multivariable conditional logistic regression analyses obtained six relevant factors and six high-risk factors. The six relevant factors included age, marriage, emotional abnormality, high prolactin, psychiatric drug intake, and sex hormone intake. Additionally, the independent high-risk factors for GLM included gestation, nipple invagination, blunt trauma, non-iatrogenic massage, lactation disorder, and nipple discharge (odds ratio (OR)=17.378, 8.518, 4.887, 3.116, 2.522, 1.685, P< 0.05). Menopause was an independent protective factor (OR=0.249, P< 0.05).
Conclusion: The factors that increase milk and secretion production in the mammary duct are the main risk factors of GLM, especially when the nipples are invaginated. These factors can obstruct the duct and induce inflammation. Additionally, hormonal disorders, extrinsic trauma, and emotional abnormalities can accelerate the occurrence of GLM.
Keywords: granulomatous lobular mastitis, GLM, predisposing factor, risk factor, case-control study, etiology
Introduction
Granulomatous lobular mastitis (GLM), known as idiopathic granulomatous mastitis, was first reported by Kessler and Wolloch in 1972.1 Epidemiological studies showed its incidence rate is 1.8% in all breast diseases and varies by race. GLM usually uses pathological examination as the diagnostic criteria, which manifests multifocal non-caseous granulomas centered around the lobules. The morbidity of GLM in Hispanic and Asian women is higher than in other races. It usually occurs in the unilateral breast among women from 20 to 40 years old, and the incidence is high within five years after delivery.2 Early manifestations of the disease include a localized painful mass and redness of the breast, which would then develop into an abscess and ulceration and could form a sinus and fistula. GLM has a long course with easy relapses, seriously affecting the patients’ breast appearance and quality of life.3
Presently, the cause of GLM is unclear. It is reported that many factors can affect the occurrence of this disease.4 The predisposing factors reported in the literature include gestation, lactation, bacterial infection, trauma, sex hormone level, oral contraceptive intake, etc.5 Among infection factors, Corynebacterium have attracted more and more attentions of researchers in recent years, and studies found that many factors such as lactation may further exacerbate the infection. The increasing incidence of GLM worldwide in recent years poses a substantial health burden to many women’s health. Therefore, it is vital to explore the predisposing factors of GLM to guide specific treatment and disease prevention strategies to reduce the incidence rate of GLM and improve the quality of life of susceptible populations.
There are few relevant studies on the predisposing factors of GLM reported in the previous literature. Most studies were retrospective analyses or small sample case-control studies, limiting the validity of their results. To the best of our knowledge, this is the first large sample case-control study on the predisposing factors of GLM. The purpose is to reduce the incidence rate of GLM through early-stage prevention and intervention.
Methods
Study Design and Participants
This case-control study was conducted from June 2018 to June 2021 at Beijing Hospital of Traditional Chinese Medicine, Capital Medical University. Patients with GLM were included as the case group, and healthy female physical examinees were selected as the control group in the same period. Participants of the two groups were matched according to gender and living area (the same prefecture-level city) in a 1:1 ratio. The histopathology diagnostic criteria for GLM patients are: The performance of microscopic pathology is atypical lobular centric nonnecrotizing granulomas in breast with or without cystic neutrophilic abscesses.6
The inclusion criteria were: (1) The pathological diagnosis of the case group was GLM, while the control group had no history of GLM; (2) People with complete clinical data; (3) People were able to communicate orally and cooperate with researchers to complete the questionnaire; (4) Participants with no severe heart, lung, liver and kidney dysfunction; (5) Participants voluntarily took part in this study, were not involved in any clinical trials in the same time and all signed informed consent.
In contrast, the exclusion criteria included: (1) Incomplete data or more than 30% of missing information in the questionnaire; (2) People with cancers or other serious diseases; (3) With a diagnosis of other autoimmune diseases, such as systemic lupus erythematosus and rheumatism arthritis.
Sample Size Estimation
According to the recommended sample size estimation method in the literature,4,5 the exposure rate of variables in the control group was set as 20% with an odds ratio (OR) of 2.0 with a 95% confidence interval (CI) and 80% power, α= 0.05, β= 0.10. Considering that the age, geographical and cultural variability of our respondents may be relatively large, we increased the sample size by 20% to ensure the stability of the analysis results. The minimum sample size was estimated to be 243 cases in each group. The formula used for the sample size calculation was as follows:
Where p0 is the incidence rate of GLM in the general population, p1 is the exposure rate of research factors in the case group, 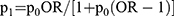 ,
, 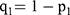 ,
, 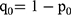 .
.
Data Collection
The occurrence of GLM is significantly associated with gestation, lactation, abortion, nipple invagination and nipple discharge caused by various reasons. Additionally, it may be associated with multiple endogenous and exogenous factors, such as dramatic changes in hormone levels, intake of sex hormones, hyperprolactinemia, bacterial infection, and blunt trauma.7–10 Based on evidence from the literature and clinical experience, we preliminarily identified 75 possible risk factors relevant to GLM and developed a questionnaire accordingly.
The survey involved completing a standard questionnaire including 75 risk factors of GLM designed by collecting literature data and combining our previous pilot study results. Trained professional researchers completed the questionnaires mainly through face-to-face interviews and collected personal information one-to-one by reviewing medical records. Data was collected on the: (1) General information of patients, including age (years), height (m), weight (kg), body mass index (BMI, kg/m2), living area (prefecture-level city), marital status, gestation history, menopause status, age of the youngest child (years), and lactation time (months); (2) Personal history, including lactation time, bilateral breast lactation disorder, nipple discharge, nipple invagination (including I, II, and III degree invagination), family history of breast benign and malignant diseases; (3) Hormone-related factors like prolactin level, psychotropic drugs intake history, sex hormone intake (including medication history of estrogen and progesterone drugs, such as contraceptive), and abortion history; (4) Exogenous factors, including history of non-iatrogenic massage on bilateral breasts (lactation and non-lactation massage) and history of trauma; (5) Emotional factors, such as emotional status (whether emotional abnormality); (6) Other factors, such as thyroid dysfunction (hyperthyroidism and hypothyroidism), sleep, and diet preference.
Considering that medications taken too long ago would not have considerable influence on the disease, and in a bit to avoid recall bias, the period of drug intake included current intake and intake in the past three months. The normal range of serum prolactin level was 3.34–26.72ng/mL before menopause and 2.74–19.64ng/mL after menopause. The standard ranges of thyroid hormones were: free triiodothyronine (FT3) 2.14–4.21ng/mL; free thyroxine (FT4) 0.59–1.25ng/mL; and thyroid-stimulating hormone (TSH) 0.56–5.91mIU/L. Body mass index (BMI) was calculated as weight (kg)/[height (m)]2. Psychotropic drugs included quetiapine, aripiprazole, ziprasidone, risperidone, olanzapine, amisulpride, and escitalopram oxalate. The definition of lactation disorders included lactation mastitis, abscess formation, breast surgery, nipple inversion, and other reasons for reduced or no breastfeeding, frequently interrupted breastfeeding, and insufficient milk supply. Nipple inversion was classified into three grades. In Grade I, the inversion is corrected simply by manipulation, and the nipple protrusion is long-lasting. In Grade II, the inversion can be corrected by manipulation, but recurrence of the inversion is frequent. Finally, in Grade III, the inversion can only be corrected through surgery. In addition, the definition of sleep disorders in this study was consistent with the Diagnostic and Statistical Manual of Mental Disorders, 5th Ed (DSM-V).
Quality Control
We assigned three trained researchers to conduct interviews and complete the questionnaire. All researchers received unified training to ensure standardization and unification of the investigation. A standardized questionnaire based on previous clinical studies was used to eliminate potential bias. During the research, we carefully communicated with the participants to gain trust and ensure the accuracy and integrity of data collection.
Statistical Analysis
IBM SPSS Statistics 26.0 software (IBM Corp., Armonk, N.Y., USA) was used for statistical analysis. Measurement data were expressed as mean ± standard deviation (mean ± SD) and compared using a t-test. Counting data was summarized as frequency and percentage, and univariate comparisons were conducted using the χ2 test. A multivariable logistic regression model was used to explore the association between the significant factors identified during univariate analyses. Adjusted ORs and their corresponding 95% CIs were obtained using binary conditional multivariable logistic regression analysis. Missing values during data collection were treated using the column deletion method. P<0.05 from two-tailed tests was considered statistically significant.
Results
Clinical Characteristics
This study included 594 female GLM patients and matched 594 healthy female physical examinees in the same period (Figure 1). On average, the case group was younger (mean age = 32.78 years) than the control group (mean age = 38.00 years), and most of the cases were 20 to 40 years old (Figure 2A). The two groups differed significantly by marital status, gestation, and age of the youngest child (P<0.05). Most patients in the case group were diagnosed within five years after the last childbirth (Figure 2B). Unilateral GLM was extremely common in the clinic, but bilateral GLM was rare. There was a significant difference in the menopausal status between the two groups (P<0.05), indicating that menopausal status may be a protective factor for GLM. In addition, there was no significant difference in BMI between the two groups (P>0.05), so the incidence of GLM may not be relevant to obesity (Table 1).
 |
Table 1 Comparisons of the Characteristics of the Study Population |
 |
Figure 1 Study flowchart for inclusion of study participants. |
 |
Figure 2 Comparisons of study groups by age (A) and age of the youngest child (B). |
Personal History
The incidence of lactation disorders in the case group was significantly higher compared with the control group (20.64% vs 4.44%, P<0.001). There was a significant difference in the history of nipple discharge (25.49% vs 5.92%, P<0.001) and nipple invagination (41.39% vs 7.13%, P<0.001) between the two groups. Mild nipple invagination was the most common among all the patients (29.22%). There were no significant differences in lactation time (12.49±8.855 months vs 11.64±8.669 months, P=0.153) and family history of benign or malignant breast diseases (P=0.424) between GLM and control groups (Table 2).
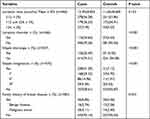 |
Table 2 Comparisons of Personal History Between the Case and Control Groups |
Hormone-Related Factors
The proportion of patients with high prolactin levels in the case group was significantly higher than in the control group (7.41% vs 1.35%, P<0.001). More GLM patients had a history of sex hormone intake, including estrogen and progesterone, compared with the control group (1.93% vs 6.32%, P=0.004). The proportion of patients with psychotropic drug intake history in the case group was significantly higher than in the control group (37.29% vs 0.68%, P=0.01). Therefore, these three factors may increase the possibility of GLM. Meanwhile, there was no significant correlation between abortion and GLM (P>0.05) (Table 3).
 |
Table 3 Comparisons of Hormone-Related Factors Between the Case and Control Groups |
Exogenous Factors
A history of non-iatrogenic breast massage was more common in the case compared to the control group (9.64% vs 45.71%, P<0.001). They sought non-iatrogenic breast massages for lactation disorder, and attempted to make the milk discharge more unobstructed (Table 4). In addition, the incidence of blunt breast trauma was higher in the case than in the control group (20.64% vs 3.02%, P<0.001). Therefore, the two factors were closely related to the occurrence of GLM.
 |
Table 4 Comparisons of Exogenous Factors Between Case and Control Groups |
Emotional and Other Factors
There was a significant difference in the proportion of people with emotional abnormalities, including depression and anxiety, between case and control groups (27.71% vs 16.00%; 48.99% vs 38.72%, P<0.001) (Table 5). However, hyperthyroidism, hypothyroidism, sleep disorder, and diet preference were not significantly relevant to GLM (P>0.05).
 |
Table 5 Comparisons of Emotional and Other Factors Between the Case and Control Groups |
Multivariable Conditional Logistic Regression Analysis
The predisposing factors associated with GLM from univariate analyses were coded as binary independent variables (Yes=1, No=0). The multivariable binary conditional logistic regression analysis showed that gestation, lactation disorder, non-iatrogenic breast massage, breast trauma, nipple discharge, and nipple invagination were independent risk factors for GLM (P<0.05). Menopause was an independent protective factor for GLM (P<0.05) (Table 6, Figure 3).
 |
Table 6 Multivariable Logistic Regression Analysis of the Risk and Protective Factors of GLM |
 |
Figure 3 Forest plot of binary conditional logistic regression analysis of risk factors of GLM. |
We plotted the ROC curve of the risk factors identified from the multivariable logistic regression analysis and calculated the area under the curve (AUC) for each variable. The results showed that nipple invagination was the most reliable risk factor to induce the disease, followed by lactation disorder, age, and non-iatrogenic breast massage (Figure 4).
 |
Figure 4 Receiver operating characteristic (ROC) curve of the predisposing factors of GLM. |
Discussion
Few studies have been published on GLM predisposing factors, and their limitations are obvious. A case-control study of 85 GLM cases emphasized that reproductive factors were the main risk factors of GLM,11 but the sample size was relatively small. In addition, some retrospective studies surveyed the risk factors of GLM occurrence and recurrence,12,13 but the lack of a control group limited the reliability of the results. Therefore, this is the first study to explore the predisposing factors of GLM using a large sample case-control design to provide effective evidence for clinical treatment and prevention.
It is reported that the ages of patients with GLM vary widely from 16 to 83.14,15 GLM mainly occurs between 20 and 40 years old,16 especially within two to five years after delivery. This study showed that the age of GLM ranges from 16 to 75, with an average age of 32.78. The incidence rate of GLM between the age of 20 to 40 was high, consistent with the literature. GLM mainly occurs in non-gestational and non-lactating women; GLM in gestational or lactating women is relatively rare.17,18 Our study suggests that the highest period of GLM occurrence is within five years after childbirth, with a median of about four years after delivery. Most GLM were unilateral breast diseases, while bilateral cases were rare. Menopause is an independent protective factor for GLM, which is congruent with the results of the patient’s age, the youngest child’s age, etc. The main reason is the relatively stable sex hormone levels in menopausal women.
Our results show that nipple discharge related to mammary duct disease was an independent risk factor for GLM. The reason may be that the secretions from the mammary duct and the stimulation of residual milk after lactation increase the permeability and obstruction of the duct, causing duct rupture and secretions overflowing to the breast mesenchyme. This process promotes humoral or cellular immunity. Local inflammation gradually expands to involve most of the breast tissues and may develop into a mass or abscess.19 The effectiveness of glucocorticoid and immunosuppressive therapy for GLM also supports this hypothesis.20 Our results indicate that lactation disorder and nipple invagination are also independent risk factors for GLM. Lactation disorder increases the milk residue in the mammary duct, and nipple invagination exaggerates the obstruction and tension of the duct, which could stimulate an inflammatory immune response and promote the onset of GLM. However, although our results suggest that nipple invagination may be one of the most important precursors of GLM, the severity of nipple invagination does not seem to have a clear causal relationship with the occurrence of GLM. The lack of an association with the severity of nipple invagination is likely because patients with severe nipple invagination are relatively rare in the clinic, and it is difficult to identify statistically significant differences. Therefore, this finding needs to be confirmed by further research.
Hormone-related factors, including changes in prolactin and other sex hormone levels, were important and independent causes of GLM. Our results showed that prolactin level is important in the pathogeny of GLM, which is consistent with the literature. Lin et al found that prolactin increase may lead to the accumulation of secretions, leading to a destruction of the epithelial cells of the mammary duct due to dilation and obstruction of the mammary duct.21 Epithelial cell destruction induces inflammatory reactions and GLM. Therefore, hyperprolactinemia was the main factor influencing GLM.22 Similarly, Sheybani et al proposed that a high level of prolactin is not only a vital factor in GLM but also a predictor of GLM recurrence.23,24 Increased prolactin is the main reason for the occurrence of GLM,8 and drugs are a cause of perturbations of hormone levels. Our results show intake of psychotropic drugs can increase the risk of GLM. A study showed that 37% of 42 patients with mastitis suffered from mental diseases and needed psychotropic drugs.25 Some psychotropic drugs have the side effect of increasing secretions in the duct, promoting mammary duct obstruction and causing GLM.26 Tollin found that the incidence rate of hyperprolactinemia was high in patients with mental disorders on risperidone,27 indicating that such psychotropic drugs could significantly promote prolactin secretion. This result is consistent with ours.
Similarly, a drastic change in other sex hormone levels (including estrogen and progesterone intake, as contraceptives) can lead to endocrine disorders and cause GLM, which is proven by our results about the significant differences in estrogen and progesterone intake between the two groups. Sex hormone disorders may lead to excessive proliferation of the mammary duct endothelium, resulting in obstruction of the duct and inflammatory reactions.28 The sex hormone levels of menopausal women decrease, so they have a reduced incidence rate of GLM. In summary, increased prolactin and the rapid change of other sex hormone levels could lead to GLM.
GLM is generally believed to be an autoimmune disease,9 and this is supported by the effectiveness of steroids and immunosuppressants in the treatment of GLM. The initial factor of GLM is the high level of prolactin or accumulation of secretions in the mammary duct, which can lead to duct damage. The damage triggers an inflammatory autoimmune reaction mediated by T cells and forms non-caseous granuloma. Cserni and Szajki believed that GLM was caused by the local autoimmune reaction of hyperprolactinemia to the fat-rich or protein-rich secretions deposited or overflowing in the mammary duct.29 Erhan et al found that most patients had T cell advantages based on immunohistochemical evaluation, indicating that the pathogenesis of GLM was closely associated with inflammatory immune response mediated by reactive T cells leading to the damage of mammary ducts and formation of centrilobular granulomas.30 It also explained the autoimmune pathophysiological results in GLM. In clinical practice, immunosuppressants and steroids can be used to achieve significant therapeutic effects.31
Based on the above research, we can infer that factors relevant to duct secretion are the main predisposing factors of GLM. These factors include gestation, lactation, nipple discharge, high prolactin level, and psychotropic drug intake, and they increase milk and secretion production in the mammary duct. Other factors like nipple invagination and sex hormone intake can aggravate the obstruction of mammary ducts. They all can induce inflammatory and autoimmune responses and cause GLM.32 This highlights the importance of measuring prolactin levels in the clinic. When treating patients with psychotropic drugs, which could increase mammary duct secretion, replacing these drugs with another without this side effect is recommended.
In addition, our results showed blunt breast trauma and non-iatrogenic massage were independent predisposing factors for GLM. The two factors meant the breasts were squeezed, rubbed, or injured by other external forces. Among various hypotheses on the etiology of GLM, inflammatory autoimmune reactions caused by duct and acinus injury are central.23 Sarkar demonstrated a robust relationship between the incidence of GLM and breast lesions.33 Local breast tissue, such as duct and acinus, would break after acute stimulation and induce an inflammatory response, eventually resulting in GLM.24,29 In cases of elevated secretion and tension in a duct, breast trauma may be the final trigger to the onset of GLM. Therefore, avoiding breast trauma would significantly reduce the risk of GLM.
Our results also showed a correlation between emotional abnormalities and GLM, including depression and anxiety. The level of inflammatory cytokines, such as TNF-α (Tumour necrosis factor-α) and IL-6 (interleukin 6), significantly correlate with negative emotions.34 The higher levels of pro-inflammatory cytokines in patients with emotional abnormalities could induce inflammatory reactions and promote the occurrence of GLM. Meanwhile, the inflammatory reaction in the pathogenesis of GLM leads to the increase of inflammatory cytokines, which can also induce emotional abnormalities. Besides, emotion can also affect the immune system through the neural-endocrine-immune network.35 Negative emotions, including depression and anxiety, could disturb the immune system and increase the risk of GLM to some extent.
We explored predisposing factors of GLM through the largest sample case-control study. Our results can guide some measures to prevent and treat GLM, such as nipple correction for nipple invagination patients and steroid support for immunologic-related GLM.
However, this study had some limitations. The conclusions of our study must be verified by further multicenter clinical trials. In addition, the mechanism of the predisposing factors of GLM is not fully understood. Besides the above predisposing factors, GLM may also be associated with Corynebacterium kroppenstedtii infection,36,37 smoking,38 α-1 antitrypsin deficiency,39 and many unknown risk factors. Corynebacterium infection and smoking were discussed in our questionnaire design. Since we could not obtain biopsy samples from the controls, it was impossible to compare Corynebacterium infection between the two groups. Although smoking has been reported in some articles, it was not associated with GLM in our preliminary research. More clinical studies are still needed. Furthermore, recall bias is not completely avoidable despite our best efforts to avoid it, such as recall bias related to lactational practices.
Conclusion
To the best of our knowledge, this is the largest sample size case-control study on the predisposing factors of GLM. We explored the risk factors of GLM through this study and identified six high-risk factors of GLM, including gestation, lactation disorder, non-iatrogenic massage, blunt trauma, nipple discharge, nipple invagination, and six relevant factors of the disease, including age, marriage, emotional abnormality, high prolactin, psychiatric drugs intake, and sex hormone intake. We believe the factors relevant to duct secretion are the main predisposing factors of GLM, such as gestation, lactation disorder, nipple discharge, nipple invagination, and high prolactin level. Hormones like estrogen and progesterone can exaggerate the process by promoting epithelial cell proliferation. Extrinsic factors like blunt trauma and non-iatrogenic massage may damage local breast lobular tissue and initiate an autoimmune system reaction. Emotional abnormalities can increase the risk of GLM by affecting immune function (Figure 5). We hope the predisposing factors identified in this study improves understanding of GLM occurrence, and offer a better clinical strategy for treating and preventing GLM, such as testing prolactin level, replacing the drugs that increase the secretion of mammary ducts, correcting nipple invagination, and maintaining good emotions. We can also take preventive measures such as avoiding exogenous breast injury and sex hormone intake to reduce the incidence rate of GLM.
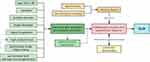 |
Figure 5 Diagram of GLM predisposing factors. |
Data Sharing Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
Ethics Statement
This study has been reviewed by the Ethics Committee of Beijing Hospital of Traditional Chinese Medicine, Capital Medical University (No: 2016BL -057-01).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Beijing Ten Million Talent Plan Project (no. 2019A31); Gansu Provincial Science and Technology Program Subsidized Projects (21JRIRG303); Research and Transformation of Clinical Diagnosis and Treatment Technology in Beijing (Z211100002921020); Young Doctor Scholar Project (2022).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Kessler E, Wolloch Y. Granulomatous mastitis: a lesion clinically simulating carcinoma. Am J Clin Pathol. 1972;58(6):642–646. doi:10.1093/ajcp/58.6.642
2. Pereira FA, Mudgil AV, Macias ES, Karsif K. Idiopathic granulomatous lobular mastitis. Int J Dermatol. 2012;51(2):142–151. doi:10.1111/j.1365-4632.2011.05168.x
3. Benson JR, Dumitru D. Idiopathic granulomatous mastitis: presentation, investigation and management. Future Oncol. 2016;12(11):1381–1394. doi:10.2217/fon-2015-0038
4. Schlesselman JJ. Case-Control Studies. Design, Conduct, Analysis. Oxford, UK: University Press; 1982.
5. Shi ZH, He Y. Statistics of Traditional Chinese Medicine and Software Application.
6. Ding H. Series of Breast Pathology Diagnosis and Differential Diagnosis. Beijing: People’s Health Publishing House; 2017.
7. Coombe RF, Hamed H. An update on granulomatous mastitis: a rare and complex condition. Br J Hosp Med. 2021;82(5):1–7. doi:10.12968/hmed.2020.0718
8. Yuan QQ, Xiao SY, Farouk O, et al. Management of granulomatous lobular mastitis: an international multidisciplinary consensus (2021 edition). Mil Med Res. 2022;9(1):20. doi:10.1186/s40779-022-00380-5
9. Altintoprak F, Kivilcim T, Ozkan OV. Aetiology of idiopathic granulomatous mastitis. World J Clin Cases. 2014;2(12):852–858. doi:10.12998/wjcc.v2.i12.852
10. Yuan Y, Zhang J, Li J. The clinicopathological characteristics and potential underlying causes of granulomatous lobular mastitis. Breast J. 2020;26(5):1099–1100. doi:10.1111/tbj.13669
11. Oltean HN, Soliman AS, Omar OS, et al. Risk factors for chronic mastitis in Morocco and Egypt. Int J Inflam. 2013;2013:184921. doi:10.1155/2013/184921
12. Huang Y, Wu H. A retrospective analysis of recurrence risk factors for granulomatous lobular mastitis in 130 patients: more attention should be paid to prolactin level. Ann Palliat Med. 2021;10(3):2824–2831. doi:10.21037/apm-20-1972
13. Tian C, Han X, Liu Z, Lv X, Ning P. Management of granulomatous lobular mastitis and risk factors associated with recurrence. World J Surg. 2022;46(11):2706–2714. doi:10.1007/s00268-022-06687-7
14. Mohammed S, Statz A, Lacross JS, et al. Granulomatous mastitis: a ten-year experience from a large inner city-county hospital. J Surg Res. 2013;184(1):299–303. doi:10.1016/j.jss.2013.06.047
15. Li J. Diagnosis and treatment of 75 patients with idiopathic lobular granulomatous mastitis. J Invest Surg. 2019;32(5):414–420. doi:10.1080/08941939.2018.1424270
16. Centers for Disease Control and Prevention (CDC). Idiopathic granulomatous mastitis in Hispanic women - Indiana, 2006–2008. MMWR Morb Mortal Wkly Rep. 2009;58(47):1317–1321.
17. Co M, Cheng VCC, Wei J, et al. Idiopathic granulomatous mastitis: a 10-year study from a multicentre clinical database. Pathology. 2018;50(7):742–747. doi:10.1016/j.pathol.2018.08.010
18. Baslaim MM, Khayat HA, Al-Amoudi SA. Idiopathic granulomatous mastitis: a heterogeneous disease with variable clinical presentation. World J Surg. 2007;31(8):1677–1681. doi:10.1007/s00268-007-9116-1
19. Lei X, Chen K, Zhu L, Song E, Su F, Li S. Treatments for idiopathic granulomatous mastitis: systematic review and meta‑analysis. Breastfeed Med. 2017;12(7):415–421. doi:10.1089/bfm.2017.0030
20. Akbulut S, Yilmaz D, Bakir S. Methotrexate in the management of idiopathic granulomatous mastitis: a review of 108 published cases and report of four cases. Breast J. 2011;17(6):661–668. doi:10.1111/j.1524-4741.2011.01162.x
21. Lin CH, Hsu CW, Tsao TY, Chou J. Idiopathic granulomatous mastitis associated with risperidone-induced hyperprolactinemia. Diagn Pathol. 2012;7:2. doi:10.1186/1746-1596-7-2
22. Nikolaev A, Blake CN, Carlson DL. Association between Hyperprolactinemia and Granulomatous Mastitis. Breast J. 2016;22(2):224–231. doi:10.1111/tbj.12552
23. Sheybani F, Sarvghad M, Naderi H, Gharib M. Treatment for and clinical characteristics of granulomatous mastitis. Obstet Gynecol. 2015;125(4):801–807. doi:10.1097/AOG.0000000000000734
24. Tasci HI, Turk E, Erinanc OH, Erkan S, Gundogdu R, Karagulle E. Factors affecting recurrence of idiopathic granulomatous mastitis. J Coll Physicians Surg Pak. 2022;32(2):161–165. doi:10.29271/jcpsp.2022.02.161
25. Wong SCY, Poon RWS, Chen JHK, et al. Corynebacterium kroppenstedtii is an emerging cause of mastitis especially in patients with psychiatric illness on antipsychotic medication. Open Forum Infect Dis. 2017;4(2):ofx096. doi:10.1093/ofid/ofx096
26. Konan A, Kalyoncu U, Dogan I, et al. Combined long-term steroid and immunosuppressive treatment regimen in granulomatous mastitis. Breast Care. 2012;7(4):297–301. doi:10.1159/000341388
27. Tollin SR. Use of the dopamine agonists bromocriptine and cabergoline in the management of risperidone-induced hyperprolactinemia in patients with psychotic disorders. J Endocrinol Invest. 2000;23(11):765–770. doi:10.1007/BF03345068
28. Bi J, Li Z, Lin X, et al. Etiology of granulomatous lobular mastitis based on metagenomic next-generation sequencing. Int J Infect Dis. 2021;113:243–250. doi:10.1016/j.ijid.2021.10.019
29. Cserni G, Szajki K. Granulomatous lobular mastitis following drug-induced galactorrhea and blunt trauma. Breast J. 1999;5(6):398–403.
30. Erhan Y, Veral A, Kara E, et al. A clinicopathologic study of a rare clinical entity mimicking breast carcinoma: idiopathic granulomatous mastitis. Breast. 2000;9(1):52–56. doi:10.1054/brst.1999.0072
31. Frozen F, Ersoy YE, Akaydin M, et al. Corticosteroid treatment and timing of surgery in idiopathic granulomatous mastitis confusing with breast carcinoma. Breast Cancer Res Treat. 2010;123(2):447–452. doi:10.1007/s10549-010-1041-6
32. Li J, McGregor HP. Idiopathic granulomatous mastitis associated with hyperprolactinemia: a nonoperative approach. Breast J. 2017;23(6):742–744. doi:10.1111/tbj.12914
33. Sarkar DK, Banerjee R, Gupta S, Singhal AK, Halder A. Management of idiopathic granulomatous mastitis: a prospective study. Ann R Coll Surg Engl. 2023;105(3):218–224. doi:10.1308/rcsann.2022.0017
34. Mihailova S, Ivanova-Genova E, Lukanov T, Stoyanova V, Milanova V, Naumova E. A study of TNF-α, TGF-β, IL-10, IL-6, and IFN-γ gene polymorphisms in patients with depression. J Neuroimmunol. 2016;293:123–128. doi:10.1016/j.jneuroim.2016.03.005
35. Wang YQ, Qiao JZ. The mechanism of cytokines influencing morbid emotional symptoms. Chin J Immunol. 2008;4:378–380+385.
36. Saydam M, Yilmaz KB, Sahin M, et al. New findings on autoimmune etiology of idiopathic granulomatous mastitis: serum IL-17, IL-22 and IL-23 levels of patients. J Invest Surg. 2021;34(9):993–997. doi:10.1080/08941939.2020.1725190
37. Stary CM, Lee YS, Balfour J. Idiopathic granulomatous mastitis associated with Corynebacterium sp. infection. Hawaii Med J. 2011;70(5):99–101.
38. Asoglu O, Ozmen V, Karanlik H, et al. Feasibility of surgical management in patients with granulomatous mastitis. Breast J. 2005;11(2):108–114. doi:10.1111/j.1075-122X.2005.21576.x
39. Zhou F, Yu LX, Ma ZB, Yu ZG. Granulomatous lobular mastitis. Chronic Dis Transl Med. 2016;2(1):17–21. doi:10.1016/j.cdtm.2016.02.004
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.