Back to Journals » Infection and Drug Resistance » Volume 16
Predictors of COVID-19 Severity in Elderly Patients Infected by Omicron in China, 18 December 2022–5 February 2023
Authors Xing Y , Li Y, Feng L , Huo R, Ma X, Dong Y, Liu D, Niu Y , Tian X , Chen E
Received 8 May 2023
Accepted for publication 6 July 2023
Published 11 July 2023 Volume 2023:16 Pages 4505—4518
DOI https://doi.org/10.2147/IDR.S418622
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Yanqing Xing,1 Yupeng Li,1 Liting Feng,1 Rujie Huo,1 Xinkai Ma,1 Yanting Dong,1 Dai Liu,1 Yuheng Niu,2 Xinrui Tian,1,* Erjing Chen1,*
1The Second Hospital of Shanxi Medical University, Taiyuan, People’s Republic of China; 2The First Hospital of Shanxi Medical University, Taiyuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xinrui Tian; Erjing Chen, The Second Hospital of Shanxi Medical University, No. 382, Wuyi Road, Xinghualing District, Taiyuan, Shanxi, People’s Republic of China, Email [email protected]; [email protected]
Purpose: To analyze the clinical characteristics and prognosis of patients hospitalized with non-severe, severe pneumonia and death in Omicron COVID-19.
Patients and Methods: We collected clinical data from 118 patients with COVID-19 in China from 18 December, 2022 and 5 February, 2023. According to the outcome, the patients were divided into non-severe group, severe group and death group. Subsequently, we statistically analyzed the general condition, clinical manifestations, laboratory parameters, NLR, MLR, PLR and HALP of these groups. We also retrospectively analyzed the possible factors affecting the prognostic regression of patients with COVID-19.
Results: A total of 118 COVID-19 patients were enrolled in this study, including 64 non-severe patients, 38 severe patients and 16 death patients. Compared with the non-severe group, T lymphocytes, B lymphocytes, Th1, Th2, Th17, Treg cells, IgA, IgG, IgM in the severe and death groups decreased more significantly (P< 0.05). The levels of myocardial markers, ALT, AST, BUN, Cr, D-dimer, fibrinogen, NLR, MLR and PLR in the severe and death groups were significantly higher than those in the non-severe group (P< 0.05). The level of HALP was significantly lower than that of non-severe group (P< 0.05). MLR is not only an independent risk factor for the transition from non-severe to severe disease, but also an independent risk factor for predicting the possibility of death in COVID-19 patients.
Conclusion: The analysis of COVID-19 patients in China showed that severe patients were older, more likely to have related complications, lower lymphocyte count, liver and kidney function disorder, glucose and lipid metabolism disorders, myocardial injury, and abnormal coagulation function, suggesting the need for early anticoagulant therapy. In addition, NLR, MLR, PLR and HALP can be used as biomarkers to evaluate the severity and prognosis of COVID-19 patients.
Keywords: COVID-19, omicron, clinical characteristics, lymphocyte subpopulation, Shanxi
Introduction
The widespread of a coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a serious global public health risk and has been classified as the sixth global public health emergency.1 COVID-19 has evolved into variant strains of different virulence and transmission, such as Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2) and Omicron (B.1.1.529).2 Omicron mutant enhances its infectivity and spreading power, causing wider spread.3,4 Omicron spread also in highly vaccinated population as health care workers (>90% immunized with 2 doses of m-RNA vaccines). Infections were all mild or asymptomatic, hence only a systematic and mandatory testing screening schedule as that enforced on health workers was able to detect all asymptomatic infections, which were by far acquired outside hospital, hence they were non-occupational.5–7 Omicron became the main circulating strain of the novel coronavirus circulating in Shanxi, China in 2022–2023.
Common symptoms in patients with Omicron COVID-19 include fever, cough and sputum, dyspnea, muscle aches, headache, fatigue, nausea and vomiting, and imaging usually shows interstitial pneumonia. Some COVID-19 patients are asymptomatic or have mild symptoms, while severe and critical with poor prognosis may develop excessive oxidative stress and cytokine release syndrome (CRS),8 which may eventually lead to acute respiratory distress syndrome (ARDS), septic shock and multi-organ failure, and even death.9 Again, in Europe in highly immunized population, by vaccination and/or natural infection, omicron was extremely infectious yet featured by low virulence. The current admission rate with Omicron is around 0.3%.10 At present, the main treatment drugs for COVID-19 pneumonia are steroid and antiviral drugs. If there is a co-infection, antibiotics are used according outcome of infected host to the results of bacterial culture.
Differences in the outcome of infected host with COVID-19 are closely related to host innate and acquired immunity. Innate immunity rapidly recognizes infection and triggers adaptive immunity. Acquired immunity includes humoral and cellular immunity, both of which work together to defend against viruses and protect the body from damage by destroying viruses in the circulatory system and killing viruses in cells.11 Humoral immunity can be acquired by natural infection and or vaccination, with evidence reporting optimal protection against with previous infection combined with 1–3 doses of COVID-19 vaccines.12 Many studies have shown that inflammation plays an important role in the progression of COVID-19 pneumonia.13,14 Severe inflammation leads to the weakening of adaptive immune response, which leads to the imbalance of immune response.15 Therefore, circulating biomarkers such as Neutrophil-to-lymphocyte ratio (NLR), Monocyte-to- lymphocyte ratio (MLR), Platelet-to-lymphocyte ratio (PLR), Hemoglobin, albumin, lymphocyte, and platelet score (HALP), which represent inflammatory and immune status, may be potential biomarkers for severity assessment and prognosis of COVID-19 patients. At present, there have been studies on NLR, MLR and PLR to predict the severity of COVID-19 patients.16,17 However, the impact of NLR, MLR and PLR on the prognosis of elderly COVID-19 patients and the impact of HALP on the severity assessment and prognosis of elderly COVID-19 patients have not been reported. Identifying early biomarkers of disease severity in COVID-19 patients has important implications for the treatment and the decrease of mortality for COVID-19.
Older patients have been reported to have higher morbidity and mortality, more comorbidities and complications, and poor prognosis.18 The elderly and people with underlying diseases are classified as high-risk groups for COVID-19.19,20 As China has a large elderly population, it is of great significance to analyze the clinical characteristics of elderly COVID-19 patients and summarize the factors that may affect their prognosis and outcomes for the guidance of treatment of elderly patients with COVID-19 and reduction of the medical economic burden. Considering the differences in genetic background, social behavior and mutations in the SARS-CoV-2 gene, and the fact that the pathogenesis of COVID-19 in severe and non-severe patients is currently not well understood, we systematically studied the clinical characteristics of omicron COVID-19 patients with different degrees of severity, and analyzed the effects of clinical metabolic, immune and other indicators. We also analyzed many factors including NLR, MLR, PLR and HALP in predicting the severity and prognosis of COVID-19. The purpose of this study is to further explore the pathogenesis of COVID-19, find biomarkers for the assessment of COVID-19, so as to provide more theoretical basis for its clinical treatment.
Materials and Methods
Study Population
We retrospectively collected clinical data from 118 patients diagnosed with Omicron COVID-19 at the Second Hospital of Shanxi Medical University between 18 December, 2022 and 5 February, 2023. The Ethics Committee of the Second Hospital of Shanxi Medical University approved the study, approval number 2023 YX 131, and received informed consent for each subject.
Diagnostic Criteria
According to the clinical classification criteria in the “Clinical Protocol for the Treatment of Novel Coronavirus Infection (Trial Version 10)” and NIH COVID-19 Treatment Guidelines,21 one or more of the following etiological and serological test results should be obtained: (1) positive nucleic acid test of novel coronavirus; (2) positive antigen test of novel coronavirus; (3) positive isolation and culture of novel coronavirus; (4) The level of novel coronavirus specific IgG antibody increased 4 times or more in the convalescent phase than in the acute phase. The classification criteria are shown in Table 1.
 |
Table 1 Classification Criteria for Severity of COVID-19 Patients |
Research Methodology
According to the clinical classification criteria in the “Clinical Protocol for the Treatment of Novel Coronavirus Infection (Trial Version 10)” and NIH COVID-19 Treatment Guidelines,21 118 patients were divided into 64 patients in the non-severe group (moderate), 38 patients in the severe group (severe + critical) and 16 patients in the death group. Demographic, clinical characteristics and laboratory test results at admission were retrieved from electronic medical records. All data were extracted by two independent researchers using a unified data collection form. Clinical data of the three groups were collected, including general information, clinical symptoms, time from onset to hospital admission, chronic underlying diseases, complications, days of hospitalisation, laboratory indicators (blood cell analysis, procalcitonin, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), liver and kidney function, myocardial enzyme profile, coagulation function, arterial blood gas analysis, Immunoglobulins (IgA, IgG, IgM), immune cytoassay, CD4+T cell subsets assay, inflammatory cytokines assay, etc.), chest CT manifestations and therapeutic drugs, etc. In addition, we added NLR, MLR, PLR, HALP to explore biomarkers that can be used to assess the severity and prognosis of COVID-19.
Statistical Analysis
SPSS24.0 and GraphPad Prism v8.3.0 were used for statistical analysis, and the measurement data were tested for normality. With data expressed as median (Interquartile range 25–75%). Comparisons between three groups of non-normally distributed continuous variables were made using the Kruskal–Wallis One Way ANOVA test. Count data were expressed as frequency (percentage), and chi-square test was used for comparison between non-severe versus (severe diseases or death). Optimal cut-off values for NLR, MLR, PLR, and HALP were calculated using receiver operating curve (ROC) analysis to maximize sensitivity and specificity based on the Youden index, and the area under the curve (AUC) was plotted. Multiple ordered logistic regression analysis was used to determine the effects of NLR, MLR, PLR and HALP on the severity of COVID-19. Kaplan-Meier analysis was used to evaluate the effects of NLR, MLR, PLR and HALP on survival time of COVID-19 patients, and to plot survival curves. Multifactorial Cox regression models were used to determine the factors associated with NLR, MLR, PLR and HALP in relation to death in patients with COVID-19, hazard ratio (HR) and 95% confidence intervals (CI) being used as common indicators to assess relative risk. Spearman correlation coefficient was used to analyze the correlation between NLR, MLR, PLR, HALP and related clinical and biochemical parameters. P<0.05 was considered statistically significant.
Results
Demographic Characteristics and Clinical Symptoms in Patients with COVID-19
Our study showed that among the 118 Omicron COVID-19 patients, there were 64 (54.2%) non-severe patients, 38 (32.2%) severe patients and 16 (13.6%) death patients. The median age of the 118 patients was 79 (68–84) years, 66 (55.9%) patients were male. There were 94 (81.0%) patients of combined chronic underlying diseases, with diabetes and hypertension as the common combined underlying diseases, 64 (54.2%) patients and 24 (20.3%) patients. Compared with the non-severe group, the median age of the severe and the death groups was older (P=0.002). During hospitalization, patients in the severe and the death groups were more likely to have complications such as respiratory failure (P<0.001), electrolyte disturbance (P<0.001), and abnormal liver function (P=0.005). Compared with the non-severe group, patients in the severe group had a longer hospital stay, while those in the death group had a shorter hospital stay, and the difference was statistically significant (P<0.001). There were no significant differences in gender, time from onset to admission, and chronic underlying diseases between the two groups (P>0.005) (Table 2).
 |
Table 2 General Demographics and Clinical Symptoms of the 118 COVID-19 Patients |
Among 118 patients, fever, dyspnea, cough and expectoration were the most common clinical symptoms, followed by chills, fatigue, poor appetite, body aches, chest tightness. Compared with the non-severe group, the incidence of cough and sputum was lower in the severe group (P=0.002 and P=0.009), and dizziness was more common in the severe group, while dizziness was not obvious in the death group (P=0.021). Patients in the death group were more likely to have chest pain symptoms than those in the non-severe and severe groups (P<0.001). There was no significant difference in other clinical symptoms between three groups (P>0.05). Detailed characteristics of the patients are provided in Table 2.
Laboratory Parameters in COVID-19 Patients
Blood Cell Analysis and Arterial Blood Gas Analysis Results and Related Derivatives Indicators Within 24 Hours After Admission
The statistical results of blood cell analysis, arterial blood gas analysis and related indicators of the three groups are shown in Table 3. Among 118 hospitalized patients, white blood cell decreased in 20 cases (16.9%) and increased in 20 cases (16.9%). Lymphocyte count decreased in 81 cases (69.5%), including 34 cases (89.5%) in severe group and 14 cases (87.5%) in death group. The results showed that compared with non-severe and severe groups, the white blood cell count and neutrophil count were significantly higher in the death group (P=0.011 and P=0.008). The lymphocyte count, platelet count in the severe and death groups was significantly lower than that in the non-severe group (P<0.001). Based on the parameters of blood cell analysis, other parameters were derived, such as NLR, MLR, PLR and HALP to explore biomarkers for assessing the disease and prognosis of COVID-19. The final results showed that NLR (P<0.001), MLR (P=0.003) and PLR (P=0.002) were significantly higher in the severe and death groups than in the non-severe group, while HALP (P<0.001) was significantly lower than in the non-severe group.
 |
Table 3 Laboratory Parameters in COVID-19 Patients Within 24 Hours After Admission |
Arterial blood of all patients was collected for blood gas analysis. The results showed that patients in the severe and death groups had lower PO2 (P<0.001), higher PCO2 (P=0.041) and lower Oxygenation index (P<0.001) compared with those in the non-severe group, and that the more severe the illness, the more significant the reduction in PO2 and Oxygenation index.
Liver and Kidney Function, Myocardial Enzyme Profile, and Coagulation Function in Patients with COVID-19
The analysis results of liver and kidney function, myocardial enzyme profile and coagulation function of patients in the three groups are shown in Table 3. The results showed that ALT (P<0.001) and AST (P<0.001) were significantly increased in the severe and death groups compared with the non-severe group, while serum total protein (P<0.001), serum albumin (P=0.003) and globulin (P=0.001) were significantly decreased in the severe and death groups. Fasting blood glucose (P<0.001) and triglycerides (P<0.001) were significantly higher in the severe and death groups than in the non-severe group. The levels of urea (P=0.003) and creatinine (P=0.008) were significantly higher in the severe and death groups than in the non-severe group, which increased as the disease progressed. CRP (P<0.001) was significantly higher in the severe and death groups than in the non-severe group, and PCT (P=0.003) was significantly higher in the death group than in the non-severe and severe groups. The increase of ESR was not statistically significant (P>0.05).
The myocardial enzyme profile and coagulation function of the three groups were statistically analyzed. The results showed that compared with the non-severe group, the myocardial markers CK (P<0.001), LDH (P<0.001), HBDH (P<0.001), CK-MB (P=0.003), Hs-cTn (P<0.001) and myoglobin (P<0.001) were significantly increased in the severe group and the death group, and the more severe the disease, the more pronounced the increase. The levels of B-type natriuretic peptide (P<0.001), D-dimer (P=0.002) and fibrinogen (P=0.015) in the severe and death groups were significantly higher than those in the non-severe group.
Immune Cell Subsets, CD4+T Cell Subsets, Cytokines and Immunoglobulins
The inflammatory storm produced by COVID–19 leads to multiple organ failure, which is an important pathogenic factor for COVID-19 critical illness and patients with long COVID-19. The T lymphocytes (P<0.001), B lymphocytes (P<0.001), Th cells (P<0.001), Ts cells (P<0.001) and NK cells (P=0.008) in the severe group and death group were significantly lower than those in the non-severe group. In CD4+T cell subsets, Th1 (P<0.001), Th2 (P=0.008), Th17 (P<0.001) and Treg (P<0.001) cells were significantly lower than those in non-severe patients. In terms of cytokines, the levels of IL-4 (P<0.001), IFN-γ (P=0.020) and TNF-α (P=0.025) were decreased in the severe and death groups, while the levels of IL-10 (P=0.002) were increased compared with those in the non-severe group. Levels of plasma immunoglobulin IgA (P<0.001), IgG (P<0.001) and IgM (P<0.001) were significantly lower in the severe group and death group than in non-severe group (Table 3).
Chest CT and Treatment in 118 Patients with COVID-19
Among the 118 patients with COVID-19, there were obvious pulmonary inflammation, among which 116 (95.8%) patients showed double-sided pneumonia on chest CT admission, 38 severe patients and 16 death patients were both double-sided pneumonia. In addition, chest CT manifestations of COVID-19 patients also include pleural thickening, hydrothorax and interstitial pneumonia. In addition to conventional symptomatic and supportive treatment, all 118 patients were given oxygen inhalation, anti-infection and antiviral therapy. The main forms of oxygen inhalation include nasal catheter oxygen inhalation, high-flow oxygen inhalation and non-invasive mechanical ventilation, in the non-severe group, nasal catheter oxygen inhalation was mainly used. With the development of the disease, only 10 patients (15.6%) needed high-flow oxygen inhalation. In the severe group, 12 patients (31.6%) needed high flow oxygen, with 4 of these patients (10.5%) requiring non-invasive mechanical ventilation as the disease progressed. In the death group, 14 patients (87.5%) required high-flow oxygen inhalation, of which 6 patients (37.5%) eventually received non-invasive mechanical ventilation. All patients received steroid therapy, including 108 (89.0%) patients with methylprednisolone sodium succinate and 10 (8.5%) patients with dexamethasone sodium phosphate. Due to the limited availability of Paxlovid,22 Azvudine was the primary antiviral drug used by patients. Since the hospitalized patients were all elderly and had more chronic underlying diseases, antibiotics were administered prophylactically, and then antibiotics should be changed according to the results of drug sensitivity. There were 58 (49.2%) patients with bacterial infection, all patients in the death group with bacterial infection, the main infection bacteria were Pseudomonas aeruginosa and Klebsiella pneumoniae. Eight patients (6.8%) had candida albicans infection and were treated with fluconazole (Table S1).
ROC Analysis of Potential Markers of NLR, MLR, PLR, and HALP to Predict Non-Severe and Severe Patients
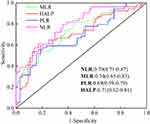
We analyzed ROC curves of the non-severe group, the severe group and the death group to predict the severity of COVID-19 (Figure 1, Table S2). Given that there is no unified laboratory reference value for NLR, MLR, PLR, and HALP to identify patients with non-severe or severe COVID-19, we performed ROC analysis to calculate the optimal threshold value. Increases in NLR, MLR, and PLR and decreases in HALP independently predicted the transition from non-severe to severe COVID-19 patients (all P<0.001). NLR had the highest AUC value and sensitivity, and HALP had the highest specificity.
Association of NLR, MLR, PLR, and HALP Results with Risk in Patients with COVID-19
The results showed that MLR [OR=0.188 (0.065–0.546), P=0.002] was an independent risk factor for the transition from non-severe to severe disease (Table 4). In addition, NLR, PLR and HALP are not independent risk factors for the transition from non-severe to severe disease.
 |
Table 4 Multivariate Ordered Logistic Regression Analysis Used to Predict the Multivariate Predictors of Different Severity Levels |
Survival Analysis
To further identify factors affecting the progression of COVID-19, four factors (NLR, MLR, PLR, and HALP) were analyzed using Kaplan-Meier curves and a multi-factor Cox regression model. After patients were classified according to Youden cutoff values obtained by the ROC curve, survival analysis revealed a significantly increased risk of death for patients with NLR>6.326, MLR>0.481, PLR>299.7 and HALP<14.474. The results were NLR (HR=0.594, 95% CI 0.377–0.933, P=0.024), MLR (HR=0.453, 95% CI 0.286–0.718, P=0.0007), PLR (HR=0.530, 95% CI 0.337–0.834, P=0.006), HALP (HR=0.479, 95% CI 0.305–0.754, P=0.002). The survival curves based on NLR, MLR, PLR and HALP are shown in Figure 2A–D. Multivariate Cox regression analysis (Figure 2E) showed that higher MLR was significantly correlated with shorter survival time (HR 0.616, 95% CI 0.402–0.943), suggesting that MLR may be an independent risk factor for predicting the likelihood of death in COVID-19.
Correlation Analysis of Potential Markers of NLR, MLR, PLR and HALP with Other Indicators
The four potential biomarkers of NLR, MLR, PLR, and HALP were evaluated for their correlation with clinical and biochemical parameters, and the results were shown in Figure S1. NLR is highly positively correlated with MLR (P<0.001), moderately positively correlated with PLR, WBC, neutrophils (P<0.001), and weakly positively correlated with BUN, LDH, myoglobin, D-dimer, and INR (P<0.001), and weakly negative correlated with lymphocytes, albumin, IgG, Th, PO2 and oxygenation index (P<0.001). MLR was moderately positively correlated with PLR and myoglobin (P<0.001), and was weakly positively correlated with WBC, neutrophils, monocytes, fasting plasma glucose, TG, LDH, D-dimer, INR and PCO2 (P<0.001), and weakly negative correlated with lymphocytes, IgA, IgG, T lymphocytes, PO2 and oxygenation index (P<0.001). PLR was weakly negatively correlated with lymphocytes, HALP, IgA, T lymphocytes, Th and NK (P<0.001). HALP was highly positively correlated to lymphocytes (P<0.001), and weakly positively correlated to Th and NK (P<0.001). Details of correlation and significance between all the collected data are show in the Supplementary File (Tables S3 and S4).
Discussion
With the continuous evolution and mutation of SARS-CoV-2, the transmission, pathogenicity and immune escape of the mutant strains are increasing,23 posing serious challenges to the global epidemic prevention and control. Omicron COVID-19 became the main circulating strain in Shanxi, China in 2022. Compared to countries in North America and Europe, where previous infection combined with 1–3 doses of COVID-19 vaccines results in high levels of pre-existing humoral immunity, the strain that first infected the elderly was Omicron COVID-19 in China, and most of them had not received the fourth dose of COVID-19 vaccine against Omicron, and the pre-existing humoral immunity was weak. There have been some studies on the clinical characteristics of COVID-19 patients.24,25 However, due to differences in genetic background and social behavior, the demographic data and clinical characteristics of elderly patients with Omicron COVID-19 in Shanxi, China have not been reported so far. Our study collected demographic and clinical data from 118 elderly patients with Omicron COVID-19 pneumonia who were admitted to the Second Hospital of Shanxi Medical University from December 2022 to February 2023, and analyzed the factors that predict the severity and prognosis of COVID-19 by NLR, MLR, PLR, and HALP. The aim is to further explore the pathogenesis of COVID-19 and find biomarkers for COVID-19 disease assessment, so as to provide reference for the prevention and treatment of COVID-19.
Several studies have previously found changes in absolute lymphocyte counts in patients with COVID-19.16,26 In the diagnosis and treatment of COVID-19 pneumonia, analysis of peripheral blood lymphocyte subsets, especially T and B lymphocytes, has become an important reference indicator in the diagnosis and treatment of COVID-19 patients.27,28 The results of blood cell analysis in 24 hours after admission showed that lymphocyte counts were significantly lower than normal in all three groups, but the decrease was more pronounced in the severe and death groups. In the immune cell subset, it was more clearly shown that T lymphocytes and B lymphocytes as well as Th and Ts cells were significantly lower in the severe and death groups than in the non-severe group, indicating that lymphocyte counts in patients with COVID-19 pneumonia correlate with the severity of the disease and decrease as the disease progresses. The greater the decrease in lymphocyte count, the more severe the patient’s disease, the more severe the lung damage, and the greater the risk of respiratory failure,29 which was also confirmed in this study. The specific mechanisms may include: (1) SARS-CoV-2 attacks the host’s immune system; (2) After SARS-CoV-2 infects the host, in order to avoid being recognized and cleared by the human immune system, the anti-inflammatory response increases and negatively regulates lymphocytes, which suppresses the function of lymphocytes and increases lymphocyte apoptosis, finally leading to a significant decrease in lymphocyte count.30 The results indicate that T lymphocytes decreased more significantly. T lymphocytes are important to prevent virus infection, and Balzanelli found31 that the CD4/CD8 ratio was low in COVID-19 patients, and Kuri-Cervantes L’s study illustrates that in COVID-19 patients, CD4+T cells may be mainly affected by pulmonary inflammatory.32 We further examined CD4+ T cell subsets in both groups of patients and found that compared with non-severe patients, Th1, Th2, Th17 and Treg cells were significantly lower in the severe and death groups. A characteristic feature of COVID-19 infection is a “cytokine storm”, or uncontrolled pro-inflammatory cytokine activity. “Cytokine storm”33,34 is associated with defects in the immune regulatory mechanisms of Treg cells, because Treg cells maintain normal self-tolerance and immune homeostasis.35–37 In patients affected by severe COVID-19, reduced Treg numbers further exacerbate the excessive inflammatory response.38,39 Host infection with SARS-CoV-2 induces virus-specific immune responses in vivo, producing large amounts of IgA, IgM and IgG in effector B cells, thereby inhibiting virus proliferation, transmission and reinfection.35 In this study, we found that the levels of IgA, IgM and IgG in the severe and death groups were significantly lower than those in the non-severe group, which may be due to the decreased number of B cells in the severe patients.
The present study also showed that patients with severe COVID-19 pneumonia were more likely to have abnormal liver and kidney function and abnormal glucose and lipid metabolism, which is consistent with the results of other studies.40,41 Therefore, elevated ALT, AST, glucose, triglycerides, and blood urea nitrogen may be indicators of poor prognosis in patients with COVID-19. In addition, we also found that CRP levels were significantly elevated in the severe and death groups, and other inflammatory markers (PCT, ESR) were also elevated to varying degrees, the more severe the disease, the more pronounced the elevation. Close monitoring of inflammatory markers is important to assess disease severity and prognosis. It has been found that patients with COVID-19 are prone to myocardial damage and even fulminant myocarditis.42 This study likewise confirmed that myocardial markers were significantly higher in the severe and death groups than in the non-severe group, therefore, it is important to prevent myocarditis during SARS-CoV-2 infection and to closely monitor patients’ cardiac function in the clinical setting. Studies have shown that B-type natriuretic peptide, D-dimer and fibrin degradation products are significantly elevated in the severe and death groups and are effective predictors of prognostic outcome in severe COVID-19 patients. Elevated D-dimer levels are associated with local pulmonary thrombosis,43,44 therefore, early anticoagulation in these patients is very important.
Many studies have shown a very high mortality rate in patients with severe COVID-1945–47 and there is an urgent need to find early indicators that predict disease progression. In order to explore biomarkers for evaluating the condition and prognosis of COVID-19 patients, we selected relevant derived indicators (NLR, MLR, PLR and HALP) in blood cell analysis and analyzed them in three groups of patients. The results showed that the NLR, MLR and PLR in the severe and death groups were significantly higher than those in the non-severe group, while HALP was significantly lower than that in the non-severe group. Analysis of NLR, MLR, PLR and HALP for predicting the severity of COVID-19 by ROC curves revealed that NLR had the highest AUC value and sensitivity, while the specificity of HALP was the highest. All of these four indexes have good predictive value for the severity of COVID-19 patients. MLR [OR=0.188 (0.065–0.546), P=0.002] was found to be an independent risk factor for the transition from non-severe to severe disease by multivariate ordered logistic regression analysis. Survival analysis showed that NLR, MLR, PLR and HALP were associated with the risk of death in patients, with statistical significance between the survival group (non-severe + severe) and the death group. Cox regression analysis suggested that MLR may be an independent risk factor for predicting the likelihood of death in COVID-19. Therefore, these four indicators can be used as biomarkers to evaluate the condition and prognosis of COVID-19 patients, and provide reference for the clinical diagnosis and treatment of COVID-19 patients. In addition, this study found that platelets were significantly lower in severe group than in non-severe group, which is consistent with other studies,48 probably because platelets play an important role in inflammatory signaling, and platelets prevent microbial invasion by combining thrombogenesis and immune recruitment functions to counteract infection factors.49
The limitation of this study lies in the single center, and the number of patients is relatively small, so further multi-center studies with a large sample size are needed to verify the results. Secondly, the subsequent quality of life of patients was not recorded in the article. We will continue to follow up and record the prognosis of these patients.
Conclusion
In summary, the analysis of Omicron COVID-19 patients with pre-existing weaker humoral immunity in Shanxi, China, found that severe COVID-19 patients were older, more likely to have related complications, lower lymphocyte count, liver and kidney function disorders, glucose and lipid metabolism disorders, myocardial damage, and abnormal coagulation function, suggesting the need for early anticoagulant therapy. Besides, the increase of NLR, MLR and PLR and the decrease of HALP can be used as biomarkers for the severity and prognosis in COVID-19 patients. Then, MLR is not only an independent risk factor for the transition from non-severe to severe disease, but also an independent risk factor for predicting the possibility of death in COVID-19 patients. Therefore, NLR, MLR, PLR and HALP can be used as practical tools to evaluate the prognosis and severity of clinical symptoms in COVID-19 patients.
Abbreviations
COVID-19, Coronavirus disease 2019; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; CRS, Cytokine release syndrome; ARDS, Acute respiratory distress syndrome; NLR, Neutrophil to lymphocyte ratio; MLR, Monocyte to lymphocyte ratio; PLR, Platelet-to-lymphocyte ratio; HALP, Hemoglobin, albumin, lymphocyte, and platelet score; CRP, C-reactive protein; ESR, Erythrocyte sedimentation rate; ALT, Alanine transaminase; AST, Aspartate aminotransferase; BUN, Blood urea nitrogen; Cr, Creatinine; PCT, Procalcitonin; CK, Creatine kinase; LDH, lactic dehydrogenase; HBDH, α-hydroxybutyrate dehydrogenase; CK-MB, Creatine kinase-MB; Hs-cTn, High sensitivity troponin; BNP, B type natriuretic peptide; PT, Prothrombin time; INR, International Normalized Ratio; ROC, receiver operating curve; AUC, area under the curve.
Data Sharing Statement
All the data of this article are available from the corresponding author upon reasonable request.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of the second Hospital of Shanxi Medical University (approval number 2023 YX 131) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all volunteers, and the anonymity of each participant was strictly preserved.
Acknowledgments
We gratefully acknowledge contributions from all authors and Funding (201903D421066 and 202203021211029).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Shanxi Provincial Key Research and Development Project (201903D421066) and Applied Basic Research Project of Shanxi Province, China (202203021211029).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Ahsan W, Alhazmi HA, Patel KS, et al. Recent advancements in the diagnosis, prevention, and prospective drug therapy of COVID-19. Front Public Health. 2020;8:384. doi:10.3389/fpubh.2020.00384
2. Wang Y, Liu M, Shen Y, et al. Novel sarbecovirus bispecific neutralizing antibodies with exceptional breadth and potency against currently circulating SARS-CoV-2 variants and sarbecoviruses. Cell Discov. 2022;8(1):36. doi:10.1038/s41421-022-00401-6
3. Zheng L, Liu S, Lu F. Impact of National Omicron Outbreak at the end of 2022 on the future outlook of COVID-19 in China. Emerg Microbes Infect. 2023;12(1):2191738. doi:10.1080/22221751.2023.2191738
4. Duan M, Duan H, An Y, et al. A booster of Delta-Omicron RBD-dimer protein subunit vaccine augments sera neutralization of Omicron sub-variants BA.1/BA.2/BA.2.12.1/BA.4/BA.5. Emerg Microbes Infect. 2023;12(1):e2179357. doi:10.1080/22221751.2023.2179357
5. Cegolon L, Ronchese F, Ricci F, Negro C, Larese-Filon F. SARS-CoV-2 infection in health care workers of Trieste (North-Eastern Italy), 1 October 2020–7 February 2022: occupational risk and the impact of the omicron variant. Viruses. 2022;14(8):1663. doi:10.3390/v14081663
6. Basso P, Negro C, Cegolon L, Larese Filon F. Risk of vaccine breakthrough SARS-CoV-2 infection and associated factors in healthcare workers of Trieste teaching hospitals (North-Eastern Italy). Viruses. 2022;14(2):336. doi:10.3390/v14020336
7. Cegolon L, Negro C, Mastrangelo G, Filon FL; ORCHESTRA working group. Primary SARS-CoV-2 infections, re-infections and vaccine effectiveness during the omicron transmission period in healthcare workers of Trieste and Gorizia (Northeast Italy), 1 December 2021–31 May 2022. Viruses. 2022;14(12):2688. doi:10.3390/v14122688
8. Elemam NM, Talaat IM, Maghazachi AA, Saber-Ayad M. Liver injury associated with COVID-19 infection: pathogenesis, histopathology, prognosis, and treatment. J Clin Med. 2023;12(5):2067. doi:10.3390/jcm12052067
9. Herrera VLM, Walkey AJ, Nguyen MQ, et al. A targetable “rogue” neutrophil-subset, [CD11b+DEspR+] immunotype, is associated with severity and mortality in acute respiratory distress syndrome (ARDS) and COVID-19-ARDS. Sci Rep. 2022;12(1):5583. doi:10.1038/s41598-022-09343-1
10. European Centre for Disease Prevention and Control. Clinical characteristics of COVID-19. Available from: https://www.ecdc.europa.eu/en/covid-19/latest-evidence/clinical.
11. Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi:10.1016/j.cell.2021.01.007
12. Stein C, Nassereldine H, Sorensen RJD; COVID-19 Forecasting Team. Past SARS-CoV-2 infection protection against re-infection: a systematic review and meta-analysis. Lancet. 2023;401(10379):833–842. doi:10.1016/S0140-6736(22)02465-5
13. Bg P, Rt C. Hyper-inflammation and complement in COVID-19. Am J Hematol. 2023;98(Suppl 4). doi:10.1002/ajh.26746
14. Aparisi Á, Martín-Fernández M, Ybarra-Falcón C, et al. Dyslipidemia and inflammation as hallmarks of oxidative stress in COVID-19: a follow-up study. Int J Mol Sci. 2022;23(23):15350. doi:10.3390/ijms232315350
15. Xiang N, Havers F, Chen T, et al. Use of national pneumonia surveillance to describe influenza A(H7N9) virus epidemiology, China, 2004–2013. Emerg Infect Dis. 2013;19(11):1784–1790. doi:10.3201/eid1911.130865
16. Ap Y, Jp L, Wq T, Hm L. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84. doi:10.1016/j.intimp.2020.106504
17. Fois AG, Paliogiannis P, Scano V, et al. The systemic inflammation index on admission predicts in-hospital mortality in COVID-19 patients. Molecules. 2020;25(23):5725. doi:10.3390/molecules25235725
18. Silverio R, Gonçalves DC, Andrade MF, Seelaender M. Coronavirus disease 2019 (COVID-19) and nutritional status: the missing link? Adv Nutr. 2021;12(3):682–692. doi:10.1093/advances/nmaa125
19. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. doi:10.1016/j.jaut.2020.102433
20. Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7(1):11. doi:10.1186/s40779-020-00240-0
21. NIH COVID-19 Treatment Guidelines. COVID-19 treatment guidelines. Available from: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/.
22. Cegolon L, Pol R, Simonetti O, Larese Filon F, Luzzati R. Molnupiravir, nirmatrelvir/ritonavir, or sotrovimab for high-risk COVID-19 patients infected by the omicron variant: hospitalization, mortality, and time until negative swab test in real life. Pharmaceuticals. 2023;16(5):721. doi:10.3390/ph16050721
23. Chaudhari AM, Joshi M, Kumar D, et al. Evaluation of immune evasion in SARS-CoV-2 delta and omicron variants. Comput Struct Biotechnol J. 2022;20:4501–4516. doi:10.1016/j.csbj.2022.08.010
24. Kannan SR, Spratt AN, Sharma K, Chand HS, Byrareddy SN, Singh K. Omicron SARS-CoV-2 variant: unique features and their impact on pre-existing antibodies. J Autoimmun. 2022;126:102779. doi:10.1016/j.jaut.2021.102779
25. Guo Y, Han J, Zhang Y, et al. SARS-CoV-2 omicron variant: epidemiological features, biological characteristics, and clinical significance. Front Immunol. 2022;13:877101. doi:10.3389/fimmu.2022.877101
26. Wang F, Nie J, Wang H, et al. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. 2020;221(11):1762–1769. doi:10.1093/infdis/jiaa150
27. Niu X, Li S, Li P, et al. Longitudinal analysis of T and B cell receptor repertoire transcripts reveal dynamic immune response in COVID-19 patients. Front Immunol. 2020;11:582010. doi:10.3389/fimmu.2020.582010
28. K A. Elucidating T cell and B cell responses to SARS-CoV-2 in humans: gaining insights into protective immunity and immunopathology. Cells. 2021;11(1). doi:10.3390/cells11010067
29. Du RH, Liu LM, Yin W, et al. Hospitalization and critical care of 109 decedents with COVID-19 pneumonia in Wuhan, China. Ann Am Thorac Soc. 2020;17(7):839–846. doi:10.1513/AnnalsATS.202003-225OC
30. Wang F, Hou H, Yao Y, et al. Systemically comparing host immunity between survived and deceased COVID-19 patients. Cell Mol Immunol. 2020;17(8):875–877. doi:10.1038/s41423-020-0483-y
31. Balzanelli MG, Distratis P, Dipalma G, et al. Immunity profiling of COVID-19 infection, dynamic variations of lymphocyte subsets, a comparative analysis on four different groups. Microorganisms. 2021;9(10):2036. doi:10.3390/microorganisms9102036
32. Kuri-Cervantes L, Pampena MB, Meng W, et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci Immunol. 2020;5(49):eabd7114. doi:10.1126/sciimmunol.abd7114
33. Helding L, Carroll TL, Nix J, et al. The cytokine storm and COVID-19. J Med Virol. 2021;93(1). doi:10.1002/jmv.26232
34. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the “Cytokine Storm” in COVID-19. J Infect. 2020;80(6):607–613. doi:10.1016/j.jinf.2020.03.037
35. Xu Z, Jiang X, Dai X, Li B. The dynamic role of FOXP3+ tregs and their potential therapeutic applications during SARS-CoV-2 infection. Front Immunol. 2022;13:916411. doi:10.3389/fimmu.2022.916411
36. Plaza-Sirvent C, Zhao B, Bronietzki AW, et al. A central role for atg5 in microbiota-dependent Foxp3+ RORγt+ treg cell preservation to maintain intestinal immune homeostasis. Front Immunol. 2021;12:705436. doi:10.3389/fimmu.2021.705436
37. Pan W, Zhu S, Dai D, et al. MiR-125a targets effector programs to stabilize Treg-mediated immune homeostasis. Nat Commun. 2015;6(1):7096. doi:10.1038/ncomms8096
38. Neurath MF. COVID-19 and immunomodulation in IBD. Gut. 2020;69(7):1335–1342. doi:10.1136/gutjnl-2020-321269
39. C S, M C, B M, et al. Regulatory T cells as predictors of clinical course in hospitalised COVID-19 patients. Front Immunol. 2021:12. doi:10.3389/fimmu.2021.789735
40. Qu J, Zhu HH, Huang XJ, et al. Abnormal indexes of liver and kidney injury markers predict severity in COVID-19 patients. Infect Drug Resist. 2021;14:3029–3040. doi:10.2147/IDR.S321915
41. He X, Liu C, Peng J, et al. COVID-19 induces new-onset insulin resistance and lipid metabolic dysregulation via regulation of secreted metabolic factors. Signal Transduct Target Ther. 2021;6(1):427. doi:10.1038/s41392-021-00822-x
42. Ammirati E, Lupi L, Palazzini M, et al. Prevalence, characteristics, and outcomes of COVID-19-associated acute myocarditis. Circulation. 2022;145(15):1123–1139. doi:10.1161/CIRCULATIONAHA.121.056817
43. Milenkovic M, Hadzibegovic A, Kovac M, et al. D-dimer, CRP, PCT, and IL-6 levels at admission to ICU can predict in-hospital mortality in patients with COVID-19 pneumonia. Oxid Med Cell Longev. 2022;2022:8997709. doi:10.1155/2022/8997709
44. T N, L D, W X, S Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4). doi:10.1111/jth.14768
45. Patel S, Truong GT, Rajan A, et al. Discharge disposition and clinical outcomes of patients hospitalized with COVID-19. Int J Infect Dis. 2023;130:1–5. doi:10.1016/j.ijid.2023.01.038
46. H C. Changes in mortality rate of the general population during the COVID-19 pandemic: an interrupted time series study in Korea. Int J Epidemiol. 2022;51(5). doi:10.1093/ije/dyac083
47. Assaad S, Avrillon V, Fournier ML, et al. High mortality rate in cancer patients with symptoms of COVID-19 with or without detectable SARS-COV-2 on RT-PCR. Eur J Cancer. 2020;135:251–259. doi:10.1016/j.ejca.2020.05.028
48. Li Q, Cao Y, Chen L, et al. Hematological features of persons with COVID-19. Leukemia. 2020;34(8):2163–2172. doi:10.1038/s41375-020-0910-1
49. Herter JM, Rossaint J, Zarbock A. Platelets in inflammation and immunity. J Thromb Haemost. 2014;12(11):1764–1775. doi:10.1111/jth.12730
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.