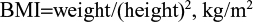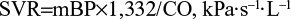Back to Journals » Vascular Health and Risk Management » Volume 12
Predictors of high central blood pressure in young with isolated systolic hypertension
Authors Radchenko G , Torbas O, Sirenko Y
Received 28 September 2015
Accepted for publication 14 April 2016
Published 2 August 2016 Volume 2016:12 Pages 321—328
DOI https://doi.org/10.2147/VHRM.S97304
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Daniel Duprez
G D Radchenko, O O Torbas, Yu M Sirenko
State Institute National Scientific Center, M.D. Strazhesko Institute of Cardiology, National Academy of Medical Science, Kyiv, Ukraine
Objective: According to the European Society of Cardiology/European Society of Hypertension 2013 guidelines, evaluation of aortic blood pressure (BP) is needed in young with isolated systolic hypertension (ISH), but using special devices is not common, especially in Ukraine, where only a few centers have these devices. The purpose of our study was to identify the simple clinical predictors for differentiation (with or without elevated aortic systolic BP [SBP]) of the young with ISH without the need for further extensive work-up.
Patients and methods: The study included 44 young men (mean age: 32.2±1.3 years) with office SBP ≥140 mmHg and office diastolic BP (DBP) <90 mmHg (average: 153.4±2.1 mmHg and 83.4±1.7 mmHg, respectively). The following procedures were performed in all the subjects: body weight and height evaluation; measurement of office SBP, DBP, and heart rate; ambulatory BP monitoring; measurement of pulse wave velocity in arteries of elastic and muscle types and central SBP (cSBP); biochemical blood tests; electrocardiography; echocardiography; and carotid ultrasound investigations. Step-by-step multifactor regression analyses were used for finding the predictors of high cSBP.
Results: Depending on the cSBP level, all the patients were divided into two groups: first group (n=17), subjects with normal cSBP, and second group (n=27), subjects with elevated cSBP. Patients in the second group were significantly older, with less height and higher body mass index; they had significantly higher levels of office SBP and DBP. Characteristics of target organ damage were within normal limits in both groups and did not differ significantly. Only pulse wave velocity in arteries of elastic type was significantly higher in the second group. The independent predictors of increased cSBP were as follows: height ≤178 cm (β=7.038; P=0.05), body weight ≥91 kg (β=5.53, P=0.033), and the level of office DBP ≥80 mmHg (β=4.43; P=0.05). The presence of two or three of these factors increased the probability of high cSBP in more than ten times (β=10.6, P=0.001). The sensitivity and specificity were 92.6% and 88.2%, respectively.
Conclusion: Thus, 38.6% of young with ISH had normal cSBP. Independent predictors of increased cSBP included height ≤178 cm, weight ≥91 kg, and the level of office DBP ≥80 mmHg. The presence of at least two of these factors indicated the need for starting the antihypertensive therapy in young with ISH. The presence of only one of these factors or none indicated the need for providing the central BP measurements in order to choose the further management strategy.
Keywords: isolated systolic hypertension, young, central blood pressure
Introduction
The population study, the National Health and Nutrition Examination Survey (NHANES; 1999–2004) reported that 5.16% of young (18–39 years) had elevated blood pressure (BP)1 and 1.57% had isolated systolic hypertension (ISH). Thus, ∼30% of hypertensive young adults had an increased level of only systolic BP (SBP), which is almost comparable with the prevalence of ISH among people aged 50–60 years. However, this type of arterial hypertension has different pathophysiological mechanisms in young and older persons.
For the first time, the phenomenon of ISH in young men was described by O’Rourke et al.2 Their conclusions were based on the results of the survey that included six young people aged 14–23 years with SBP 150–176 mmHg and diastolic BP (DBP) 50–85 mmHg. All of them were asymptomatic, and their height was a little higher for their age. The main instrumental method in this research was applanation tonometry for determining central SBP (cSBP). Applanation tonometry was used to obtain pulse wave curves (changes in BP in site) on the carotid, femoral, and radial arteries. Calibration of these curves is performed automatically depending on the BP measurements of the brachial artery. In all the six subjects with ISH, the level of cSBP was within normal limits (<126 mmHg). O’Rourke et al explained this by the pulse wave amplification effect. In another study, Mahmud and Feely3 reported that ISH is not a rare condition. They found that 12% of 174 medical students had this type of hypertension: mean office BP was 147/70 mmHg, the average central pressure was 116/70 mmHg, and all of them were male. None of these students smoked, while in a group of students with normal office BP 15% were smokers; all the subjects with ISH were involved in sport activities compared with only 40% of those with normal office BP. Moreover, subjects with ISH had lower heart rate (HR). Authors pointed out that these young people had no other health-related problems except elevated office SBP. Applanation tonometry revealed that the amplification effect was more pronounced in boys with ISH. The mean difference between peripheral BP and central BP was ∼31 mmHg in young with ISH, compared with individuals having normal BP (not >20 mmHg).
These two observations initiated further investigation to identify the prevalence of ISH in young, to investigate the factors associated with this phenomenon, and to evaluate the importance of such type of hypertension for prognosis. The results of these studies should give an answer for the question whether all young patients with ISH should be treated by antihypertensive drugs or not. There are only a few observations shown that young people with elevated cSBP should be treated, but with normal cSBP it would be better just to follow-up the patients and modify their lifestyle.4 The European Society of Cardiology and European Society of Hypertension (ESH) 2013 guidelines indicated how important it is to determine the level of cSBP in addition to traditional BP measurements (office, ambulatory, home) for pathophysiology, pharmacology, and therapeutics, but, in fact, ISH in young is the only one clinical indication for the measurements of aortic pressure.5
Because of the necessity of valid technique, there are some limitations for wide spreading of aortic BP measurement method for the correct diagnosis of arterial hypertension, especially in Ukraine, where only a few centers have special devices. Thus, it was interesting for us to identify simple clinical predictors for differentiation (with or without elevated aortic SBP) of the young patients with ISH without the need for further extensive (and expensive, and not always available) work-up. This could help practitioners to stratify patients and to make a choice whether they need to be treated by antihypertensive drugs or only to follow up.
Patients and methods
Subjects
We included 44 young (according to the World Health Organization criteria in 2012) untreated patients, who were referred to outpatient department of State Institute “National Scientific Center ‘Institute of Cardiology named after academician. M D Strazhesko’ National Academy of Medical Science” for the evaluation of their arterial hypertension. Some patients were referred by their family physicians, and others by the physicians of their sport teams. The average age was 32.2±1.3 years. The inclusion criteria were: office SBP ≥140 mmHg and office DBP <90 mmHg (average values: 153.4±2.1 and 83.4±1.7 mmHg, respectively). All the patients were men. The protocol of this study was approved by the local ethics committee of the National Scientific center, The Strazhesko Institute of Cardiology of the National Academy of Medical Science of Ukraine, and all patients signed an informed consent form. For the elimination of concomitant factor influences, we excluded patients with established diagnoses of diabetes, secondary hypertension, arrhythmia, or with other clinical significant comorbidities. In addition, we excluded patients with “white coat” hypertension. All our patients had ISH, which was confirmed by ambulatory BP monitoring (ABPM).
Methods
The following clinical examinations were performed: height and body weight measurements with body mass index (BMI) calculation, office SBP, DBP, and HR measurements, and ABPM. The target organ damages were evaluated by the following: determination of pulse wave velocity in arteries of elastic (PWVe) and muscle (PWVm) types, creatinine to calculate glomerular filtration rate, and albuminuria, electrocardiography, echocardiography with tissue Doppler, carotid ultrasound investigation, and ankle-brachial index evaluation. We also performed biochemical blood tests (levels of potassium, sodium, uric acid, alanine transaminase, aspartate transaminase, bilirubin, glucose, total cholesterol, triglycerides, and cholesterol of high- and low-density lipoprotein) and cSBP evaluation.
The levels of office SBP and DBP were recorded at baseline (OMRON 705IT device; Omron Healthcare Co., Ltd., Kyoto, Japan). We took into account the average of three sequenced measurements. HR was determined after the second measurement.
BMI was calculated using the following formula:
|
|
To exclude the presence of “white coat” hypertension, we performed ABPM using a portable ABPM-04 device (Meditech, Budapest, Hungary). Final ABPM report included: average SBP and DBP (24-hour SBP and 24-hour DBP), daytime SBP, nighttime SBP, and maximum levels of SBP and DBP and HR. We used the standard protocol for monitoring: every 15 minutes during the day and every 30 minutes during the night (from 10 pm to 6 am). If ABPM was unsuccessful, we provided repeated ABPM and excluded patients who were unsuccessful in the second ABPM. We used ABPM with the number of readings not <70% of expected measurements. Patients were informed to spend a usual day activity without overworking and psycho-emotional stress.6
Biochemical analyses were performed using automatic photometer (Cormay Livia Chemistry Analyzer, Lublina, Poland) in the certified laboratory of State Institute “National Scientific Center ‘Institute of Cardiology named after academician M D Strazhesko’ National Academy of Medical Science” of Ukraine. Glomerular filtration rate was calculated using the CKD-EPI formula, approved in Kidney disease improving global outcomes 2013, The Kidney Disease Outcomes Quality Initiative (K/DOQI).7
PWVe, PWVm, cSBP, central pulse pressure, and augmentation index (Aix) adjusted to HR 75 per 1 minute (Aix@75) were determined using Sphygmocor-PVx device (AtCor Medical Pty Ltd., Sydney, Australia). Piezoelectric probes were installed on the common right carotid, femoral, and radial arteries under visual (on the monitor) and automatic quality controls, which were carried out by systemic support of the device. The distance between probes was measured by centimeter ribbon. For the evaluation of the elastic artery stiffness (PWVe), segment from carotid artery to femoral artery was used, and for the assessment of muscular artery stiffness (PWVm), segment from carotid artery to femoral artery was used. cSBP, central pulse BP, and Aix@75 were calculated automatically after entering of brachial SBP and DBP data. Normal level of cSBP was considered automatically according to the device’s definition with age adjustment.
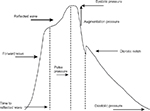
Aix is a percentage ratio of the difference between first and second systolic pulse wave peaks and pulse BP (Figure 1). It could be typically negative in young with elastic vessels. Aix depends on the intensity of pulse wave reflection, ventricular ejection time, and the time of pulse wave reflection (time of pulse wave spreading from the heart to periphery and back). The pulse wave reflection closely connected with PWV. The higher the velocity, the earlier reflection wave meets direct wave; thus, first systolic peak appears earlier and the difference between second and first systolic peak is more. The intensity of reflection depends on vessel diameter and arteriole elasticity. Aix increases with the elevation of mean BP and decreases with the elevation of HR.8,9 It has negative correlation with height.10,11 This fact could explain more Aix value in women. Pulse wave analysis in patients with various risk factors showed that Aix increases with age, in diabetic and hypercholesterolemia patients independently on BP.12,13 Prognostic value of Aix was studied in some prospective clinical trials (ASCOT, SEARCH, FIELD). One study showed that 10% Aix increment was associated independently with 1.28-fold increase in cardiovascular events.14
Intima-media thickness was measured by Sonos 5500 (Hewlett Packard, Palo Alto, CA, USA) three times with the calculation of average value in the right coronary artery and in the left carotid artery according to the consensus of the American Society of Echocardiography 2008.15 Ankle-brachial index was determined by the automatic device Omron M-10 (Omron Healthcare Co., Ltd.), and three consecutive measurements were performed to determine the mean value.
Echocardiography was performed using Sonos 5500 according to the extended protocol recommended by the European Association of Cardiovascular Imaging with the definition of main dimensions and volumes of the heart chambers, and major vessels, ejection fraction, diastolic function of the left ventricle, and left ventricle myocardial mass index according to the formula of the American Society of Echocardiography,16 which were recommended by the ESH in 2013.5 Stroke volume (SV) was calculated as the difference between end-diastolic volume and end-systolic volume, cardiac output (CO) was defined as the product between the SV and HR, and systemic vascular resistance (SVR) was calculated by the following formula:17
|
|
where mBP is the mean BP and 1,332 is the coefficient.
The evaluation of left ventricle diastolic function included measurements such as the peak early mitral inflow velocity (E), the peak velocity during atrial contraction (A), E/A ratio, peak early diastolic mitral annular velocity (E′), and E/E′ ratio.
Statistics
Statistical analysis was performed using the software, IBM Statistics SPSS 21.0 (SPSS Inc., Chicago, USA), to calculate the mean values (M) and the mean absolute error (m) by independent samples t-test. Values of P<0.05 were considered statistically significant. We assessed parameters that were significantly different in the group of patients without and with high cSBP. Relationships between these variables and the presence of high cSBP were assessed by Spearman’s correlation (all the parameters were abnormally distributed) and then by binary logistic univariate regression analysis. Statistically significant correlated variables were included in the regression model, and stepwise multivariate backward regression analyses with CI 95% were used for finding independent predictors of high cSBP.
Results
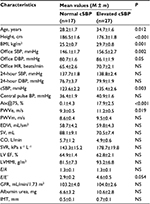
According to the results of central BP measurements (mean cSBP: 129.5±2.2 mmHg), all the patients with ISH were divided into two groups: first group, with normal aortic SBP (n=17), and second group, with high cSBP (n=27). Thus, 38.6% of young persons with ISH had normal levels of cSBP. The clinical characteristics of patients are shown in Table 1.
Patients in both the groups were matched with inclusion criteria. However, the levels of office SBP and DBP in the second group were significantly higher. The presence of ISH was confirmed by the ABPM data. But, despite the differences in the office BP, there were no any significant differences between groups in 24-hour SBP and DBP levels.
There were no significant differences between groups for target organ damage, except for large artery stiffness. PWVe was significantly higher in patients with elevated cSBP. Calculated SV, SVR, and CO in both groups were within normal limits, but in the first group SV and CO values were closer to the upper limit of normal (60–90 mL and 4.5–6.0 L/min, respectively). There were not significant differences, but only tentency to slightly higher CO and SV in the first group and higher SVR in the second group.
It was found that there were significant differences between groups in age, height, and BMI. Patients with elevated cSBP were older, with less height and higher BMI. No biochemical parameter was correlated with elevated cSBP.
The other part of our work was to identify the predictors of high cSBP in young patients with ISH. The Spearman’s analysis showed significant positive correlation of cSBP with age (r=0.31, P<0.04), weight (r=0.32, P<0.034), BMI (r=0.45, P<0.002), office SBP (r=0.38, P<0.01), and office DBP (r=0.57, P<0.01) and negative correlation with height (r=-0.30, P<0.05).
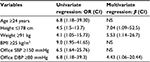
To identify the specific variables significantly associated with abnormal cSBP, we carried out stepwise univariate regression analysis (Table 2). It was found that the probability of high aortic pressure was significantly increased at the age of ≥24 years (OR =6.8, P=0.032), height of ≤178 cm (OR =4.5, P=0.042), weight of ≥91 kg (OR =4.1, P=0.042), BMI of ≥25 kg/m2 (OR =9.0, P=0.005), the level of office SBP of ≥150 mmHg (OR =6.5, P=0.008), and the level of office DBP of ≥80 mmHg (OR =6.8, P=0.032).
The data of multivariate regression analysis are presented in Table 2. The independent predictors of elevated aortic BP were height ≤178 cm (β=7.038; P=0.05), body weight ≥91 kg (β=5.53, P=0.033), and the level of office DBP ≥80 mmHg (β=4.43; P=0.05). The presence of two or three of these factors increased the probability of high cSBP ten times (β=10.6, P=0.001). In our sample, only two patients (11.8%) from the first group had two or more of these factors, whereas in the second group it was 25 patients (92.6%). The sensitivity, specificity, positive predictive value, and negative predictive value were 92.6%, 88.2%, 92.6%, and 88.2%, respectively.
Discussion
O’Rourke18 indicated that the pulse wave is the result of direct and reflected pulse wave summation. Aortic SBP may increase when the peaks of the pulse wave (direct and reflected) occur in systole. It happens in older people due to increasing arterial stiffness and high speed of the pulse wave returning. However, in the young people, there is the other mechanism of amplification.
Three main factors affect the pulse wave contour form: PWV, the reflected pulse wave distance, and HR.19 PWV depends on BP and arterial stiffness that are influenced by the age and the presence of concomitant diseases (diabetes, atherosclerosis, hypertension, etc). In young subjects, it takes more time to reflect a pulse wave and direct and reflected waves meet in diastole. The distance of pulse wave passing depends on the height. It takes a longer time for pulse wave to return in higher subjects. Thus, two waves meet in diastole in healthy young persons. When HR is increased, the systole is shorter and reflected pulse wave will meet the direct wave in diastole.8 In young with ISH, it takes much more time for pulse wave returning to the ascending part of the aorta than in young people without hypertension. Thus, two of the three factors (low PWV and increased height) contribute to pulse wave amplification, while low HR contributes to lowering it. The possible explanation for the increased brachial SBP is the significant SV. If CO at resting conditions is normal and HR is low, SV is compensatory. Young subjects with ISH often involved in sport activities and may have some signs of “athletic heart”. If their aorta has a very high compliance, the increased SV occurs without aortic SBP elevation, but due to amplification it may lead to the elevation of peripheral SBP. McEniery et al20,21 demonstrated that younger persons with ISH had increased SV and CO than subjects with essential hypertension, who had higher SVR. In our study, the young with ISH had high enough SV, especially in the group with normal cSBP. But mean values of SV were within the normal range. In comparison with the literature data,17,21 SVR was much lower in both of our groups than in essential hypertensive patients (>220 kPa·s−1·L−1). Normal SV and PWVe were found in patients with normal cSBP. Patients with elevated cSBP had significant higher PWVe compared with patients with normal cSBP. In patients in the second group, the aortic elastic properties were significantly worse. This fact, worsening of aortic elastic properties, may be considered the reason for the elevation of cSBP in young.
Our groups had significant differences in Aix@75. As mentioned earlier, this index depends on the intensity of pulse wave reflection, ventricular ejection time, and time of pulse wave reflection. The time of pulse wave reflection closely connected with arterial stiffness (positive correlation) and height (negative correlation). It was confirmed by our data that patients with elevated cSBP had significant higher PWVe and less height.
In the previous studies, the prevalence of ISH in young subjects was torn between 6.9% and 16.6%.22,23 Some clinicians believe that ISH in young subjects is an intermediate state between normal BP and persistent hypertension. In order to diagnose this state, several repeated BP measurements should be provided. Mahmud and Feely3 indicated that repeated BP measurements at intervals of 1 minute after 5 minutes of rest reduced the ISH rate in young from 12.6% to 8.5%. In another observation,23 it was decreased from 16.6% to 10.6% in young, but state unchanged in persons aged 55–60 years (14.6% and 13.9%) after all repeated measurements. This state could be explained by the presence of stress response during the initial medical examination. Repeated measurements or ambulatory BP monitoring, as was used in our study, should help to identify the “white coat” hypertension, in which the prevalence is high among young hypertensives.24,25 In our study, we excluded patients with white coat hypertension and did not find any differences between groups in ABPM results. But we noted significant higher office SBP and DBP in patients with elevated cSBP. It could be explained by the presence of stress effect during office measurements. This stress influence was not so significant for office BP in patients with ISH and normal cSBP. The last ones had less aortic stiffness. In our further study, we plan to evaluate relationships between stress reactivity and arterial stiffness in young patients with ISH. We hypothesize that artery damage could lead to more prominent stress influence on office BP.
In the NHANES study (1999–2004 years), the obesity (OR =2.68), smoking (OR =2.06), and low socioeconomic status (OR =2.98) were major factors associated with the identification of ISH, which allowed the authors to point out the necessity of lifestyle modification in these patients.1 At the same time, in the Mahmud and Feely’s3 observational study, it was reported that the young people did not have any of additional risk factors. In our study, all subjects had elevated office and 24-hour SBP, and increased BMI was associated with increased risk of abnormal cSBP. Wilkinson et al,26 reported that the level of office DBP, but not SBP, in young was correlated better with the level of central BP. This was confirmed in our study, where the level of office DBP was independently associated with increased risk of high aortic BP.
In the study by Saladini et al,4 young subjects (n=61) with ISH 30 (49.2%) had normal level of central BP, which was higher than in our study (only 38.6%). In these patients, the compliances of large and small arteries were the same as in subjects with normal BP. In patients with high aortic BP, the stiffness was increased. In our study, we evaluated arterial stiffness by PWV measurements. In young with elevated cSBP, the mean PWVe was increased. On the one hand, it may indicate a role of central BP in the damage of arteries and necessity of antihypertensive therapy for this group of patients with ISH. On the other hand, as was mentioned earlier, aortic stiffness could increase the level of the central BP in young persons. The HARVEST study has shown that in almost all young people with ISH and elevated cSBP, the chronic hypertension was diagnosed after 10 years of follow-up and antihypertensive treatment was started. In contrast, in the group of patients with normal cSBP, the chronic hypertension rate did not differ significantly from normotensive group.4
In our study, the characteristics of target organ damage did not differ significantly between groups, except for arterial stiffness. Moreover, they were within the normal range. It could be explained by short-time hypertension (mean duration: 2.8 years) in both the groups. In addition, in patients with normal cSBP, there was no main pathophysiological factor for target organ damage – elevated aortic pressure. No biochemical parameter was correlated with cSBP. Theoretically, glucose or cholesterol levels could influence on arterial stiffness and central BP, but at baseline they were within normal range in most of our patients. The patients were young, and the time of ISH existence was very short for the manifestation of negative metabolic effects.
Our study showed that young with ISH and height ≤178 cm, weight ≥91 kg, and office DBP ≥80 mmHg, had the high probability of elevated aortic pressure level. In some papers,5,22,23 the necessity of discussing about starting the antihypertensive therapy in patients with high central BP after cardiovascular risk stratification was pointed out. Our study showed that young with ISH and three factors such as height >178 cm, weight <91 kg, and office DBP <80 mmHg had the high probability of the normal cSBP level. They most likely do not need to take drug therapy. The other young with ISH need to be provided the central BP measurements in order to choose the further management strategy.
Current European Society of Cardiology/ESH 2013 guidelines recommend lifestyle modification for young people with ISH.5 It is not still clear whether it is necessary to initiate the treatment or what antihypertensive drug of choice should be for young with ISH and elevated central BP. Morgan et al22 consider calcium antagonists, renin–angiotensin system blockers, and diuretics, the most preferable for lowering the central BP. Nair23 pointed out the sympathetic hyperactivity in young people and administration of calcium antagonists could increase BP more. In his opinion, highly selective β-blockers could be more favorable. In spite of providing some studies with ISH in young subjects, we could conclude that all statements about ISH treatment are only the expert’s opinions. There were no studies demonstrating the favorable effects of the certain antihypertensive drugs in such kind of patient’s cohort.
In addition, using central BP measurements is not routine in clinical practice and it could not be recommended for all patients with ISH. Thus, our results are important, because they simplify the stratification of young with ISH – who need to be provided the central BP measurements or be discussed about treating with antihypertensive drugs.
Disclosure
The authors report no conflicts of interest in this work.
References
Grebla R, Rodriguez C, Borrell L, et al. Prevalence and determinants of isolated systolic hypertension among young adults: the 1999–2004 U.S. National Health and Nutrition Examination Survey. J Hypertens. 2010;28(1):15–23. | ||
O’Rourke M, Vlachopoulos C, Graham R. Spurious systolic hypertension in youth. Vasc Med. 2000;5(3):141–145. | ||
Mahmud A, Feely J. Spurious systolic hypertension of youth: fit young men with elastic arteries. Am J Hypertens. 2003;16(3):229–232. | ||
Saladini F, Santonastaso M, Mos L, et al; HARVEST Study Group. Isolated systolic hypertension of young-to-middle-age individuals implies a relatively low risk of developing hypertension needing treatment when central blood pressure is low. J Hypertens. 2011;29(7):1311–1319. | ||
Mancia G, Fagard R, Narkiewicz K, et al; Task Force Members. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertension. 2013;31(7):1281–1357. | ||
White W. Blood Pressure Monitoring in Cardiovascular Medicine and Therapeutics. Clifton, NJ: Humana Press; 2001:308. | ||
Levey A, Stevens L, Schmid C, et al; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. | ||
Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525(Pt 1):263–270. | ||
Williams B, Lacy P; for the CAFE and the ASCOT (Anglo- Scandinavian Cardiac Outcomes Trial) Investigators. Impact of heart rate on central aortic pressures and hemodynamics analysis from the CAFE (conduit artery function evaluation) study: CAFE-heart rate. J Am Coll Cardiol. 2009;54(8):705–713. | ||
Nakamura M, Sato K, Nagano M. Estimation of aortic systolic blood pressure in community-based screening: the relationship between clinical characteristics and peripheral to central blood pressure differences. J Hum Hypertens. 2005;19(3):251–253. | ||
Nelson MR, Stepanek J, Cevette M, Covalciuc M, Hurst RT, Tajik AJ. Noninvasive measurement of central vascular pressures with arterial tonometry: clinical revival of the pulse pressure waveform? Mayo Clin Proc. 2010;85(5):460–472. | ||
Cameron JD, McGrath BP, Dart AM. Use of radial artery applanation tonometry and a generalized transfer function to determine aortic pressure augmentation in subjects with treated hypertension. J Am Coll Cardiol. 1998;32(5):1214–1220. | ||
McVeigh GE, Bratteli CW, Morgan DJ, et al. Age-related abnormalities in arterial compliance identified by pressure pulse contour analysis: aging and arterial compliance. Hypertension. 1999;33(6):1392–1398. | ||
Chirinos J, Zambrano J, Chakko S, et al. Aortic pressure augmentation predicts adverse cardiovascular events in patients with established coronary artery disease. Hypertension. 2005;45(5):980–985. | ||
Stein J, Korcarz C, Hurst R, et al; American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography carotid intima-media thickness task force endorsed by the society of vascular medicine. J Am Society Echocardiogr. 2008;21(2):93–111. | ||
Foppa M, Duncan B, Rohde L. Echocardiography-based left ventricular mass estimation. How should we define hypertrophy? Cardiovasc Ultrasound. 2005;3:17. | ||
Lenter C. Geigy Scientific Tables. Basel: CIBA-GEIGY Corporation; 1990:278. | ||
O’Rourke MF. From theory into practice: arterial haemodynamics in clinical hypertension. J Hypertens. 2002;20(10):1901–1915. | ||
Avolio A, Van Bortel L, Boutouyrie P, et al. Role of pulse pressure amplification in arterial hypertension experts’ opinion and review of the data. Hypertension. 2009;54(2):375–383. | ||
McEniery CM, Qasem A, Schmitt M, Avolio AP, Cockcroft JR, Wilkinson IB. Endothelin-1 regulates arterial pulse wave velocity in vivo. J Am Coll Cardiol. 2003;42(11):1975–1981. | ||
McEniery C, Wallace Y, Maki-Petaja K, et al; ENIGMA Study Investigators. Increased stroke volume and aortic stiffness contribute to isolated systolic hypertension in young adults. Hypertension. 2005;46(1):221–226. | ||
Morgan T, Lauri J, Bertram D, Anderson A. Effect of different antihypertensive drug classes on central aortic pressure. Am J Hypertens. 2004;17(2):118–123. | ||
Nair T. Isolated systolic hypertension (ISH) of the young – shifting focus from father to son. Health Sci. 2013;2(2):JS003. | ||
Reynolds K, Bowling CB, Sim JJ, Sridharan L, Harrison TN, Shimbo D. The utility of ambulatory blood pressure monitoring for diagnosing white coat hypertension in older adults. Curr Hypertens Rep. 2015;17(11):86. | ||
de la Sierra A, Vinyoles E, Banegas JR, et al. Short-term and long-term reproducibility of hypertension phenotypes obtained by office and ambulatory blood pressure measurements. J Clin Hypertens (Greenwich). Epub 2016 Feb 18:doi: 10.1111/jch.12792. | ||
Wilkinson I, Franklin S, HallI R, Tyrrell S, Cockcroft JR. Pressure amplification explains why pulse pressure is unrelated to risk in young subjects. Hypertension. 2001;38(6):1461–1466. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.