Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 13
Predictors of pneumonia on routine chest radiographs in patients with COPD: a post hoc analysis of two 1-year randomized controlled trials
Authors Rubin DB, Ahmad HA, O'Neal M, Bennett S, Lettis S , Galkin DV , Crim C
Received 24 May 2017
Accepted for publication 25 August 2017
Published 8 January 2018 Volume 2018:13 Pages 189—201
DOI https://doi.org/10.2147/COPD.S142530
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Video abstract presented by David B Rubin
Views: 444
David B Rubin,1 Harris A Ahmad,2 Michael O’Neal,2 Sophie Bennett,3 Sally Lettis,3 Dmitry V Galkin,1 Courtney Crim1
1Respiratory R&D, GSK, Research Triangle Park, NC, 2Medical Affairs, Medical Imaging and Biomarkers, BioClinica Inc., Princeton, NJ, USA; 3Statistics and Programming, GSK, Stockley Park, Uxbridge, UK
Background: Patients with COPD are at risk for life-threatening pneumonia. Although anatomical abnormalities in the thorax may predispose to pneumonia, those abnormalities identified on routine chest X-rays (CXRs) in patients with COPD have not been studied to better understand pneumonia risk.
Methods: We conducted a post hoc exploratory analysis of data from two replicate year-long clinical trials assessing the impact of fluticasone furoate–vilanterol versus vilanterol alone on COPD exacerbations (GSK studies: HZC102871/NCT01009463 and HZC102970/NCT01017952). Abnormalities on baseline CXRs from 179 patients who developed pneumonia and 50 randomly selected patients who did not were identified by blinded consensus readings conducted by two radiologists. Positive and negative likelihood ratios and diagnostic odds ratios (ORs) were calculated to evaluate the markers for subsequent pneumonia development during the 1-year study period.
Results: Baseline characteristics distinguishing the pneumonia and non-pneumonia groups included a lower body mass index (24.9 vs 27.5 kg/m2, P=0.008), more severe airflow obstruction (mean post-bronchodilator forced expiratory volume in 1 second [FEV1]/forced vital capacity ratio: 42.3% vs 47.6%, P=0.003), and prior pneumonia (36% vs 20%, P=0.030). Baseline CXR findings with the highest diagnostic ORs were: elevated hemi-diaphragm (OR: 6.87; 95% CI: 0.90, 52.26), thick tracheal-esophageal stripe (OR: 4.39 [0.25, 78.22]), narrow cardiac silhouette (OR: 2.91 [0.85, 9.99]), calcified pleural plaque/mid-chest pleural thickening (OR: 2.82 [0.15, 53.76]), and large/prominent pulmonary artery shadow (OR: 1.94 [0.95, 3.97]). The presence of a narrow cardiac silhouette at baseline was associated with a statistically significant lower mean pre-bronchodilator FEV1 (P=0.040). There was also a trend for a lower mean pre-bronchodilator FEV1 in patients with a large/prominent pulmonary artery shadow at baseline (P=0.095).
Conclusion: Findings on routine CXR that relate to pathophysiological mechanisms of pneumonia could help determine pneumonia risk in patients with COPD.
Keywords: COPD, pneumonia, chest X-rays, predictors of risk
Introduction
COPD is more commonly associated with pneumonia compared with other chronic diseases.1 Patients with COPD are at an increased risk of developing community-acquired pneumonia (CAP)2–4 – a phenomenon thought to be linked to altered host innate immune mechanisms leading to susceptibility for pathogen colonization.5 The risk of developing CAP appears to be elevated with an increasing airflow limitation severity of COPD.6,7 A retrospective, UK population-based cohort study of 40,414 patients with COPD estimated the overall incidence of CAP at 22.4 episodes/1,000 person-years; corresponding rates were 18.2, 19.2, and 35.9 episodes/1,000 person-years in patients with mild, moderate, and severe COPD, respectively.6 Furthermore, a US-based longitudinal study reported higher rates of pneumonia hospitalizations in patients with more severe COPD; rates were 22.7/1,000 person-years in patients with Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 3/4 compared with 6.9, 6.0, and 2.9/1,000 person-years in those with GOLD stages 2, 1, and 0 (normal lung function), respectively.7 Moreover, there are data to suggest that patients with COPD are at an increased risk of pneumonia-associated mortality. A retrospective, observational study of 744 hospitalized patients with CAP reported significantly higher 90-day mortality rates in patients with COPD, compared to those without (18.6% vs 11.7%; P=0.013).8 Thus, predicting pneumonia risk in patients with COPD is of clinical importance.
Aside from severe airflow obstruction,9 factors associated with increased pneumonia risk in patients with COPD include low body mass index (BMI),5,9 older age,5,6 use of psychoanaleptics,9 presence of gastroesophageal reflux disease (GERD),10 and use of inhaled corticosteroids (ICS).5 Furthermore, the presence of anatomical abnormalities in the lung or thorax could render the airway parenchyma prone to respiratory infection11–13 or reflect underlying disease severity.
The chest radiograph (chest X-ray; CXR) is the most common clinical test used to screen for such anatomical abnormalities; however, other than in cystic fibrosis,14 very little is known about how CXR findings in patients with chronic respiratory diseases, including COPD, might relate to the subsequent risk of developing pneumonia. An opportunity to retrospectively assess this risk is possible by utilizing baseline CXRs collected during clinical trials in COPD.
To investigate whether abnormalities detected on routine CXRs could be associated with subsequent pneumonia development in patients with COPD, we conducted a post hoc analysis of data from two previously published randomized controlled trials that assessed the effect of the ICS/long-acting β2 agonist (LABA) combination of fluticasone furoate (FF) plus vilanterol (VI) versus VI alone in preventing exacerbations of COPD.15
Methods
This post hoc exploratory analysis of the association between baseline CXR findings and subsequent development of pneumonia was conducted using data from two replicate multicenter, double-blind, parallel-group, 1-year, randomized controlled trials that evaluated the effect of once-daily FF (50, 100, or 200 μg) plus VI 25 μg versus VI 25 μg alone on the prevention of exacerbations of COPD (NCT01009463 and NCT01017952).15 The trial protocols were approved by relevant institutional review boards/independent ethics committees, details of which are provided in the Supplementary Materials. The trials were conducted in accordance with Good Clinical Practice guidelines and the principles founded in the Declaration of Helsinki. All patients provided written informed consent.15
The trial population comprised outpatients aged ≥40 years with a diagnosis of COPD, who had a smoking history of ≥10 pack-years, a forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio ≤0.70 after bronchodilators, a post-bronchodilator FEV1 ≤70% of predicted, and a documented history of ≥1 moderate/severe exacerbations of COPD in the previous year. Baseline CXRs (posterior–anterior and lateral views) were obtained at screening. Adverse events of pneumonia were coded per MedDRA version 14.1. In total, 3,255 patients were randomized to treatment across the two studies; of these, 181 developed pneumonia during the 1-year study period. All but two patients who developed on-treatment pneumonia had a baseline CXR available for independent radiologic review as part of this study.
The objective of the present analysis was to compare baseline CXR findings in patients who developed on-treatment pneumonia (n=179; pneumonia group) with a randomly selected subset of patients who did not (n=50; non-pneumonia group).
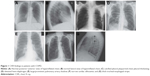
A subjective qualitative review of all 229 baseline CXRs was first undertaken by a pulmonologist (D.B.R.) and an infectious disease specialist (D.V.G), with both physicians blinded to treatment arm and chronological identity of the images. CXR findings were categorized into 21 groups according to pre-specified definitions (normal posterior–anterior and lateral views from COPD patients are shown in Figure 1A and B, respectively): 1) abnormal cardio/thoracic ratio measurement; 2) atelectasis or other evidence of volume loss; 3) basilar lines of COPD; 4) blurring or loss of diaphragmatic shadow; 5) calcified pleural plaque over the diaphragm (on posterior–anterior view) or mid-chest pleural thickening (Figure 1C); 6) elevation of hemi-diaphragm (indirect sign of volume loss [any lobe]) (Figure 1D); 7) evidence of hiatal hernia; 8) focal or diffuse reticular-nodular or “ground glass” shadows; 9) hyperinflated chest; 10) indirect signs of upper lobe volume loss (right hilum equal to left hilum position, right upper lobe atelectasis, and/or elevation of minor fissure on posterior–anterior view); 11) large cardiac silhouette; 12) large/prominent pulmonary artery shadow along the right mainstem bronchus (Figure 1E); 13) narrow cardiac silhouette (Figure 1F); 14) normal heart size in a hyperinflated chest (normal cardiac silhouette); 15) pleural thickening (any pleural change); 16) pleural thickening at apices; 17) pleural thickening at bases including costophrenic angle blunting; 18) signs of bronchiectasis; 19) signs of congestive heart failure including basilar effusion and Kerly B lines; 20) thick tracheal-esophageal stripe on lateral view (Figure 1G); and 21) vascular calcifications: coronary, carotid, or aortic.
A blinded independent subjective qualitative review of all CXRs was then conducted by two radiologists (H.A. and M.O.), who reviewed the CXR films together and determined a final interpretation of CXR findings by reaching a consensus.
The pneumonia and non-pneumonia groups were statistically compared for a variety of parameters: Student’s t-test was used for between-group comparisons of mean values for age, BMI, and lung function parameters at screening. Between-group differences in the proportions of patients with/without a particular CXR finding or medical history were tested using the chi-square test or Fisher’s exact test (the latter was used if cell values were <5). Positive likelihood ratios (LR+), negative likelihood ratios (LR−), and diagnostic odds ratios (OR) were also calculated. No adjustments were made for other baseline factors in these analyses. Similar analyses were conducted using history of pneumonia as a potential predictive marker.
Results
Patient demographics and baseline characteristics are summarized in Table 1. Compared with the non-pneumonia group, patients in the pneumonia group had a significantly lower mean BMI (24.9 vs 27.5 kg/m2; P=0.008) and mean post-bronchodilator FEV1/FVC ratio (42.3% vs 47.6%; P=0.003), and there was a trend for a lower baseline percent predicted post-bronchodilator FEV1 (FEV1%; 41.7% vs 45.2%; P=0.096). A higher proportion of patients in the pneumonia group had a history of pneumonia (36% vs 20%; P=0.030; OR: 2.28; Table S1). The baseline characteristics of the subset of 50 patients included in the non-pneumonia group appeared similar to the overall population of patients who did not develop pneumonia (Table S2).
A significantly higher proportion of patients in the pneumonia group than in the non-pneumonia group had a baseline CXR finding of elevated hemi-diaphragm (12% vs 2%, P=0.033; LR+: 6.15; LR−: 0.89; OR: 6.87; 95% CI: 0.90, 52.26); overall, this finding had the highest values for LR+ and diagnostic OR (Table 2). However, the opposite was evident for an alternative indirect sign of upper lobe volume loss (elevation of the right hilum), which was significantly less prevalent at baseline in the pneumonia group than in the non-pneumonia group (11% vs 22%, P=0.048; LR+: 0.51; LR−: 1.14; OR: 0.45 [95% CI: 0.20, 1.01]). Other baseline CXR findings with LR+ and diagnostic OR values >2 were a thick tracheal-esophageal stripe (4% vs 0%, P=0.352; OR: 4.39), narrow cardiac silhouette (16% vs 6%, P=0.101; OR: 2.91), and calcified pleural plaque over the diaphragm/mid-chest pleural thickening (8% vs 0%, P=0.578; OR: 2.82). In addition, the finding of large/prominent pulmonary artery shadow aligning the right mainstem bronchus was identified as having a diagnostic OR of ~2 (38% vs 24%, P=0.067; OR: 1.94).
We explored whether the presence of certain baseline CXR findings could be associated with subsequent pneumonia localization within the lung tissue (Table S3). We hypothesized that pneumonia would occur in the same hemi-thorax as did the findings of elevated hemi-diaphragm and calcified pleural plaque over the diaphragm/mid-chest pleural thickening; however, this ipsilateral relationship was found in only 10/20 and 2/9 cases, respectively. Furthermore, we examined whether the presence of a thick tracheal-esophageal stripe was associated with the presence of lower lobe pneumonia that reflected aspiration; this lower lobe relationship was observed in only 4/7 cases.
As the majority of patients had hyperinflation and the population had, on average, severe airflow obstruction, there was a rationale to try and relate lung function to the finding of narrow cardiac silhouette (indicative of significant obstruction with possible hyperinflation resulting in a shift in mediastinal structures). We observed a statistically significant higher mean pre-bronchodilator FEV1 in patients without a narrow cardiac silhouette at baseline than in patients who did have this finding (ratio: 1.16 [95% CI: 1.01, 1.34], P=0.040; Figure 2A). Furthermore, as the presence of a large/prominent pulmonary artery shadow may reflect pulmonary hypertension secondary to airflow obstruction and tissue hypoxia, it was also of interest to try and relate lung function to this CXR finding. We observed a trend toward a higher mean pre-bronchodilator FEV1 in patients without a large/prominent pulmonary artery shadow at baseline than in patients with this finding (ratio: 1.09 [95% CI: 0.99, 1.21], P=0.095; Figure 2B).
Discussion
While previous studies in COPD have investigated the relationship between patient demographics/baseline disease characteristics and risk for pneumonia, the association between anatomical abnormalities of the lung as detected on routine CXRs and risk of developing pneumonia has not, to our knowledge, been studied.
This exploratory post hoc analysis of data from two previous randomized clinical trials in COPD15 sought to investigate whether baseline CXR findings could predict subsequent pneumonia development. Baseline characteristics significantly associated with development of pneumonia were lower BMI, lower post-bronchodilator FEV1/FVC ratio, and prior history of pneumonia. The diagnostic OR for history of pneumonia was 2.28, with a CI that excluded unity. Furthermore, there was a trend for lower baseline post-bronchodilator FEV1% in patients who developed pneumonia compared with those who did not. These findings are consistent with two previous studies of pneumonia risk in patients with COPD,5,6 suggesting that more severe airflow obstruction at baseline is associated with an increased risk of developing pneumonia.
In this analysis, the presence on baseline CXR of an elevated hemi-diaphragm, thick tracheal-esophageal stripe, narrow cardiac silhouette, calcified pleural plaque/mid-chest pleural thickening, or large/prominent pulmonary artery shadow was associated with subsequent development of pneumonia; however, 95% CIs for the diagnostic ORs included unity in all cases. The LR+ was >2 for each of these findings (with the exception of large/prominent pulmonary artery shadow; LR+ of ~2), suggesting that an individual with pneumonia is more likely to test positive for these CXR findings than an individual without pneumonia; however, the LR− values were close to unity, meaning a negative finding would not be informative.
An elevated hemi-diaphragm, indicative mostly of lower lobe volume loss, supports the hypothesis that non-inflated lung tissue is prone to pneumonia. In contrast, upper lobe volume loss, reflected by elevation of the right hilum, appears to be associated with a lower risk of developing pneumonia.11,13 While there is no clear pathophysiological explanation for the latter phenomenon, it has been reported that lung volume reduction surgery improves the respiratory status of COPD patients with upper lobe emphysema;16 however, it is unknown whether lung volume reduction lowers the risk of developing pneumonia.
There was no difference in the distribution of abnormal or normal cardio/thoracic ratios between the pneumonia and non-pneumonia groups; however, the presence of a narrow cardiac silhouette did appear to predict for pneumonia risk. A narrow cardiac silhouette on CXR represents torsion of the mediastinal structures due to hyperinflation and suggests severe obstructive disease. Low FEV1 has been previously reported to be a risk factor for pneumonia in patients with COPD.5 Accordingly, in the present analysis we observed a statistically significantly lower mean pre-bronchodilator FEV1 at baseline in patients with a narrow cardiac silhouette as compared with patients without this finding.
The finding of a thick tracheal-esophageal stripe was only observed in the pneumonia group. One reported cause of this CXR finding is esophageal stricture secondary to GERD,17 a common comorbidity of COPD18 that increases patients’ risk for aspiration-induced bronchitis and/or pneumonia.10 The presence of pleural shadows on the lateral wall of a posterior–anterior radiograph signifies a prior pneumonic process, for example, asbestosis-related disease, which could predispose to future infection.
Finally, the finding of an enlarged pulmonary artery shadow is indicative of pulmonary hypertension. If COPD-related hypoxia were to be a cause of pulmonary hypertension, the enlarged pulmonary artery shadow, like the narrow cardiac silhouette, could be indicative of disease severity, which is itself a risk factor for pneumonia. Notably, we observed a trend for a lower mean pre-bronchodilator FEV1 in patients with a large/prominent pulmonary artery shadow at baseline, compared with patients without this finding.
While CXR findings of elevated hemi-diaphragm, thick tracheal-esophageal stripe, or calcified pleural plaque/mid-chest pleural thickening were identified as potential markers for the development of pneumonia, additional exploratory analyses did not identify any clear relationship between these findings and subsequent pneumonia localization within the lung tissue.
There are several limitations to the present study that should be considered: 1) This was a post hoc retrospective analysis of data from two previously published trials, and our results require further exploration in a larger, prospectively designed study. 2) Due to the small sample size included in this analysis, there was no adjustment for baseline factors that may have been associated with development of pneumonia. ICS use has been associated with increased pneumonia risk in COPD,5 and it is worth noting that, in the included trials, three times as many patients were randomized to FF/VI than to VI. Of the 181 patients who developed on-treatment pneumonia, 85% were treated with an ICS-containing regimen compared with only 15% in the control arm.15 3) Another limitation relates to the small number of patients randomly selected for inclusion in the non-pneumonia group and whether this subgroup is representative of the overall patient population that did not develop pneumonia; however, the two populations were found to be similar in terms of baseline demographics and lung function. Furthermore, while the control group did not develop pneumonia during the 1-year study period, they may not be truly representative of a patient population who never develop pneumonia (ie, they may not be true “negatives”). 4) The ratio of patients with and without pneumonia in the present analysis is not representative of the real-world scenario, and this impacts upon the ability to assess predictive measures such as positive predictive value, as prevalence is not reflective of the everyday clinical setting. 5) In this study design, there was no matching of time-at-risk between pneumonia cases and controls; an alternative approach would have been to identify a control at the time a case of pneumonia was found. 6) Consideration of the reliability of the association between baseline CXR findings and the timing of pneumonia development during the 1-year study period is also warranted. It is possible that the CXR findings detected at the baseline assessment may have differed to those detected at the time of pneumonia diagnosis. 7) The CXR films reviewed in this analysis were derived from various imaging machines and study sites; consequently, there was no standardized radiographic magnification or kilovoltage exposure. Accordingly, we did not specify quantifying measures for CXR findings (eg, for height of diaphragm elevation, or size of pulmonary artery aligning the right mainstem bronchus) or assess CXR findings using a severity scale. Instead, the study relied on the subjective analysis of experienced radiologists to simulate “real-world” CXR review, and the practicality of reviewing many CXR images should be highlighted. 8) Finally, as a variety of baseline CXR findings were examined in this analysis, it is possible that some findings could have been observed by chance; therefore, this study should be viewed as hypothesis generating and not confirmatory.
The routine CXR has not commonly been used in the assessment of pneumonia risk. The extent of bronchiectatic and post-pneumonia alterations detected on CXR has been identified as a predictor of pneumonia in patients with cystic fibrosis;14 however, we are not aware of any similar studies in other chronic respiratory diseases. Understanding the increased risk for development of pneumonia based on a common clinical test could have an impact on the management of patients with COPD. If subsequent prospective studies confirm any of these anatomical abnormalities on CXR as being predictive of the likelihood of developing pneumonia, both patients and physicians could be especially vigilant for the signs and symptoms of incipient pneumonia, prompting earlier diagnostic evaluation of suspected cases. In addition, physicians may be encouraged to redouble their efforts to prevent pneumonia (eg, via methods such as vaccination, improving treatment adherence, home respiratory therapy, and employing prophylactic measures [such as avoiding ICS where possible]).
Conclusion
Our findings suggest that the presence of an elevated hemi-diaphragm, thick tracheal-esophageal stripe, narrow cardiac silhouette, calcified pleural plaque/mid-chest pleural thickening, or large/prominent pulmonary artery shadow on routine CXR may be associated with the subsequent development of pneumonia in patients with COPD. These results warrant further exploration and confirmation in a prospectively designed study or a well-controlled retrospective cohort design in a larger patient population.
Acknowledgments
Editorial support (in the form of writing assistance, collating author comments, assembling tables and figures, grammatical editing, and referencing) during the development of this manuscript was provided by Emma Landers, PhD, of Gardiner-Caldwell Communications, Macclesfield, UK, and was funded by GSK. The authors were fully responsible for the decision to submit the article for publication and for all content and editorial decisions, were involved at all stages of manuscript development, and approved the final version for submission. This analysis was funded by GSK (data were derived from GSK studies HZC102871/NCT01009463 and HZC102970/NCT01017952). Employees of the sponsor were involved in study concept, data collection, data analysis/review, and manuscript writing/review. Medical writing support during the development of this manuscript was funded by GSK.
Disclosure
DBR, SL, DVG, and CC: employment and stock ownership (GSK). SB: employment (GSK) at the time the analysis was conducted. HAA: employment and shareholder (Bristol-Myers Squibb). The current affiliation for HAA is Headquarters Medical, Immunoscience Marketed Products Development, Bristol-Myers Squibb, Princeton, NJ, USA. The authors report no other conflicts of interest in this work.
References
Chatila WM, Thomashow BM, Minai OA, Criner GJ, Make BJ. Comorbidities in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(4):549–555. | ||
Jackson ML, Neuzil KM, Thompson WW, et al. The burden of community-acquired pneumonia in seniors: results of a population-based study. Clin Infect Dis. 2004;39(11):1642–1650. | ||
Curcio D, Cané A, Isturiz R. Redefining risk categories for pneumococcal disease in adults: critical analysis of the evidence. Int J Infect Dis. 2015;37:30–35. | ||
Torres A, Blasi F, Dartois N, Akova M. Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease. Thorax. 2015;70(10):984–989. | ||
Crim C, Calverley PM, Anderson JA, et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J. 2009;34(3):641–647. | ||
Müllerova H, Chigbo C, Hagan GW, et al. The natural history of community-acquired pneumonia in COPD patients: a population database analysis. Respir Med. 2012;106(8):1124–1133. | ||
Mannino DM, Davis KJ, Kiri VA. Chronic obstructive pulmonary disease and hospitalizations for pneumonia in a US cohort. Respir Med. 2009;103(2):224–229. | ||
Restrepo MI, Mortensen EM, Pugh JA, Anzueto A. COPD is associated with increased mortality in patients with community-acquired pneumonia. Eur Respir J. 2006;28(2):346–351. | ||
DiSantostefano RL, Li H, Hinds D, Galkin DV, Rubin DB. Risk of pneumonia with inhaled corticosteroid/long-acting β2 agonist therapy in chronic obstructive pulmonary disease: a cluster analysis. Int J Chron Obstruct Pulmon Dis. 2014;9:457–468. | ||
DiBardino DB, Wunderink RG. Aspiration pneumonia: a review of modern trends. J Crit Care. 2015;30(1):40–48. | ||
van Kaam AH, Lachmann RA, Herting E, et al. Reducing atelectasis attenuates bacterial growth and translocation in experimental pneumonia. Am J Respir Crit Care Med. 2004;169(9):1046–1053. | ||
Kwon KY, Myers JL, Swensen SJ, Colby TV. Middle lobe syndrome: a clinicopathological study of 21 patients. Hum Pathol. 1995;26(3):302–307. | ||
Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109(5):571–577. | ||
Terheggen-Lagro S, Truijens N, van Poppel N, Gulmans V, van der Laag J, van der Ent C. Correlation of six different cystic fibrosis chest radiograph scoring systems with clinical parameters. Pediatr Pulmonol. 2003;35(6):441–445. | ||
Dransfield MT, Bourbeau J, Jones PW, et al. Once-daily inhaled fluticasone furoate and vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med. 2013;1(3):210–223. | ||
Fishman A, Martinez F, Naunheim K, et al; National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348(21):2059–2073. | ||
Palayew MJ. The tracheal-esophageal stripe and the posterior tracheal band. Radiology. 1979;132(1):11–13. | ||
Casanova C, Baudet JS, del Valle Velasco M, et al. Increased gastro-oesophageal reflux disease in patients with severe COPD. Eur Resp J. 2004;23(6):841–845. |
Supplementary materials
List of approving institutional review boards/independent ethics committees for GSK study HZC102871 (NCT01009463)
Argentina
Comite de Etica Independiente en Investigacion Clinica (CEIC), Buenos Aires
Comite Independiente de Etica para Ensayos en Farmacologia Clinica, Buenos Aires
Comite de Docencia e Investigacion INSARES, Mendoza
Comite Independiente de revision y etica (CIRE), Buenos Aires
Ave Pulmo.Comite de docencia e investigacion, Mar del Plata, Buenos Aires
Comite de Etica en Investigacion, Insituto de Investigaciones Clinicas, Mar del Plata, Buenos Aires
Comite de Docencia e Investigacion Instituto InAER, Buenos Aires
Comite de Docencia e Investigacion Policlinico, Rio Negro
Australia
ACT Health Human Research Ethics Committee, Canberra, ACT
Austin Health Human Research Ethics Committee, Heidelberg, VIC
Flinders Clinical Research Ethics Committee, Bedford Park, Adelaide, SA
Metro North Health Service District – Redcliffe Caboolture Human Research Ethics Committee, Redcliffe, Brisbane, QLD
Peninsula Health, Human Research and Ethics Committee, Mount Eliza, VIC
Cairns & Hinterland Health Service District Human Research Ethics Committee, Cairns, QLD
Bellberry Human Research Ethics Committee, Dulwich, Adelaide, SA
Canada
IRB Services, Aurora, ON
Comite d’ethique de la recherche de l’hopital, Quebec City, QC
Chile
Comite de Evaluacion Etico Cientifico, Servicio Salud, Valparaiso San Antonio, Valparaiso
Comite de Evaluacion Etica Cientrfica, Temuco
Comite de Etica de la Investigacion Servicio de Salud Metropolitano Norte, Santiago
Comite de etica de Investigacion en seres humanos, Facultad Medicina Universidad de Chile, Santiago
Estonia
Tallinn Medical Research Ethics Committee, National Institute for Health Development, Tallinn
Germany
Ethik-Kommission der Arztekammer Schleswig-Holstein, Bad Segeberg
Italy
Comitato Etico Centrale, Fondazione Salvatore Maugeri I.R.C.C.S, Pavia
Comitato Etico Aziendale A.O. Ospedale S. Martino e Cliniche Universitarie Convenzionate di Genova, Genova
Comitato di Etica della ASL di Salerno, Nocera Inferiore
Comitato Etico I.R.C.C.S. San Raffaele Pisana, Roma
Comitato Etico dell’Azienda Ospedaliera San Paolo, Milano
Comitato Etico dell’Azienda Ospedaliera Complesso San Filippo Neri di Roma, Roma
Comitato Etico A.O. Umberto I e Facolta’ di Medicina e Chirurgia Università degli Studi di Ancona, Torette
Comitato Etico Provincia di Ferrara-Azienda Ospedaliero Universitaria, Corso della Giovecca, Ferrara
Mexico
Comite de Etica Cardiolink Clin Trials, Monterrey
Comite da Bioetlca del Instituto, IMER de Oocidente, Guadalajara, Jalisco
Comite de Etica dellnstituto Jalisciense de Investigacion Clfnica, Guadalajara, Jalisco
Cornite de Etica del Hospital General de Tijuana, Baja California Norte
Comite Bioeticopara Ia lnvestigacion Clinica, Roma
The Netherlands
Independent Review Board, Amsterdam
Peru
Comite Institucional de Etica, Universidad Peruana Cayetano Heredia, San Martin de Porres, Lima
Comite Inslitucional de Etica en la Investigacion, del Hospital Nacional Cayetano Heredia, San Martin de Porres, Lima
Comite de Etica del Hospital Edgardo Rebagliati Martins, Jesus Maria, Lima
Philippines
Research And Ethics Committee, Veterans Memorial Medical Center, Quezon City
Ethics Committee, Lung Centre of the Philippines, Quezon City
Hospital Ethics Committee, Quirino Memorial Medical Centre, Quezon City
Ethics Committee, Dagupan Doctors Villaflor Memorial Hospital, Dagupan City
South Africa
Pharma Ethics Committee, Pretoria
Medicines Control Council, Clinical Trials Unit, Pretoria
Health Research Ethics Committee, University Of Stellenbosch, Tygerberg
Sweden
Regionala Etikprovningsnamnden i Goteborg, Goteberg
United Kingdom
Oxfordshire Research Ethics Committee B, Bicester
United States
Quorum Review, Inc., Seattle, WA
University of Texas Health Science Center San Antonio, San Antonio, TX
Birmingham VA Institutional Review Board, Department of Veterans Affairs Medical Centre, Birmingham, AL
Human Studies Subcommittee, Bay Pines VA Healthcare System, Research & Development Service (151), Bay Pines, FL
Western Institutional Review Board, Olympia, WA
Research Compliance Office, Stanford, CA
Committee for the Protection of Human Subjects, Dartmouth College, Hanover, NH
Human Studies Subcommittee, VA Long Beach Health Care System, Long Beach, CA
Phoenix VA Health Care System IRB, Phoenix, AZ
Institutional Review Board, Program for the Protection of Human Subjects, Mount Sinai School of Medicine, New York, NY
University of Florida Institutional Review Board-01, Gainesville Health Science Centre, Gainesville, FL
University of Miami, Human Subject Research Office, Miami, FL
Scott & White Institutional Review Board, Temple, TX
List of approving institutional review boards/independent ethics committees for GSK study HZC102970 (NCT01017952)
Argentina
Comite de Etica Independiente en Investigacion Clinica, Buenos Aires
Comite de Docencia e Investigacion Fundacion CIDEA, Buenos Aires
Comite de Revision Institutional del Instituto Medico Especializado, Buenos Aires
Comite de Docencia e Investlgacion CEMIT, Medicos Investigatores Tucuman, Tucuman
Comite de Docencia e Investigacion de Investigaciones en Patologias Respiratorias, Tucuman
Australia
Uniting Care Health Human Research Ethics Committee, The Wesley Hospital, Auchenflower, QLD
Royal Adelaide Hospital Research Ethics Committee, Adelaide, SA
Bellberry Human Research Ethics Committee, Dulwich, Adelaide, SA
Sir Charles Gairdner Group Human Research Ethics Committee, Sir Charles Gairdner Hospital, Nedlands, WA
Gold Coast Health Service District Human Research Ethics Committee, Southport, QLD
Eastern Health Research and Ethics Committee, Box Hill, VIC
Sydney West Area Health Service Human Research Ethics Committee, Westmead Hospital, Westmead, NSW
Tasmania Health & Medical Human Research Ethics Committee, University of Tasmania, Hobart, TAS
Canada
REB Biomedical-C, Montreal Chest Institute, Montreal, QC
Institutional Review Board Services, Aurora, ON
Colchester East Health Authority Ethics Board, Truro, NS
Concordia Hospital Ethics Committee, Concordia Hospital, Winnipeg, MB
Chile
Comite Etico Cientifico, Servicio de Salud Metropolitano Oriente, Santiago
Comite Etico Cientifico, Secretaria Ministerial de Salud, Concepcion
Denmark
Videnskabsetiske Komiteer for Region Hovedstaden, Hillerod
Germany
Ethik-Kommission der Landesarztekammer Hessen, Frankfurt
Italy
Comitato di Etica A.S.L. Salemo, Nocera Inferiore, Salemo
Comitato Etico Aziende Sanitarie dell’Umbria, Segreteria Scientifico-Amministrativa, Ellera di Corciano (PG)
Comitato Etico, Azienda Sanitaria Locale 1, Caserta
Comitato Etico Unico per la Provincia di Parma, Azienda Ospedaliero-Universitaria di Parma, Parma
Comitato Etico dell’Azienda Ospedaliera Universitaria Policlinico Paolo Giaccone dee’Universita degi Studi di Palermo, Palermo
Comitato Etico Provinciale di Modena, Azienda Ospedaliera universitaria Poloclinico di Modena, Modena
Comitato Etico, Azienda Ospedaliero-Universiatria, Ospedali Reuniti di Foggia, Foggia
Comitato Etico Azienda Ospedaliera Universitaria Pisana, Pisa
Comitato Etico dell’Universita’ Cattolica del Sacro Cuore – Policlinico Universitario A. Gemelli di Roma, Roma
Comitato Etico Per La Sperimentazione Clinica Dei Medicinali Azienda Ospedaliera Universitaria Pisana, Pisa
Mexico
Comite de Bioetica del Instituto IMER de Occidente, Guadalajara, Jalisco
Comite de Etica del Instituto Jalisciense de Investigacion Clinica, Guadalajara, Jalisco
Comite de Etico de la Facultad de Medicina de Medicina de la UANL y Hospital Universitario, Monterrey
The Netherlands
Independent Review Board, Amsterdam
Peru
Comite Institucional de Etica de la Universidad Peruana Cayetano Heredia, San Martin de Porres, Lima
Comite de Bioetica de la Red Asistencial Sabogal, Essalud, Callao
South Africa
Pharma Ethics Committee, Pretoria
Medicines Control Council, Pretoria
University of Cape Town, Human Research Ethics Committee, Cape Town
University of the Witwatersrand, Human Research Ethics Committee, WITS Health Consortium, Parktown
Spain
Comite Etico de Investigacion Clinica, Hospital Clínico San Carlos, Madrid
Comite Etico de Investigacion Clinica, Hospital General Santa Maria del Rosell, Cartegena, Murcia
Comite Etico de Investigacion Clinica, Hospital de Elda, Elda, Alicante
Comite Etico de Investigacion Clinica, Clinica Mediterranea de Neurociencias, Camino Viejo Alicante-Elche, Alicante
Comite Etico de Investigacion Clinica, Hospital Universitario Puerta de Hierro, Majadahonda
CEIC Hospital de Galdakano, Galdakano, Vizcaya
CEIC Autonomica Pais Vasco, Dpto. Sanidad Gobierno Vasco, Vitoria, Alava
Comite Etico de Investigacion Clinica de Cáceres, Hospital San Pedro de Alcántara, Caceres
Comite Etico de Investigacion Clinica, Islas Baleares Consell, Palma de Mallorca
Sweden
Regionala etikprovningsnamnden i Linkoping, Halsouniversitetets Kansli, Linkoping
United Kingdom
Oxfordshire Research Ethics Committee B, Bicester
United States
Quorum Review, Inc., Seattle, WA
Dean Institutional Review Board, Middleton, WI
Health Sciences Institutional Review Board, Columbia, MO
Berkshire Medical Center Institutional Review Board, Pittsfield, MA
Western Institutional Review Board, Olympia, WA
McGuire Institutional Review Board, McGuire VA Medical Center, Richmond, VA
Institutional Review Board Committee, University of Arizona Human Subjects Protection Program, Tucson, AZ
Department for Human Research Protections, University of Toledo Medical Center, Toledo, OH
Institutional Review Board for Human Subject Research for Baylor College of Medicine and Affiliated Hospitals, Houston, TX
Vanderbilt Institutional Review Board, Nashville, TN
Texas Health Resources, Institutional Review Board, Arlington, TX
Saint Francis Hospital and Medical Centre IRB, Hartford, CT
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.