Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 12
Predictors of the Efficacy of Dipeptidyl Peptidase-4 Inhibitors in Taiwanese Patients with Type 2 Diabetes Mellitus
Received 21 June 2019
Accepted for publication 9 December 2019
Published 24 December 2019 Volume 2019:12 Pages 2725—2733
DOI https://doi.org/10.2147/DMSO.S220180
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Muthuswamy Balasubramanyam
Yi-Hsin Lin,1 Hsuan Huang2
1Division of Endocrinology and Metabolism, Department of Internal Medicine, Taiwan Adventist Hospital, Taipei, Taiwan (R.O.C.); 2Division of Pediatric Surgery, Department of Surgery, Mackay Memorial Hospital, Taipei, Taiwan (R.O.C.)
Correspondence: Hsuan Huang
Division of Pediatric Surgery, Department of Surgery, Mackay Memorial Hospital, Taipei, Taiwan (R.O.C.)
Email [email protected]
Background/purpose: Dipeptidyl peptidase-4 (DPP-4) inhibitors are the most popular oral antidiabetic drugs (OADs) in recent 20 years because of the low risk of hypoglycemia, intermediate efficacy to lower glycated hemoglobin (△HbA1c): 0.5–0.9%, neutral effect on body weight change, convenience for usage (mostly once daily), and rare occurrence of major side effects. The purpose of this study was to determine the important predictors of the efficacy of naïve use of DPP-4 inhibitors in Taiwanese patients with type 2 diabetes mellitus (T2D).
Methods: A retrospective observational study was conducted. Of the T2D patients, 193 (122 men) naïve DPP-4 inhibitor users with an age of 58.0 ± 12.6 years, disease duration 5.4 ± 4.7 years, body mass index (BMI) 26.1 ± 4.3 kg/m2, and estimated glomerular filtration rate 95.9 ± 27.0 mL/min/1.73M2 were assessed for △HbA1c in 6 months.
Results: After 6 months of DPP-4 inhibitors use, mostly second or third line of OADs (2.8 ± 0.7 kinds of OADs), 193 T2D patients (mean baseline HbA1c: 8.4 ± 1.4%) had △HbA1c 1.1 ± 1.2% on average (P < 0.01). The group with a higher baseline HbA1c level had more effective efficacy (△HbA1c ≥0.5%) in lowering HbA1c. Single regression analysis showed that the change in HbA1c after 6 months of treatment was positively associated with the baseline HbA1c level (R = 0.71, P < 0.001). In addition, multiple regression analysis showed that contributors to decrease HbA1c level after 6 months were high baseline HbA1c level, low BMI, short T2D duration, and fewer kinds of OADs.
Conclusion: Our study suggested that high baseline HbA1c level, low BMI, short T2D duration, and fewer kinds of OADs are the predictors of the efficacy of DPP-4 inhibitors in Taiwanese patients with T2D. The baseline HbA1c level, in particular, played the most important role in effective efficacy (△HbA1c ≥0.5%).
Keywords: dipeptidyl peptidase-4 inhibitor, type 2 diabetes, HbA1c, predictor of efficacy
Introduction
Dipeptidyl peptidase-4 (DPP-4) inhibitors are the most popular oral antidiabetic drugs (OADs) in recent 20 years.1 They mainly inhibit DPP-4 enzymes and elevate the concentrations of endogenous incretins, which increase insulin secretion and decrease glucagon secretion;2 therefore, they decrease plasma glucose levels. In clinical practice, DPP-4 inhibitors have a low risk of hypoglycemia, neutral effect on body weight change, and intermediate efficacy to lower glycated hemoglobin (△HbA1c: 0.5–0.9% on average) as second and third lines of OADs according to the previous clinical trials.3–6 Most importantly, they are convenient for usage (usually taken once daily) and have rare major adverse effects.7 However, DPP-4 inhibitors may non-selectively inhibit other member proteins of the DPP-4 gene family, including DPP-8, DPP-9, and fibroblast activation protein. The biological effects of the above inhibition remain unclear.8,9 The long-term safety of DPP-4 inhibitors, as well as their high cost and lack of definite cardiovascular benefits,10 is a serious concern, which all physicians face the dilemma of whether to continue the use of DPP-4 inhibitors especially when the efficacy is not as good as expected (△HbA1c <0.5%).
The purpose of the study was to find out the important predictors for the efficacy of the naïve use of DPP-4 inhibitors in Taiwanese patients with type 2 diabetes mellitus (T2D).
Research Design and Methods
A retrospective observational study was carried out from the principal investigator’s outpatient department in Taiwan Adventist Hospital between January 2013 and November 2016. The study enrolled T2D patients with a naïve prescription for DPP-4 inhibitors, despite taking other kinds of OADs (metformin, pioglitazone, acarbose, sulfonylurea, etc.) before. Patients who were on current insulin treatment were not included in order to simplify the variants. The patients were followed up at the clinic every 2–3 months. The glycemic control was monitored by blood HbA1c level before and after the prescription of DPP-4 inhibitors. The follow-up period was 6 months. During these 6 months, there was no dose adjustment for the other kinds of OADs or no additional new OAD prescriptions. △HbA1c for 6 months was calculated (△HbA1c = baseline HbA1c–post 6-month HbA1c). △HbA1c ≥0.5% was defined as effective and △HbA1c <0.5% as noneffective. The characteristics of patients (age, gender, body mass index [BMI], T2D duration, baseline HbA1c level, renal function, and the number of OADs used [including DPP-4 inhibitors]) were analyzed. Renal function was recorded as estimated glomerular filtration rate (eGFR) using the modification of diet in renal disease study equation.11
In order to simplify and minimize the variants, the patients were excluded if they were hospitalized or suffered from systemic diseases such as acute cardiac insults (acute heart failure and acute myocardial infarction), hyperglycemic crisis, acute kidney injury (serum creatinine level increased to two times higher or eGFR decreased to >50%), acute liver failure (serum glutamic pyruvic transaminase or serum glutamic-oxaloacetic transaminase increased to two to three times higher), cancer, sepsis, acute gastrointestinal bleeding with severe anemia, and major surgery during the 6-month period. We also excluded patients with intolerance to the side effects of DPP-4 inhibitor (headache, flu-like symptoms, etc.) and who have no regular clinic follow-up or blood test. This study was approved by the Institutional Review Board of Taiwan Adventist Hospital and conducted in accordance with the Declaration of Helsinki. Although the institutional review board specifically waived the need for patient consent due to the retrospective nature of the review, we confirmed that the data were anonymized and maintained with confidentiality during the study.
Statistical Analysis
Numeric values are presented as mean ± standard deviation and categorical values as n (%). Paired t-test was used to analyze the differences of clinical and laboratory characteristics between effective and noneffective patients, analysis of variance for numeric data, and Chi-square test for some categorical data. The degrees of association among independent variables for HbA1c level after 6 months, including age, gender, BMI, eGFR level, T2D duration, and the number of kinds of OADs, were assessed by multiple regression analyses. A P-value of <0.05 was considered statistically significant.
Results
Patient Disposition and Characteristics
There were 296 naïve DPP-4 inhibitor users with T2D initially enrolled in the study. We traced 6-month follow-up in chart records, and 103 patients were excluded (12 patients due to acute cardiac insults, 1 for cancer, 1 for sepsis, 2 for hyperglycemic crisis, 4 for acute kidney injury, 2 for acute liver failure, 2 for DPP-4 inhibitors side effect intolerance, and 79 for irregular clinic follow-up; Figure 1). Clinical and laboratory characteristics of 193 T2D patients were summarized (Table 1). Most of the 193 T2D patients (mean baseline HbA1c: 8.4 ± 1.4%) used DPP-4 inhibitors as a second or third line of OADs (2.8 ± 0.7 kinds of OADs), and the efficacy was △HbA1c: 1.1 ± 1.2% on average. Of these 193 patients, 71 (37%) were women and had older age than men (61.1 ± 11.5 vs 57.4 ± 13.0 years, P = 0.009). Other characteristics were similar. Over the 6-month DPP4-inhibitor treatment period, none of the 193 patients reported experiencing any side effects, such as severe hypoglycemia or pancreatitis.
 |
Table 1 Clinical and Laboratory Characteristics of 193 Patients with T2D |
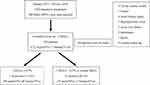 |
Figure 1 Study flow. Abbreviations: DPP4-i, dipeptidyl peptidase-4 inhibitors; T2D, type 2 diabetes mellitus; HbA1c, glycated hemoglobin. |
The Effective (△HbA1c ≥0.5%) Group Had a Significantly Higher Baseline HbA1c Level
Of these 193 patients, 138 (71.5%) were effective (△HbA1c ≥0.5%) with mean △HbA1c: 1.5 ± 1.1% compared with noneffective (△HbA1c <0.5%) group with mean △HbA1c: 0.0 ± 0.4% (P < 0.001; Table 2). The effective group had a significantly higher baseline HbA1c level in statistic. The age, BMI, T2D duration, eGFR level, and the number of kinds of OADs played no significant roles in effective efficacy (△HbA1c ≥0.5%).
A High Baseline HbA1c Level, Low BMI, Short T2D Duration, and Fewer Kinds of OADs are Predictors to Decrease HbA1c Levels After Treatment with DPP-4 Inhibitors
Stepwise multiple regression analysis was performed to elucidate independent determinants for decreased HbA1c level 6 months after the start of treatment. It was shown that the baseline HbA1c level was a positive contributor to change in HbA1c at 6 months after the start of treatment and that BMI, T2D duration, and the number of kinds of OADs were negative contributors to change (Table 3). Single regression analysis showed that the change in HbA1c after 6 months of treatment was positively associated with the baseline HbA1c level (R = 0.71, P < 0.001) and it was negatively associated with BMI (R = 0.43, P < 0.001), T2D duration (R = 0.23, P < 0.001), and kinds of OADs (R = 0.43, P < 0.001) (Figure 2). This implies that the decrease in HbA1c level is greater in patients with high baseline HbA1c level, low BMI, short T2D duration, and fewer kinds of OADs. Associations with age, gender, and eGFR level were statistically excluded.
 |
Figure 2 (Continued). |
Discussion
Nowadays, except for metformin, which is the first drug of choice, there is still lacking evidence on what kind of glucose-lowering agents need to be chosen as the second, third, and fourth lines of OADs. The challenge of a combination treatment for T2D is to find out the best balance between better glycemic responses and fewer side effects via appropriate individualized therapy. Knowing the phenotype of patients who will respond to a drug might be the most important information for drug selection. It has not been established which patients will benefit from the treatment of DPP-4 inhibitors, especially in Taiwan.
In this retrospective observational study, DPP-4 inhibitors were effective (△HbA1c ≥0.5%) in decreasing the HbA1c level after 6 months of treatment in 71.5% of Taiwanese patients with T2D. We pointed out that the contributors that decrease HbA1c levels after treatment with DPP-4 inhibitors were high baseline HbA1c level, low BMI, short T2D duration, and fewer kinds of OADs. High baseline HbA1c level was the most significant predictor for effective efficacy (△HbA1c ≥0.5%) of DPP-4 inhibitors in Taiwanese patients with T2D.
For a higher baseline HbA1c level with a greater reduction in HbA1c, our study focused on its role in effective efficacy (△HbA1c ≥0.5%) and provided Taiwanese patient-level data, consistent with previous studies of other countries, which were described by Nomiyama et al,12 Maeda et al,13 and Yagi et al14 Furthermore, Esposito et al reported the meta-regression of a 0.26% greater reduction in HbA1c with every increase of 1% of baseline HbA1c, a response to DPP-4 inhibitors.15 Little is known on this subject, but it is clear that a baseline HbA1c level is the most readily identifiable predictor of response to most antidiabetic drugs.16,17 The reasons why we suggest using DPP-4 inhibitors preferentially are its low risk of hypoglycemia, neutral effect on body weight change, rare occurrence of major side effects and recent evidence to improve long-term glycemic durability, consistent with “VERIFY” study with the strategy of an early combination treatment approach with DPP-4 inhibitors plus metformin.18 However, more consensus is needed to determine what a good response means, such as a great △HbA1c level or an HbA1c achievement <7%, which is a treatment target of the current guidelines.19,20 It is difficult to answer that reduction of HbA1c from 9% to 8% or from 7.5% to 6.8% is a better response. More comprehensive studies and analyses are required for the influence of a baseline HbA1c level in long-term glycemic control.21,22
Lower BMI predicted better efficacy of DPP-4 inhibitors in our study. It has been suggested that leaner individuals with T2D tend to have a greater defect in insulin secretion than obese ones, instead of insulin resistance. Thus, in patients with lower BMI, the insulin secretory stimulation by DPP-4 inhibitors may respond better. Kirino et al showed a high BMI correlation with an increased DPP-4 activity.23 The Danish ADDITION-PRO study reported that obese and overweight individuals had up to 20% reduction in plasma glucagon-like peptide-1 (pGLP-1) response to an oral glucose tolerance test compared with those with normal weight.24 pGLP-1 is an insulinotropic incretin produced by enteroendocrine L cells in the small intestine, and the pGLP-1 receptor system has been shown to affect central satiety process and feeding behavior.25,26 DPP-4 enzyme is in charge of rapid degradation of pGLP-1 and DPP-4 inhibitors, which primarily inhibit this pathway for the treatment of T2D.27,28 The studies of both Kirino and the ADDITION-PRO accounted for less efficacy of DPP-4 inhibitors in high BMI groups. In addition, a meta-analysis study reported by Cai et al showed greater glucose-lowering efficacy of DPP-4 inhibitors in Asian patients than in Caucasian patients.29 Plausible explanations for the observed differences may be a clear separation of baseline BMI (25.55 ± 1.44 kg/m2 in Asian studies and 31.13 ± 1.36 kg/m2 in Caucasian studies) and a meaningful increase of DPP-4 inhibitors to about 20–30% in lower BMI groups, which affected the pharmacokinetic concentrations and pharmacodynamic responses.30,31
It is a common consent that baseline HbA1c level is a predictor of response to DPP-4 inhibitors, but BMI had been rarely reported as a positive association.22 Yagi et al reported low baseline BMI as a predictor of efficacy in Japanese patients,14 the same as in our study in Taiwanese patients, which means that baseline BMI is a unique predictor for Asian patients treated with DPP-4 inhibitors.
The issue of early combination treatment in patients with short duration of type 2 diabetes compared with standard-of-care initial metformin monotherapy following by initial failure and sequential combination with other OADs requires careful consideration. Early synergistic combination treatment potentially enhances the clinic efficacy, which is well established from many studies and significantly increases glycemic durability, which is supported by the recent “VERIFY” study.18 This may be attributed to the complementary mechanism of combination treatment and relatively preserved β-cell function in patients with short duration of T2DM. Long-term T2D duration entails a progressive decline in β-cell function and failure in glycemic control due to the impaired capacity of insulin secretion by insulin secretagogues (sulfonylureas) and DPP4-inhibitors, which was described by Brath et al32 Moreover, while long duration of T2DM might develop hyperglycemic memory and epigenetic alterations, standard-of-care initial monotherapy following by initial failure and sequential combination might be introspected in the future. Our DPP-4 inhibitor study showed T2D duration as a negative contributor to the change in the HbA1c level. We also found fewer kinds of OADs as another negative contributor that decreases HbA1c level, which indicated that the early usage of DPP4-inhibitors benefits glycemic control, which is consistent with the previous recommendations of Del Prato et al.33
Our study had several limitations. This was a retrospective study with a small sample size and only 6 months follow-up period in our hospital. Some kinds of DPP4-inhibitors (sitagliptin and alogliptin) were not available in our hospital during the enrolling period. The study contained a patient selection bias. Also, we could not compare the different kinds of DPP-4 inhibitors and other OADs, including the use of insulin, as the characteristics of the patients. We excluded patients with coadministration of insulin because frequently adjusting the dosage of insulin was supposed to increase the possibility of bias. Further long-term and large randomized controlled trials are needed to investigate the clinical significance of these findings.
Conclusion
The study suggested that high baseline HbA1c level, low BMI, short T2D duration, and fewer kinds of OADs are the predictors of the efficacy of DPP-4 inhibitors in Taiwanese patients with T2D. Baseline HbA1c level played the most important role in effective efficacy (△HbA1c ≥0.5%).
Ethics Approval
The Taiwan Adventist Hospital Ethics Committee approved this study. The study was conducted in accordance with the Declaration of Helsinki. Although the institutional review board specifically waived the need for patient consent due to the retrospective nature of the review, we confirmed that the data were anonymized and maintained with confidentiality during the study.
Acknowledgments
The authors would like to thank all the people with T2DM who participated in this study, Department of Education and Research, Taiwan Adventist Hospital for support during data collection and Enago for revision of the English. The authors were also grateful to Prof. WS Yang (National Taiwan University Hospital) for his teaching and suggestion during this study.
Author Contributions
Both Yi-Hsin Lin and Hsuan Huang had substantial contributions to
1. Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data;
2. Drafting the article or revising it critically for important intellectual content;
3. Final approval of the version to be published; and
4. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
The authors declare that there are no conflicts of interest.
References
1. Turner LW, Nartey D, Stafford RS, Singh S, Alexander GC. Ambulatory treatment of type 2 diabetes in the U.S., 1997–2012. Diabetes Care. 2014;37:985–992. doi:10.2337/dc13-2097
2. McIntosh CH, Demuth HU, Pospisilik JA, Pederson R. Dipeptidyl peptidase IV inhibitors: how do they work as new antidiabetic agents? Regul Pept. 2005;128:159–165. doi:10.1016/j.regpep.2004.06.001
3. Mikhail N. Combination therapy with DPP-4 inhibitors and pioglitazone in type 2 diabetes: theoretical consideration and therapeutic potential. Vasc Health Risk Manag. 2008;4:1221–1227. doi:10.2147/VHRM
4. Wu D, Li L, Liu C. Efficacy and safety of dipeptidyl peptidase-4 inhibitors and metformin as initial combination therapy and as monotherapy in patients with type 2 diabetes mellitus: a meta-analysis. Diabetes Obes Metab. 2014;16:30–37. doi:10.1111/dom.2014.16.issue-1
5. Wang F, He Y, Zhang R, Zeng Q, Zhao X. Combination therapy of metformin plus dipeptidyl peptidase-4 inhibitor versus metformin plus sulfonylurea and their association with a decreased risk of cardiovascular disease in type 2 diabetes mellitus patients. Medicine (Baltimore). 2017;96:e7638. doi:10.1097/MD.0000000000007638
6. White WB, Heller SR, Cannon CP, Howitt H, Khunti K, Bergenstal RM; EXAMINE Investigators. Alogliptin in patients with type 2 diabetes receiving metformin and sulfonylurea therapies in the EXAMINE trial. Am J Med. 2018;131:813–819. doi:10.1016/j.amjmed.2018.02.023
7. Deacon CF. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes Metab. 2011;13:7–18. doi:10.1111/dom.2011.13.issue-1
8. Kirby M, Yu DM, O’Connor S, Gorrell MD. Inhibitor selectivity in the clinical application of dipeptidyl peptidase-4 inhibition. Clin Sci (Lond). 2009;118:31–41. doi:10.1042/CS20090047
9. Han R, Wang X, Bachovchin W, Zukowska Z, Osborn JW. Inhibition of dipeptidyl peptidase 8/9 impairs preadipocyte differentiation. Sci Rep. 2015;5:12348. doi:10.1038/srep12348
10. Wu S, Hopper I, Skiba M, Krum H. Dipeptidyl peptidase-4 inhibitors and cardiovascular outcomes: meta-analysis of randomized clinical trials with 55,141 participants. Cardiovasc Ther. 2014;32:147–158. doi:10.1111/cdr.2014.32.issue-4
11. Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi:10.7326/0003-4819-145-4-200608150-00004
12. Nomiyama T, Akehi Y, Takenoshita H, et al. Contributing factors related to efficacy of the dipeptidyl peptidase-4 inhibitor sitagliptin in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2012;95:e27–8. doi:10.1016/j.diabres.2011.08.016
13. Maeda H, Kubota A, Tanaka Y, Terauchi Y, Matsuba I, ASSET-K study group. The safety, efficacy and predictors for HbA1c reduction of sitagliptin in the treatment of Japanese type 2 diabetes. Diabetes Res Clin Pract. 2012;95:e20–2. doi:10.1016/j.diabres.2011.10.011
14. Yagi S, Aihara K, Akaike M, et al. Predictive factors for efficacy of dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes mellitus. Diabetes Metab J. 2015;39:342–347. doi:10.4093/dmj.2015.39.4.342
15. Esposito K, Chiodini P, Capuano A, Maiorino MI, Bellastella G, Giugliano D. Baseline glycemic parameters predict the hemoglobin A1c response to DPP-4 inhibitors: meta-regression analysis of 78 randomized controlled trials with 20,053 patients. Endocrine. 2014;46:43–51. doi:10.1007/s12020-013-0090-0
16. Bloomgarden ZT, Dodis R, Viscoli CM, Holmboe ES, Inzucchi SE. Lower baseline glycemia reduces apparent oral agent glucose-lowering efficacy: a meta-regression analysis. Diabetes Care. 2006;29:2137–2139. doi:10.2337/dc06-1120
17. Esposito K, Chiodini P, Bellastella G, Maiorino MI, Giugliano D. Proportion of patients at HbA1c target <7% with eight classes of antidiabetic drugs in type 2 diabetes: systematic review of 218 randomized controlled trials with 78945 patients. Diabetes Obes Metab. 2012;14:228–233. doi:10.1111/j.1463-1326.2011.01512.x
18. Matthews DR, Paldánius PM, Proot P, Chiang Y, Stumvoll M, Del Prato S. VERIFY study group. Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): a 5-year, multicentre, randomised, double-blind trial. Lancet. 2019;394:1519–1529. doi:10.1016/S0140-6736(19)32131-2
19. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the american diabetes association and the european association for the study of diabetes. Diabetes Care. 2015;38:140–149. doi:10.2337/dc14-2441
20. American Diabetes Association. Standards of medical care in diabetes; 2018. Available from: https://professional.diabetes.org/slidelibrary/standards-medical-care-diabetes-2018.
21. Shaw JE, Bloomgarden ZT. Individualizing therapy: do we have the tools to do it? J Diabetes. 2015;7:1–2. doi:10.1111/1753-0407.12218
22. Bihan H, Ng WL, Magliano DJ, Shaw JE. Predictors of efficacy of GLP-1 agonists and DPP-4 inhibitors: a systematic review. Diabetes Res Clin Pract. 2016;121:27–34. doi:10.1016/j.diabres.2016.08.011
23. Kirino Y, Sei M, Kawazoe K, Minakuchi K, Sato Y. Plasma dipeptidyl peptidase 4 activity correlates with body mass index and the plasma adiponectin concentration in healthy young people. Endocr J. 2012;59:949–953. doi:10.1507/endocrj.EJ12-0158
24. Færch K, Torekov SS, Vistisen D, et al. GLP-1 response to oral glucose is reduced in prediabetes, screen-detected type 2 diabetes, and obesity and influenced by sex: the ADDITION-PRO study. Diabetes. 2015;64:2513–2525. doi:10.2337/db14-1751
25. Turton MD, O’Shea D, Gunn I, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi:10.1038/379069a0
26. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi:10.1053/j.gastro.2007.03.054
27. Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab. 1995;80:952–957. doi:10.1210/jcem.80.3.7883856
28. Mortensen K, Christensen LL, Holst JJ, Orskov C. GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul Pept. 2003;114:189–196. doi:10.1016/S0167-0115(03)00125-3
29. Cai X, Han X, Luo Y, Ji L. Efficacy of dipeptidyl-peptidase-4 inhibitors and impact on β-cell function in Asian and Caucasian type 2 diabetes mellitus patients: A meta-analysis. J Diabetes. 2015;7:347–359. doi:10.1111/1753-0407.12196
30. Graefe-Mody U, Retlich S, Friedrich C. Clinical pharmacokinetics and pharmacodynamics of linagliptin. Clin Pharmacokinet. 2012;51:411–427. doi:10.2165/11630900-000000000-00000
31. Hu P, Yin Q, Deckert F, et al. Pharmacokinetics and pharmacodynamics of vildagliptin in healthy Chinese volunteers. J Clin Pharmacol. 2009;49:39–49. doi:10.1177/0091270008325152
32. Brath H, Paldánius PM, Bader G, Mathieu C. Relationship between duration of type 2 diabetes and effectiveness of DPP-4 inhibitor versus sulfonylurea as add-on therapy: a post hoc analysis. Diabetes Ther. 2017;8:829–836. doi:10.1007/s13300-017-0276-1
33. Del Prato S, Felton AM, Munro N, Nesto R, Zimmet P, Zinman B. Global partnership for effective diabetes management. Improving glucose management: ten steps to get more patients with type 2 diabetes to glycaemic goal. Int J Clin Pract. 2005;59:1345–1355. doi:10.1111/j.1742-1241.2005.00674.x
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



