Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Predictors of Success of Inpatient Pulmonary Rehabilitation Program in COPD Patients
Authors Hafner T , Pirc Marolt T, Šelb J, Grošelj A, Kosten T, Simonič A, Košnik M , Korošec P
Received 26 June 2023
Accepted for publication 17 October 2023
Published 8 November 2023 Volume 2023:18 Pages 2483—2495
DOI https://doi.org/10.2147/COPD.S425087
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Tomaž Hafner,1,2 Tinkara Pirc Marolt,1 Julij Šelb,1,2 Anja Grošelj,1 Tatjana Kosten,1 Anja Simonič,1 Mitja Košnik,1,2 Peter Korošec1,3
1University Clinic of Respiratory and Allergic Diseases Golnik, Golnik, Slovenia; 2Medical Faculty, University of Ljubljana, Ljubljana, Slovenia; 3Faculty of Pharmacy, University of Ljubljana, Ljubljana, Slovenia
Correspondence: Tomaž Hafner, UKPA Golnik, Golnik 36, 4204 Golnik, Slovenia, Tel +386 4 2569100, Fax +386 5 2569117, Email [email protected]
Purpose: Pulmonary rehabilitation programs (PR) are an important part of the comprehensive treatment of patients with chronic pulmonary diseases. Patients respond individually to PR. The aim of this study is to identify potential predictors of success of PR to recognise patients who benefit most and to uncover possible reasons for poor response to PR.
Patients and Methods: We included 121 patients with chronic obstructive pulmonary disease (COPD) who completed our 4-week inpatient PR without any exacerbations of disease during PR that could potentially affect PR outcomes. Improvement in distance of ≥ 30 m on the 6-minute walk test (6MWT) after PR was chosen as a primary marker of physical success. Ninety-one patients achieved improvement of ≥ 30 m on the 6MWT and were thus considered good responders, and 30 patients were poor responders with improvement in the distance of < 30 m on the 6MWT.
Results: We compared baseline clinical characteristics, medication, lung function, physical capacity, body composition, and laboratory blood tests between groups of good and poor responders. The most prominent differences between groups were associated with differences in baseline body composition and erythrocyte-related parameters. Good responders had significantly lower body water content (p = 0.042) and higher body weight (p = 0.036), body fat content (p = 0.049), dry lean mass (p = 0.021), haemoglobin levels (p = 0.040), erythrocyte count (p = 0.017), haematocrit (p = 0.030) and iron level (p = 0.028).
Conclusion: A more muscular body composition and a higher ability to transport oxygen from the blood to the muscles could be beneficial for the outcome of PR.
Plain Language Summary: Pulmonary rehabilitation programs (PR) are important part of management of patients with chronic obstructive pulmonary diseases and other chronic pulmonary diseases. Nevertheless, PR are sparsely available to patients, and patients respond to PR individually. Our study will help identify patients who benefit most from PR and find possible reasons why the physical condition of some patients does not improve with PR. Only patients with chronic obstructive pulmonary disease who completed inpatient pulmonary rehabilitation program without any exacerbations of disease that could potentially affect PR outcomes were included in this study to determine what baseline patient characteristics could predict good and poor responders to PR. The results of our study suggest that a more muscular body composition and a higher ability to transport oxygen from the blood to the muscles could be beneficial to the outcome of PR. We suggest that before sarcopenic or anaemic patients are referred for PR, special care should first be taken to address and remedy their condition to maximise their physical gain in PR.
Keywords: pulmonary rehabilitation, COPD, predictors, responders
Introduction
Pulmonary rehabilitation programs (PR) are an important part of the comprehensive treatment of patients with chronic obstructive pulmonary disease (COPD). It is a multidisciplinary program involving interventions from doctors, physical therapists, nurses, dietitians, social workers, psychologists, and occupational therapists. PR also yielded favourable effects in other chronic pulmonary diseases, such as interstitial lung diseases, asthma and bronchiectasis, by ameliorating symptoms, increasing physical performance, reducing exacerbations and medical costs, and thereby improving patient quality of life.1–3 However, the response to PR varies individually, as only some of the expected results and even no improvement at all have been observed in the so-called “nonresponders” or “poor responders”. The exact reasons for such variation are poorly understood.4–6
Although several predictors of success of PR have been identified in studies, their application and comparison are often limited because of differences in rehabilitation programs, enrolled patients, duration of programs, and markers of success. In a study by Troosters and coworkers, ventilatory reserve, inspiratory muscle strength and peripheral muscle strength were found to be significant predictors of training success.7 Using multidimensional response profiling, Spruit and coworkers showed that patients in the “very good responder” cluster had a higher number of dyspnoea symptoms, a higher number of hospitalisations in the past 12 months, poorer physical performance, poorer performance and satisfaction scores for problematic activities of daily life, more symptoms of anxiety and depression, poorer health status, and a higher proportion of patients following an inpatient PR program compared with patients in the other three clusters.5 Garrod and coworkers found that patients baseline characteristics were poor predictors of response to PR, and less improvement was detected only in patients with a high Medical Research Council dyspnoea scale (MRC) grade 5.4 Jones and coworkers also showed that sarcopenia did not affect the response to PR.8 Walsh and coworkers recognised lower baseline quadriceps strength as an independent predictor of response to PR, whereas baseline physical activity or dyspnoea grade could not identify responders.9 Furthermore, Tunsupon and coworkers found that physical capacity improved in patients regardless of body composition.10 Moreover, Barberan-Garcia and coworkers reported a negative association between nonanaemic iron deficiency and aerobic capacity before and after endurance training in COPD patients.11
Since PR is sparsely available to patients, the objective of this study is to identify potential clinical, physiological, or biochemical markers associated with the success of PR. Our study will help identify patients who benefit most from PR and possible reasons why the condition of some patients does not improve with PR. One of the most commonly used markers of success in PR, improvement in the 6-minute walk test (6MWT),12 was chosen as the primary marker of success in this study.
Subjects and Methods
Subjects
We collected data from 192 consecutive patients with chronic pulmonary diseases who enrolled in our inpatient PR program from May 2017 to August 2021. Only COPD patients who completed PR without an exacerbation of the disease during PR that could affect their progress were included in the final analysis (Figure 1). Inclusion criteria include all consecutive patients with COPD who were identified as PR candidates and enrolled in our PR program. All patients with other chronic pulmonary diseases without concomitant COPD, patients with COPD who ended the PR program prematurely or who completed the PR program but had exacerbation of disease during the PR due to which the PR program had to be stopped or modified were excluded from the study.
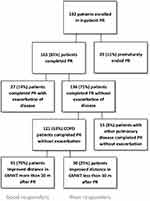 |
Figure 1 Patient inclusion flow chart. |
Methods
We used the improvement in distance walked on the 6MWT to identify baseline clinical, physiological, or biochemical parameters potentially associated with a gain in physical performance during PR. An increase in distance of ≥30 m on the 6MWT is an appropriate marker of success according to the guidelines and other similar studies.13–16 Patients who improved their distance on the 6MWT by ≥30 m after PR were identified as good responders, others were identified as poor responders. Data were collected and analysed retrospectively. All patients signed an informed consent form and agreed to anonymous analysis of their data for study purposes. The study was approved by The National Medical Ethics Committee of the Republic of Slovenia, Ministry of Health of the Republic of Slovenia (approval no. 0120–284/2020/6). Our study fully complies with the Declaration of Helsinki.
We performed our standard 4-week inpatient PR at the University Clinic of Respiratory and Allergic Diseases Golnik. Before and after the PR, the COPD assessment test (CAT), MRC, St. George’s respiratory questionnaire (SGRQ), 6MWT, incremental shuttle walk test (ISWT), endurance shuttle walk test (ESWT), cycle endurance test (CET), cycloergospirometry, maximal inspiratory pressure (MIP), maximal expiratory pressure (MEP), dynamometry, and assessment of body composition were performed. Laboratory tests and respiratory function tests were performed only before PR.
Our multidisciplinary team includes a physical therapist, doctor, nurse, clinical dietitian, clinical psychologist and social worker. We assessed the physical status of the patients, and the doctor prescribed an appropriate training level according to the initial test before PR. During PR, all physical exercises were performed under the supervision of physical therapists. The program was balanced and included strength and endurance exercises on bicycle, treadmill, and leg press, each alternating 2–3 times per week, muscle electrostimulation twice daily, and daily breathing exercises, balance exercises, stair walking and relaxation exercises. Before PR, a clinical psychologist performed individual talks with patients. Thereafter, patients had group sessions twice a week and individual sessions as needed. The social worker performed individual interviews to identify possible social problems and provided social help as needed. The nurse educated patients about their disease and checked their inhalation therapy techniques. The clinical dietitian measured weight and height to calculate body mass index (BMI). Body composition was determined by bioelectrical impedance (BIA) using Bodystat QuadSCAN 4000 (Bodystat Ltd. Isle of Man, UK).17 Those patients who had lost weight in the past or whose FFMI was below 17 kg/m2 were prescribed nutritional supplements during and after PR. Laboratory data were obtained in our accredited laboratory using standard methods and validated tests.
Statistical Analysis
Data were collected and analysed using Microsoft Excel 2016 (Microsoft Corporation, Washington, USA), GraphPad Prism for Windows 9.4.1.681 (GraphPad Software, California, USA), and R Statistical Software 2020 (R Foundation for Statistical Computing, Vienna, Austria). Normality was assessed using the Shapiro‒Wilk test. Paired data were compared using the paired-t/Wilcoxon signed-rank test as appropriate. Differences between samples were evaluated using the t/Mann–Whitney U-test. A p value of ≤.05 was considered statistically significant. Data in the tables are presented as the mean ± standard deviation (SD) or frequencies and percentages. To account for interrelations between the observed variables that differed significantly between the good and the poor responders, the variables were used in multiple logistic regression model. We also performed backward and forward selection of predictors using full and null models, respectively (R Foundation for Statistical Computing, Vienna, Austria).
Results
Good and Poor Responders Based on 6MWT
Our results showed a beneficial effect of PR in COPD patients based on the 6MWT. Before PR, the distance walked on the 6MWT (342.9 ± 108.8 m; min 110 m, max 600 m) was significantly less (p ≤.0001) than the distance walked after PR (400.1 ± 106 m; min 160 m, max 635 m). Patients improved their distance by 57.2 ± 54.8 m (min −127 m, max 240 m). Overall, 91 patients improved their distance by 30 m or more (good responders), and 30 patients improved their distance by less than 30 m (poor responders). In the good responder group, patients walked a distance of 334.3 ± 112 m (min 110 m, max 600 m) before PR and a distance of 411.9 ± 104 m (min 160 m, max 635 m) after PR on the 6MWT. The distance walked on the 6MWT in the poor responder group was 368.8 ± 95.5 m (min 182 m, max 555 m) before PR and 364.2 ± 105.6 m (min 200 m, max 570 m) after PR. In contrast to the difference walked after PR (p = 0.045), there was no statistically significant difference in the distance walked on the 6MWT between groups before PR (p = 0.133).
Basic Clinical Characteristics and Therapy
A total of 163 (85%) of the 192 patients with chronic pulmonary diseases completed PR, and 121 (63%) patients with COPD completed PR without exacerbations of COPD that could potentially affect the outcome of rehabilitation. Therefore, only these 121 patients were included in the study (Figure 1): 79 (65%) men with a mean age of 65.9 ± 7.1 years and 42 (35%) women with a mean age of 63.9 ± 7.6 years. Twenty-three (19%) patients received long-term oxygen therapy (LTOT), and 43 (36%) patients were prescribed nutritional supplements during rehabilitation. Each patient smoked at least once in the lifetime; 96 (79%) patients were ex-smokers, and 25 (21%) were current/active smokers who smoked 42.9 ± 26.5 packs/year (min 1.25, max 150).
Besides COPD, 93 (77%) patients had at least one other diagnosis. The most common comorbidities included decreased bone mineral density – osteopenia, diagnosed in 42 (35%) patients, and osteoporosis in 54 (45%) patients. Moreover, 53 (44%) patients had arterial hypertension, and 15 (12%) suffered from diabetes. Psychological examination revealed anxiety in 11 (9%) patients, depression in 15 (12%), and combined anxiety and depression disorder in 33 (27%) patients. In addition, 26 (21%) patients lived alone, while others lived with family members or partners.
There were no statistically significant differences between the good responder group and poor responder group in the abovementioned basic clinical parameters as detailed in Table 1.
 |
Table 1 Clinical Characteristics of Patients |
During PR, patients received ongoing medical therapy as prescribed by their pulmonologist. Bronchodilators were prescribed to 118 (97%) patients, either long-acting muscarinic antagonists (LAMA) (110 patients; 91%) or beta-2 agonists (118 patients; 97%). Fourteen (12%) patients received dual bronchodilator therapy, 102 (84%) patients received inhaled corticosteroid, and 95 (78%) patients received triple inhaled therapy including inhaled steroids. Thirty-eight (31%) patients received theophylline and 13 (11%) patients received other pulmonary therapy in addition to inhaled therapy (7 (6%) azitromicin, 2 (2%) roflumilast, 1 (1%) mucolytic, 1 (1%) nintedanib, 1 (1%) omalizumab, 1 (1%) montelukast, 2 (2%) metilprednisolone). Combined inhaled therapy was as follows: 4 (3%) patients used only a bronchodilator or LAMA, 14 (12%) used a beta-2 agonist bronchodilator and LAMA, 7 (6%) used an inhaled steroid and a beta-2 agonist bronchodilator, and 95 (78%) patients received triple inhaled therapy with an inhaled steroid, a beta-2-adrenergic agonist, and LAMA. Prescribed medical therapy did not affect the response to PR between groups, either by individual drug class or by combination of inhaled therapy. Detailed data can be found in Table 2.
 |
Table 2 Medical Therapy of Patients Before/During PR |
Physiological Characteristics
Pulmonary Function
In terms of patients baseline pulmonary function, the groups of good responders and poor responders differed significantly only in absolute vital capacity (VC) (3230 ± 970 mL and 2837 ± 855 mL, p = 0.048), while VC expressed as % of predicted did not show statistical significance (86 ± 22% and 81 ± 17%, p = 0.269). Other parameters of lung function also did not reach statistical significance, despite the trend towards reduced lung function in the poor responder group, as shown in Table 3.
 |
Table 3 Physiological Characteristics of Patients Evaluated Before PR |
Physical Performance
A significant improvement in physical performance during PR was seen in both groups. Nevertheless, in the group of good responders, patients improved in all measured parameters of physical performance, while in the group of poor responders, patients improved only in some parameters, and the improvement was less obvious.
Before PR, no significant differences were detected between groups of good and poor responders, but after PR, the good responders had significantly better results in ESWT (327.5 ± 157.3 s and 304.8 ± 123.6 s, p = 0.013) and exhibited more power in cycloergometry (67.9 ± 26.8 W and 51.5 ± 16.9 W, p = 0.007) as demonstrated in Table 3 and Table 4. Patients’ overall physical performance improved during PR, as presented in Table 5 further demonstrating the beneficial effect of PR in COPD patients.
 |
Table 4 Physiological Characteristics of Patients Evaluated After PR |
 |
Table 5 Absolute Effects (Changes/Gains in Tests Before and After) of PR in COPD Patients |
Body Composition
The overall body composition of the patients did not change significantly during PR. However, we found some differences between the good responder and poor responder groups in body composition before PR. Good responders had higher body weight (77.4 ± 19.2 kg and 69.5 ± 15.3 kg, p = 0.036), reduced content of water (51 ± 6% and 54 ± 8%, p = 0.042), higher fat content (26.6 ± 9.0 kg and 23.4 ± 8.3 kg, p = 0.049) and higher dry lean mass (11.7 ± 5.2 kg and 9.3 ± 4.3 kg, p = 0.021). Even more differences between groups were observed after PR. In addition to the changes in the abovementioned parameters, patients in the good responder group also had higher BMI (27.2 ± 5.6 kg/m2 and 24.8 ± 5.1 kg/m2, p = 0.025), FFMI (17.8 ± 3.5 kg/m2 and 16.4 ± 3.2 kg/m2, p =0.040) and lean mass (51.4 ± 13.5 kg and 45.2 ± 10.1 kg, p = 0.027) after PR. All data are presented in detail in Tables 3–5.
Laboratory Blood Tests
Detailed data on laboratory blood tests before PR can be found in Table 6. Electrolytes, renal and liver function tests, CRP, NTproBNP, and HbA1c did not differ statistically between the good and poor responder groups. Statistically significant changes were observed only in erythrocyte-related parameters, including increased iron serum concentration (19.65 ± 7.67 µmol/L and 16.79 ± 6.45 µmol/L, p = 0.028), higher number of erythrocytes (4.68 ± 0.47 × 1012/L and 4.48 ± 0.45 × 1012/L, p = 0.017), higher haemoglobin concentration (145.6 ± 13.7 g/L and 139.7 ± 11.6 g/L, p = 0.040) and higher haematocrit level (0.43 ± 0.03 L/L and 0.41 ± 0.03 L/L, p = 0.030) in the group of good responders.
 |
Table 6 Laboratory Tests Measured Before PR |
Despite statistical significance, the differences were not clinically important. There were only 3 patients with haemoglobin levels below normal (male 130 g/l, female 120 g/L) in the poor responder group (min 114 g/L, max 123 g/L) and 5 patients (min 99 g/L, max 127 g/L) in the good responder group. The number of erythrocytes (male < 4.5 × 1012/L, female < 3.8 × 1012/L) was diminished in seven patients in the poor responder group and in 17 patients in the good responder group. The haematocrit level was below normal (male < 0.40 L/L, female < 0.36 L/L) in 5 patients in the poor responder group and 10 patients in the good responder group. Low serum iron was detected only in one patient (male/female < 5.8 μmol/L) in the good responder group, who was also the only patient with a decrease in all four blood parameters that were statistically significantly reduced. In the poor responder group, 3 parameters (erythrocytes, haemoglobin and haematocrit) were below normal values in 3 patients compared with 5 patients in the good responder group.
Interrelations Between the Observed Variables
To account for interrelations between the observed variables that differed significantly between the good and the poor responders, the variables were used in a multiple logistic regression model. In the full model, none of the modelled variables was significant in predicting good responder status. The results can be seen in Table 7. We therefore performed backward and forward selection of predictors using full and null models, respectively. The results of best fit of the backward model demonstrated the importance of body fat with p=0.051, iron p=0.054 and erythrocytes with p=0.040 and forward model demonstrated the importance of iron with p = 0.084 and dry lean mass p = 0.047 (Table 8 and Table 9).
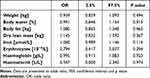 |
Table 7 Multiple Logistic Regression Full Model |
 |
Table 8 Multiple Logistic Regression Backward Selection Model |
 |
Table 9 Multiple Logistic Regression Forward Selection Model |
Discussion
The strength of our study is the recruitment of consecutive real-life COPD patients who were clinically and not for study purposes identified as candidates for PR and who completed the program without any exacerbation of their disease during PR that could affect the course and outcomes of rehabilitation.
As previously found in other studies, basic clinical characteristics and comorbidities did not influence the success of PR.4,5,7,18,19 In contrast, Crisafulli and coworkers20 reported that osteoporosis was independently associated with worse rehabilitation outcomes, but our study did not confirm this finding. Nevertheless, caution should be taken when comparing the studies because of differences in pulmonary rehabilitation time, place of PR (inpatient/outpatient) and criteria for successful completion of PR.
Our study confirmed that lung function determined before PR was not a good predictor of success, as has been shown in other studies.4,6,14 However, there was a trend revealing a possible association between better VC and improved 6MWT, but statistical significance was observed only in VC expressed in absolute values. Sahin and coworkers21 demonstrated that COPD patients with severe diffusion defects in diffusing capacity for carbon monoxide (DLCO) experienced a better pulmonary rehabilitation outcome in terms of improvement in dyspnoea level, but there was no significant difference between groups in terms of 6MWT as in our study.
Baseline physical performance did not differ between groups of good and poor responders. Similar results have been shown by others, although some studies revealed a correlation when baseline physical performance was combined with factors including dyspnoea and health status.5,18
Body composition evaluated before PR noticeably affected the outcome of PR. Statistically significant differences between good and poor responders were found in body weight, water and fat content, and dry lean mass. BMI showed only a tendency towards statistical significance (p = 0.067), although heavier patients with more fat had greater chances of improving their physical condition after PR. In accordance, most studies found that BMI or obesity had no effect on PR outcomes.8,10,14,22
Since dry lean mass is the total body mass without water and fat, it represents mainly proteins and minerals. Taking into account the high prevalence of osteopenia and osteoporosis in our COPD patients, who consequently have reduced mineral content, dry lean mass is a good indicator of muscle mass in the body. Therefore, it is possible that patients with lower muscle mass respond poorly to PR. However, Jones and coworkers8 reported that sarcopenia defined by the EWGSOP did not affect the response to PR. Moreover, Tunsupon and coworkers10 showed that muscle depletion or obesity had no effect on the percentage of patients achieving the MCID as a measure of quality of life and physical tolerance after PR. In contrast to our study, a distance of 26 m on the 6MWT was used as the threshold to divide patients into groups, and only BMI, FFM and FFMI were analysed.
We speculate that the higher percentage of body water in the group of poor responders could be the result of water retention as a consequence of congestive heart failure, since there is also a trend towards increased NT-proBNP in poor responders (p = 0.056). However, we did not detect important decompensation of congestive heart failure in any patient during PR. Body fat and dry lean mass were also significant predictors in multiple logistic regression analysis.
Scores on the MRC, CAT, and SQRQ questionnaires acquired before PR were not significantly different between the groups of good and poor responders and were not good predictors of success as also found in other studies.5,14 A similar questionnaire to MRC, mMRC, in combination with baseline physical performance based on the 6MWT, yielded success in predicting clinically meaningful changes after PR.18 Moreover, Garrod and coworkers4 demonstrated that patients with MRC grade 5 correlate with less improvement than patients with less severe MRC score grades.
Regarding laboratory tests, the most striking differences between good and poor responders were erythrocyte-related. Anaemia is common in COPD patients.23–25 There is evidence that iron deficiency affects physical activity in COPD patients.26,27 Furthermore, nonanaemic iron deficiency has been associated with poorer physical performance and response to training.11 Our data confirmed that a reduction in erythrocyte-related factors, including the number of erythrocytes, haematocrit, haemoglobin and iron, might be associated with an unsuccessful physical response. Notably, erythrocytes and iron were also significant predictors in multiple logistic regression analysis.
There are several limitations of our study. First, a relatively small number of patients were included in the study due to overall limited number of patients that we can include in our PR and further limitation to COPD patients who concluded PR without exacerbation of the disease. Also, greatly reduced access to PR because of COVID-19 hospital reorganisations in past years contributed in part to reduced cohort size. Furthermore, we could not ignore the fact that in our country only inpatient PR is available and it lasts only 4 weeks, which is shorter than is common in other countries. Nevertheless, even with this shorter program, we showed that most patients improved their physical condition. Another limitation relevant to interpretation is that we selected only one parameter to identify patients with good response in physical gain. However, we believe that the difference in distance gained in 6MWT after PR is a good overall marker of patients’ physical improvement.
Conclusion
Our study revealed that baseline physical status, dyspnoea level, lung function, comorbidities, social status, and smoking status were not good predictors of improvement in physical performance after PR based on the 6MWT in COPD patients. We found that COPD patients with higher body weight, more body fat—but not obese (did not have higher body fat %), higher dry lean mass, higher haemoglobin levels, more erythrocytes, higher haematocrit and higher iron level may benefit more than others. We can conclude that more muscular body composition and a higher ability to transport oxygen from the blood to the muscles may be associated with better physical improvement during PR in COPD patients as measured with the 6MWT. Our results should be confirmed in larger studies and with other PR settings (place of rehabilitation, duration of rehabilitation, etc.). Nevertheless, we suggest that before sarcopenic or anaemic patients are referred to PR, special care should first be taken to address and remedy their condition to maximise their physical gain in PR.
Abbreviations
1-min STS, 1 minute sit-to-stand test; 6MWT, 6-minute walk test; ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; BFMI, body fat mass index; BMI, body mass index; CAT, COPD assessment test; CET, cycle endurance test; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; DLCO, diffusing capacity for carbon monoxide; eGF, estimated glomerular filtration; ESWT, endurance shuttle walk test; FEV1, forced expiratory volume in 1 second; FFMI, fat-free mass index; gammaGT, gamma-glutamyl transferase; HbA1c, A1c glycosylated haemoglobin; HDL, high-density lipoprotein cholesterol; ISWT, incremental shuttle walk test; LAMA, long-acting muscarinic antagonist; LDL, low-density lipoprotein cholesterol; LTOT, long-term oxygen treatment; MCH, mean cell haemoglobin; MCHC, mean cell haemoglobin concentration; MCID, minimal clinically important difference; MCV, mean cell volume; MEP, maximal expiratory pressure; MIP, maximal inspiratory pressure; MPV, mean platelet volume; MRC, medical research council dyspnoea scale; NTproBNP, N-terminal pro-Brain natriuretic peptide; PR, pulmonary rehabilitation program; RDW, red cell distribution width; SGRQ, St. George’s Respiratory Questionnaire; TIBC, total iron-binding capacity; UIBC, unsaturated iron-binding capacity; VC, vital capacity.
Data Sharing Statement
Original study data supporting the results can be found at corresponding author on request and are available if needed only to the reviewers for review purposes of this article and no other sharing, comparing or publication of our original data is permitted.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors declare that they have no conflicts of interest in this work.
References
1. Vogiatzis I, Rochester CL, Spruit MA, Troosters T, Clini EM. Increasing implementation and delivery of pulmonary rehabilitation: key messages from the new ATS/ERS policy statement. Eur Respir J. 2016;47(5):1336–1341. doi:10.1183/13993003.02151-2015
2. Spruit MA, Singh SJ, Garvey C, et al. An official American thoracic society/European respiratory society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–e64. doi:10.1164/rccm.201309-1634ST
3. Cornelison SD, Pascual RM. Pulmonary rehabilitation in the management of chronic lung disease. Med Clin North Am. 2019;103(3):577–584. doi:10.1016/j.mcna.2018.12.015
4. Garrod R, Marshall J, Barley E, Jones PW. Predictors of success and failure in pulmonary rehabilitation. Eur Respir J. 2006;27(4):788–794. doi:10.1183/09031936.06.00130605
5. Spruit MA, Augustin IML, Vanfleteren LE, et al. Differential response to pulmonary rehabilitation in COPD: multidimensional profiling. Eur Respir J. 2015;46(6):1625–1635. doi:10.1183/13993003.00350-2015
6. Brown AT, Hitchcock J, Schumann C, Wells JM, Dransfield MT, Bhatt SP. Determinants of successful completion of pulmonary rehabilitation in COPD. Int J COPD. 2016;11(1):391–397. doi:10.2147/COPD.S100254
7. Troosters T, Gosselink R, Decramer M. Exercise training in COPD: how to distinguish responders from nonresponders. J Cardiopulm Rehabil. 2001;21(1):10–17. doi:10.1097/00008483-200101000-00004
8. Jones SE, Maddocks M, Kon SSC, et al. Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax. 2015;70(3):213–218. doi:10.1136/thoraxjnl-2014-206440
9. Walsh JR, Morris NR, McKeough ZJ, Yerkovich ST, Paratz JD. A simple clinical measure of quadriceps muscle strength identifies responders to pulmonary rehabilitation. Pulm Med. 2014;2014:1–8. doi:10.1155/2014/782702
10. Tunsupon P, Mador MJ. The influence of body composition on pulmonary rehabilitation outcomes in chronic obstructive pulmonary disease patients. Lung. 2017;195(6):729–738. doi:10.1007/s00408-017-0053-y
11. Barberan-Garcia A, Rodríguez DA, Blanco I, et al. Non-anaemic iron deficiency impairs response to pulmonary rehabilitation in COPD. Respirology. 2015;20(7):1089–1095. doi:10.1111/resp.12591
12. Issues S, Test MW, Equipment R, Preparation P. American thoracic society ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi:10.1164/rccm.166/1/111
13. Singh SJ, Puhan MA, Andrianopoulos V, et al. An official systematic review of the European Respiratory Society/American thoracic society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1447–1478. doi:10.1183/09031936.00150414
14. Giallauria F, Souto-Miranda S, Mendes MA, et al. Functional status following pulmonary rehabilitation: responders and non-responders. Journal of Clinical Medicine. 2022;11(3):518. doi:10.3390/jcm11030518
15. Bohannon RW, Crouch R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract. 2017;23(2):377–381. doi:10.1111/jep.12629
16. Holland AE, Nici L. The return of the minimum clinically important difference for 6-minute-walk distance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(4):335–336. doi:10.1164/rccm.201212-2191ED
17. Maddocks M, Kon SSC, Jones SE, et al. Bioelectrical impedance phase angle relates to function, disease severity and prognosis in stable chronic obstructive pulmonary disease. Clin Nutr. 2015;34(6):1245–1250. doi:10.1016/j.clnu.2014.12.020
18. Costi S, Crisafulli E, Trianni L, et al. Baseline exercise tolerance and perceived dyspnea to identify the ideal candidate to pulmonary rehabilitation: a risk chart in COPD patients. Int J COPD. 2019;14:3017–3023. doi:10.2147/COPD.S223038
19. Selzler AM, Simmonds L, Rodgers WM, Wong EYL, Stickland MK. Pulmonary rehabilitation in chronic obstructive pulmonary disease: predictors of program completion and success. COPD J Chronic Obstr Pulm Dis. 2012;9(5):538–545. doi:10.3109/15412555.2012.705365
20. Crisafulli E, Gorgone P, Vagaggini B, et al. Efficacy of standard rehabilitation in COPD outpatients with comorbidities. Eur Respir J. 2010;36(5):1042–1048. doi:10.1183/09031936.00203809
21. Sahin H, Naz I, Varol Y, Aksel N, Tuksavul F, Ozsoz A. COPD patients with severe diffusion defect in carbon monoxide diffusing capacity predict a better outcome for pulmonary rehabilitation. Rev Port Pneumol (English Ed. 2016;22(6):323–330. doi:10.1016/j.rppnen.2016.03.003
22. Ramachandran K, McCusker C, Connors M, ZuWallack R, Lahiri B. The influence of obesity on pulmonary rehabilitation outcomes in patients with COPD. Chron Respir Dis. 2008;5(4):205–209. doi:10.1177/1479972308096711
23. Pizzini A, Aichner M, Sonnweber T, Tancevski I, Weiss G, Löffler-Ragg J. The significance of iron deficiency and anemia in a real-life COPD cohort. Int J Med Sci. 2020;17(14):2232–2239. doi:10.7150/ijms.46163
24. Yohannes AM, Ershler WB. Anemia in COPD: a systematic review of the prevalence, quality of life, and mortality. Respir Care. 2011;56(5):644–652. doi:10.4187/respcare.01002
25. Cavaillès A, Brinchault-Rabin G, Dixmier A, et al. Comorbidities of COPD. Eur Respir Rev. 2013;22(130):454–475. doi:10.1183/09059180.00008612
26. Martín-Ontiyuelo C, Rodó-Pin A, Sancho-Muñoz A, et al. Is iron deficiency modulating physical activity in COPD? Int J COPD. 2019;14:211–214. doi:10.2147/COPD.S182700
27. Boutou AK, Stanopoulos I, Pitsiou GG, et al. Anemia of chronic disease in chronic obstructive pulmonary disease: a case-control study of cardiopulmonary exercise responses. Respiration. 2011;82(3):237–245. doi:10.1159/000326899
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
