Back to Journals » International Journal of General Medicine » Volume 17
Predictors and Clinical Significance of Myocardial Injury in Elderly Patients Under the COVID-19 Pandemic
Received 6 January 2024
Accepted for publication 27 March 2024
Published 6 April 2024 Volume 2024:17 Pages 1369—1379
DOI https://doi.org/10.2147/IJGM.S458365
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sandul Yasobant
You Zhou,1,* Jing Dong,1,* Hao Lu2
1Department of Cardiology, Shanghai Geriatric Medical Center, Shanghai Institute of Cardiovascular Diseases, Zhongshan Hospital, Fudan University; National Clinical Research Center for Interventional Medicine; Shanghai Clinical Research Center for Interventional Medicine, Shanghai, 200032, People’s Republic of China; 2Department of Cardiology, Shanghai Institute of Cardiovascular Diseases, Zhongshan Hospital, Fudan University; National Clinical Research Center for Interventional Medicine; Shanghai Clinical Research Center for Interventional Medicine, Shanghai, 200032, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hao Lu, Department of Cardiology, Shanghai Institute of Cardiovascular Diseases, Zhongshan Hospital, Fudan University; National Clinical Research Center for Interventional Medicine; Shanghai Clinical Research Center for Interventional Medicine, 180 Fenglin Road, Shanghai, 200032, People’s Republic of China, Tel +86-21-64041990 ext 2728, Email [email protected]
Background: The elderly patients are at increased high risk of myocardial injury and mortality after the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. This study aims to investigate the prevalence, predictors and prognostic implications of myocardial injury in the elderly patients with SARS-CoV-2 infection.
Methods: Patients aged over 65 years were consecutively recruited between April to May, 2022. Myocardial injury was assessed using the high-sensitivity cardiac troponin T (hs-cTnT) assay. The primary endpoint was in-hospital mortality.
Results: A total of 347 patients were recruited with a median age of 81 years. 45.8% were male and 18 (5.2%) deceased before discharge. Myocardial injury (hs-cTnT over 99% upper reference limit [URL]) was detected in 202 (58.2%) of patients. Predictors of myocardial injury included age (per 5-year increase), hypertension, vaccination, creatine, and neutrophil-to-lymphocyte ratio. hs-cTnT over 3 × URL was independently correlated with in-hospital mortality (adjusted odds ratio [adOR], 13.21; 95% confidence interval [CI], 2.11– 87.1; p = 0.005) in comparison to hs-cTnT > URL (adOR, 0.66; 95% CI, 0.09– 5.92; p = 0.680).
Conclusion: Myocardial injury was a common phenomenon and prognostic predictor in elder patients after SARS-CoV-2 infection. Higher threshold of myocardial injury may be considered to improve risk stratification.
Keywords: COVID-19, SARS-CoV-2, myocardial injury, mortality
Introduction
In the past years, the coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a world-wide pandemic affecting the entire population. Among all those infected patients, the elderly have been acknowledged as the most vulnerable subgroup with worse clinical outcomes due to high prevalence of comorbidities, inherent fragility and low vaccination coverage.1,2 In the very recent wave of infection of the Omicron variant of SARS-CoV-2 in Hong Kong, China, 96% mortality occurred in persons aged ≥60 years.3 Since healthcare facilities could be overwhelmed facing the exponential increase of infected patients, efficient risk stratification of elderly patients and providing matched surveillance and proper treatments are crucial in the management of COVID-19.
Cardiovascular involvement is one of the most common extrapulmonary manifestations of COVID-19. Viral presence and inflammatory infiltrates have been detected within myocardium.4,5 Myocardial injury, defined by elevation of cardiac troponins, is demonstrated as a frequent complication of SARS-CoV-2 infection, independently associated with increased risks of adverse clinical outcomes.6–9 Multiple etiology of myocardial injury under the circumstance of COVID-19 has been suggested, including direct damage by viral invasion, acute myocardial infarction, particularly type 2 myocardial infarction and nonischemic causes such as myocarditis and stress cardiomyopathy.10–12 The prevalence of myocardial injury is higher in elderly patients because of comorbidities and impaired compensatory mechanisms.2,13 Meanwhile, it has also been proposed age-specific threshold of cardiac troponins should be used to better portray clinical features of the elderly.13 Hence, it would be important to investigate the characteristics and prognostic implications of myocardial injury in the elderly to permit precise risk stratification and early intervention.
In this study, we recruited patients aged >65 years who had high-sensitivity cardiac troponin T (hs-cTnT) measured during the hospitalization for COVID-19. We investigated the incidence, determinants and prognostic significance of myocardial injury. This study aims to describe the clinical characteristics of myocardial injury and acquire a deeper understanding of the cardiac involvement in elderly patients with SARS-CoV-2 infection.
Methods
Study Design and Participants
Geriatric Medical Center affiliated to Zhongshan Hospital is a designated hospital to admit aged patients infected by the omicron variant of SARS-CoV-2 in the most recent epidemic of COVID-19 in Shanghai, China.14 In this retrospective study, we consecutively recruited 347 patients who are over 65 years old and had hs-cTnT tested at least once during hospitalization from April 12, 2022, to May 16, 2022. The viral genomes were clustered into the SARS-CoV-2 BA.2.2 sub-lineage. For patients with concurrent ST-segment elevation myocardial infarction (STEMI) would be emergently transferred to other specialized medical centers for revascularization, they were excluded because of censored outcome. Besides, patients with missing cardiac troponin values, deceased within 2 hours after admission and those who were not capable of finishing informed consent were also excluded. This study was approved by the Ethics Committee of Zhongshan Hospital, Fudan University (No.: B2022-238R) and conducted in accordance with the guidelines of the Declaration of Helsinki. All participants were given written informed consent at the time of index hospitalization.
Laboratory Measurements
The earliest venous blood samples for hs-cTnT measurements were obtained within 48 hours after admission and the following tests were ordered according to patients’ condition. Troponin T was measured by an automated analyzer using a high-sensitivity assay (Roche Diagnostics) and the 99th percentile upper reference limit (URL) was 0.014 ng/mL. The presence of SARS-CoV-2 was semi-quantitatively detected by real-time reverse-transcription polymerase-chain-reaction (RT-PCR) assay for nasopharyngeal swab specimens. The open reading frame 1ab (ORF1ab) and nucleocapsid protein (N) were simultaneously tested and reported as cycle threshold (Ct) values. Clinical and biochemical data were recorded prospectively for all patients.
Definitions
Myocardial injury was defined as hs-cTnT levels above the 99th percentile URL.15 Patients were diagnosed according to the guideline on prevention and control of COVID-19 (eighth edition) established by the National Health Commission of China.16 Symptoms in mild illness included cough, sore throat, fever, cough, sputum, myalgia, fatigue, diarrhea and so on. Moderate illness was diagnosed with chest computed tomography confirmed pneumonia. Patients with COVID‐19 having severe illness were defined having one of the following criteria: (1) respiratory frequency more than or equal to 30/min, (2) oxygen saturation less than or equal to 93% at rest, (3) arterial partial pressure of oxygen/inspired oxygen fraction [PaO2/FiO2]) less than or equal to 300 mm Hg, or (4) acutely deteriorated clinical condition and rapid expansion of lesions >50% in chest imaging within 24–48 hours. Patients were considered critical meeting any of the following: (1) respiratory failure and need for mechanical ventilation, (2) shock; or (3) vital organ failure in need of intensive care. Discharge criteria were normal body temperature lasting longer than 3 days; respiratory symptoms improved significantly; Ct values of ORF1ab and N over 35 in two consecutive SARS-CoV-2 RNA tests at least 24 hours apart. In-hospital death was the primary endpoint in this study.
Statistical Analysis
Continuous and categorical variables were summarized as median (interquartile range [IQR]) and n (%), respectively. Comparison between groups was conducted by Mann–Whitney U-test, χ²-test, or Fisher’s exact test, as applicable. To explore the risk factors of myocardial injury and in-hospital death, univariable and multivariate logistic regression models were used. A spline curve analysis was used to assess the association between the continuous values of hs-cTnT and in-hospital mortality. Subsequently, the hs-cTnT level was dichotomized at the cut-off value derived from receiver operating characteristics curve and included as a binary variable in the multivariate model. Cumulative survival curves were derived from the Kaplan–Meier method and differences between curves were analyzed using multivariate Cox regression. A two-sided p value of less than 0.05 was considered statistically significant. Statistical analyses were done using R software (version 4.1.3).
Results
Study Population
A total of 347 patients were included in the final analysis. The median age was 81 years old (IQR 66.8–89.3), and 45.8% were male. Comorbidities were present in over half of patients, with hypertension being the most common comorbidity, followed by diabetes, coronary artery disease (CAD) and chronic obstructive pulmonary disease (COPD). 73.8% patients were not vaccinated, 95 (27.4%) received oral antivirals for SARS-CoV-2 (nirmatrelvir-ritonavir). Among all patients, 35 (10.2%) were severe or critical, 19 (5.5) were admitted in intensive care unit, and 7 (2.0%) were mechanically ventilated through intubation or tracheotomy. Eighteen (5.2%) patients died during hospitalization and 329 were discharged. The other clinical characteristics of patients are shown in Table 1 and Table 2.
 |
Table 1 Baseline Demographics and Clinical Characteristics According to Myocardial Injury |
 |
Table 2 Baseline Demographics and Clinical Characteristics According to Survival |
Characteristics of Patients with Myocardial Injury
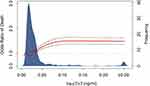
The distribution of hs-cTnT values is displayed in Figure 1. According to the fourth universal definition of myocardial infarction, myocardial injury was defined as hs-cTnT levels above 99% URL. Two hundred and two (58.2%) had concomitant myocardial injury on admission. The comparison of characteristics and outcomes of patients with and without myocardial injury is shown in Table 1. Patients with myocardial injury were older, more frequently complicated by hypertension, diabetes and CAD. Moreover, creatinine, high-sensitivity C-reactive protein (hs-CRP), neutrophil-to-lymphocyte ratio (NLR), D-dimer, and N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels were substantially higher in patients with myocardial injury. Of note, patients with myocardial injury had lower body mass index (BMI) and lower levels of albumin. Compared with patients without myocardial injury, the vaccination ratio was lower, while the rate of critical illness, ICU admission and death was significantly higher. The load of SARS-CoV-2 was semi-quantitatively assessed by Ct values. Temporal changes in Ct values from admission are demonstrated in Figure 2. Although the length of hospital stay was not significantly different between groups, the overall levels of Ct values were higher and rate of recovery were faster in patients without myocardial injury.
Risk Factors of Myocardial Injury
Based on differences between patients with and without myocardial injury observed in Table 1, univariate logistic regression was used to filtrated candidate risk factors (p value < 0.05). Afterwards, multivariate analysis was conducted to identify independent predictors of myocardial injury. As shown in Figure 3, we found that older age (per 5-year increase), hypertension, creatinine and NLR were associated with increased odds of myocardial injury, while vaccination was a major protective factor.
Characteristics of Survivors and Non-Survivors
Patients deceased during hospitalization were much older (median age: 91.0 vs 79.0 years old, p = 0.004). Compared with survivors, levels of creatine, hs-CRP, D-dimer, white blood cell count, NLR, hs-cTnT, NT-proBNP were clearly elevated in non-survivors (Table 2). In contrast, hemoglobin, platelet count and albumin were decreased in non-survivors. The difference in sex, comorbidities, vaccination and use of antivirals was not significant between groups.
Myocardial Injury and Clinical Endpoint
As shown in Figure 1, spline curve indicated that the odds of death increased with the levels of hs-cTnT. Potential predictors of mortality were evaluated with univariate logistic regression models and those with p value <0.05 entered multivariate analysis (Table 3). It was implied that hs-cTnT > URL was not associated with higher risk of mortality after adjustment (adjusted odds ratio [adOR], 0.66; 95% confidence interval [CI], 0.09–5.92; p = 0.680). We plotted receiver operating characteristic curve (Figure 4A) and found hs-cTnT above 0.052 ng/mL (3-fold URL) had the most balanced sensitivity and specificity. Multivariate regression analysis revealed hs-cTnT > 3 × URL was an independent predictor of mortality (adOR, 13.21; 95% CI, 2.11–87.1; p = 0.005). Moreover, survival curve confirmed patients with hs-cTnT more than 3-fold URL were at significantly increased risk of in-hospital death (Figure 4).
 |
Table 3 Predictors of Mortality in Multivariate Logistic Regression Models |
Discussion
In this study, we demonstrated that myocardial injury was present in over half of the elder patients with COVID-19. Older age (per 5-year increase), hypertension, creatinine, NLR and vaccination were dependently associated with myocardial injury. hs-cTnT above 3 × URL was an independent prognostic predictor of in-hospital mortality and improved risk stratification of SARS-CoV-2 infected elders.
Substantially elevated rates of morbidity and mortality have been observed in elder patients with the outbreak and fast spreading of SARS-CoV-2. Over 95% of deaths occurred in person aged ≥60 years during the fifth wave of COVID-19 in Hong Kong, China.3 From late February 2022, an epidemic of SARS-CoV-2 infection cause by the Omicron variant swept Shanghai, China. Among over 600,000 cases, a total of 568 people died with or from COVID-19 with an average age >65 years, as of May 13, 2022.14,17,18 The low coverage of vaccination and high mortality in elder patients required us to more exquisitely treat this special population on the occasion of exponentially increased burden to medical and health system.
It was acknowledged that myocardium was frequently involved in COVID-19, leading to poor prognosis. Due to inconsistency in inclusion criteria and detection assay, the reported prevalence of myocardial injury ranged from 1% to 100%.8,11,19–22 In our study, the incidence of myocardial injury was 58.2%. This high rate of myocardial injury indicated myocardium was more vulnerable in older patients. The elder patients were considered “frail” characterized by more comorbidities, poor cognitive status, weakened resistance to stressors and impaired compensatory capability.23 Vasculature aging, myocardium remodeling and immunological senescence made elders more susceptible to myocardial injury.2,13 A number of theories had been proposed accounting for myocardial injury, in which non-plaque-related causes, including type 2 myocardial infarction, myocarditis and collateral damage from the systemic immune response, seemed to be more prevalent.10,12,21 Microcirculatory dysfunction is also an important mechanism responsible for myocardial injury.24 The exaggerated systemic inflammatory response, microthrombi formation could lead to microcirculatory impairment. In addition, SARS-CoV-2 could also directly damage the microcirculation vessels. The prevalence of arrhythmia could also increase the risk of myocardial injury and poor prognosis.25 These etiologies would have an exaggerated detrimental effect on older patients compared with other populations. In this sense, the presence of myocardial injury should alert physicians to cautious assessment.
However, it has also been suggested that 99th percentile values of cardiac troponins increased with age and age-specific thresholds should be used.26,27 Over-interpretation of elevated cardiac troponins could lead to unnecessary downstream testing and excess use of anti-platelet therapy, anti-coagulants, or early coronary angiography.28 This implied maybe we should be more tolerant with elevation of troponins in elder patients. It was imperative to determine a most balanced cut-off value of cardiac troponins to facilitate clinical practice. In this study, we found the odds of death increased together with hs-cTnT values in the spline curve. Partially due to that fact that there were less cases as hs-cTnT value went higher, the curve was flattened when hs-cTnT was above 10-fold URL. Analysis of ROC implied hs-cTnT > 3 × URL was the cut-off value of with best diagnostic performance. Multivariate logistic and Cox regression models confirmed hs-cTnT > 3 × URL was superior to hs-cTnT > URL to independently predict mortality. This would inspire physicians to more rationally evaluate cardiac troponin elevation and improve risk stratification of the elder patients with COVID-19. Still, larger-scale researches are needed to draw more concise and plausible conclusions on this topic.29,30
We noticed the absolute and relative changes in hs-cTnT values were not statistically significant between groups. Since not all patients had repeated troponin measurement during hospitalization in this study, we should acknowledge the fact that patients with elevated baseline troponin levels and critical conditions were more likely to have multiple tests. This could cause bias and required further researches concerning the relationship between troponin changes and outcomes. The prevalence of use of antiplatelet drugs was higher in patients with myocardial injury, but not anticoagulation therapy (oral anticoagulation or low molecular weight heparin). This could be due to two possible reasons. On the one hand, anticoagulation was suggested as an important therapy in the treatment of patients with COVID-19, especially those with high risk of thrombosis. Older patients with high incidence of comorbidities and immobility were prone to be treated with anticoagulation medication, this could close the gap between groups. Besides, considering the increased risk of bleeding, patients revealing myocardial injury may be more likely to be prescribed with antiplatelet drugs rather than anticoagulation. The effects of different therapies in cardiovascular events and prognosis need to be validated in the future studies.
In this study, creatine was significantly associated with the risk of in-hospital mortality in univariate models but not after adjustment for cTnT > 3 × URL. We believe there could be several explanations. First, the level of cTnT was closely related with the kidney function. The worse the kidney function is, the higher cTnT tends to be. Hence, adjustment for both cTnT and creatine would weaken the impact of kidney function. Besides, we noticed the values of creatine varied greatly in patients who deceased during hospitalization. This could partially be due to the moderate population size. The deviation of creatine values would influence the odds ratio of kidney function. Further studies are pending to provide more evidence in the future.
Among independent determinants of myocardial injury, high NLR was associated with increased odds of myocardial injury. NLR was considered a surrogate marker of systemic inflammation with combined information of neutrophils and lymphocytes. Decreased lymphocytes, especially CD8+ T cells, was significantly associated with inflammatory status, COVID-19 severity and treatment efficacy.31 Besides, it was reported that NLR was positively correlated with disease severity in COVID-19 and could be a potential predictor of prognosis.32 In this study, the classic inflammatory biomarker, that is, hs-CRP, was elevated in patients with myocardial injury but not an independent risk factor. Since NLR could be conveniently derived from blood routing results, NLR seemed to be a promising maker of inflammation and disease severity to guide clinical practice in treating COVID-19.
Vaccination was shown to be the only protective factor of myocardial injury in older patients infected by the omicron variant of SARS-Cov-2. Although the omicron variant was reported to be less virulent, the incidence of myocardial injury was still substantial in elderly patients. The prevalence of patients receiving 2 to 3 doses of vaccination was much higher in those without myocardial injury. In addition, we found that in patients without myocardial injury, not only the baseline Ct values was higher, the rate of increase of Ct values was also faster. Ct value was a semi-quantitative measure of viral RNA levels. Hence, this suggested the extent of myocardial involvement was associated with virus load and the rate of clearance, and vaccination could play a cardioprotective role by decreasing peak virus load and accelerating virus clearance. The omicron variant of SARS-Cov-2 has been reported to be more competent in immune escape. Our findings implied that vaccination, maybe at least two doses were needed, was still effective in cardiac protection. Containing the virus’s spread and enhancing immunity were crucial measurement to protect the elder patients.
The level of albumin was lower in both patients with myocardial injury and non-survivors. Multivariate analysis also indicated serum albumin was inversely associated with the risk of in-hospital death. Albumin used to be considered as an indicator of malnutrition. As an acute-phase reactant, low levels of albumin could reflect the underlying status of activated inflammation. However, albumin levels should not be merely interpreted as an epiphenomenon.33 Albumin served as an antioxidant protein, which could bind reactive oxygen species and nitrogen reactive species, modifying the redox state in the plasma so as to protect against the cytokine storm.34,35 Moreover, albumin not only exerted antiplatelet properties to inhibit platelet inactivation and clotting but also played an important role in maintaining osmolality and microcirculatory homeostasis.36,37 It was reported that low levels of albumin were associated with longer recovery from pathology and increased risk of mortality.38 Otherwise, supplement of albumin revealed controversial results.39,40 Deeper investigation of albumin in elder patients is pending in the future.
This study has some limitations. First, this is a single-centered study with a moderate size. Multi-centered researches are needed to draw more persuasive conclusions. Second, in this study, the median age of participants was over 80 years. Patients with STEMI were excluded, so data regarding drastically elevated values of hs-cTnT was lacking. Of note, the Omicron variant of SARS-CoV-2 was the predominant pathogen in this epidemic of COVID-19 outbreak. Therefore, the results should be interpreted cautiously when applied to other populations. Third, cardiac ultrasound and magnetic resonance imaging were not available when the study was conducted. Researches providing these data would help us to have a better understanding of cardiac involvement in COVID-19. Finally, our results are more hypothesis-generating than conclusive, studies in larger scale with longer duration are pended to complement our research.
Conclusions
In conclusion, we reported that myocardial injury was a prevalent and prognostically significant phenomenon in elder patients with COVID-19. hs-cTnT > 3 × URL maybe more appropriate to stratify patients at higher mortality risk.
Data Sharing Statement
The data supporting this article would be available upon reasonable request by contacting the corresponding authors.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No:82370261).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi:10.1016/S0140-6736(20)30566-3
2. Moccia F, Gerbino A, Lionetti V, et al. COVID-19-associated cardiovascular morbidity in older adults: a position paper from the Italian Society of Cardiovascular Researches. Geroscience. 2020;42(4):1021–1049. doi:10.1007/s11357-020-00198-w
3. Smith DJ, Hakim AJ, Leung GM, et al. COVID-19 mortality and vaccine coverage - Hong Kong Special Administrative Region. MMWR Morb Mortal Wkly Rep. 2022;71(15):545–548. doi:10.15585/mmwr.mm7115e1
4. Lindner D, Fitzek A, Brauninger H, et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5(11):1281–1285. doi:10.1001/jamacardio.2020.3551
5. Schurink B, Roos E, Radonic T, et al. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe. 2020;1(7):e290–e299. doi:10.1016/S2666-5247(20)30144-0
6. Nuzzi V, Merlo M, Specchia C, et al. The prognostic value of serial troponin measurements in patients admitted for COVID-19. ESC Heart Fail. 2021;8(5):3504–3511. doi:10.1002/ehf2.13462
7. Poterucha TJ, Elias P, Jain SS, et al. Admission cardiac diagnostic testing with electrocardiography and troponin measurement prognosticates increased 30-day mortality in COVID-19. J Am Heart Assoc. 2021;10(1):e018476. doi:10.1161/JAHA.120.018476
8. Arnau-Barres I, Pascual-Dapena A, Lopez-Montesinos I, et al. Prevalence and prognostic value of myocardial injury in the initial presentation of SARS-CoV-2 infection among older adults. J Clin Med. 2021;10(16):3738. doi:10.3390/jcm10163738
9. Maloberti A, Biolcati M, Giannattasio C. Troponin elevation in COVID-19 patients: an important stratification biomarker with still some open questions. Int J Cardiol. 2021;341:107–109. doi:10.1016/j.ijcard.2021.07.049
10. Cheng R, Leedy D. COVID-19 and acute myocardial injury: the heart of the matter or an innocent bystander? Heart. 2020;106(15):1122–1124. doi:10.1136/heartjnl-2020-317025
11. Sandoval Y, Januzzi JJ, Jaffe AS. Cardiac troponin for assessment of myocardial injury in COVID-19. J Am Coll Cardiol. 2020;76(10):1244–1258. doi:10.1016/j.jacc.2020.06.068
12. Imazio M, Klingel K, Kindermann I, et al. COVID-19 pandemic and troponin: indirect myocardial injury, myocardial inflammation or myocarditis? Heart. 2020;106(15):1127–1131. doi:10.1136/heartjnl-2020-317186
13. Obas V, Vasan RS. The aging heart. Clin Sci. 2018;132(13):1367–1382. doi:10.1042/CS20171156
14. Zhang X, Zhang W, Chen S. Shanghai’s life-saving efforts against the current omicron wave of the COVID-19 pandemic. Lancet. 2022;399:2011–2012. doi:10.1016/S0140-6736(22)00838-8
15. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction. J Am Coll Cardiol. 2018;72(18):2231–2264. doi:10.1016/j.jacc.2018.08.1038
16. Chinese National Health Commission. Guideline on prevention and control of COVID‐19. Available from: http://www.nhc.gov.cn/yzygj/s7653p/202008/0a7bdf12bd4b46e5bd28ca7f9a7f5e5a/files/a449a3e2e2c94d9a856d5faea2ff0f94.pdf.2022.
17. Shanghai Municipal Health Commission. Home page; 2022. Available from: https://wsjkw.sh.gov.cn/yqtb/index.html.
18. Shanghai Municipal Health Commission. Shanghai Municipal Administrator of Traditional Chinese Medicine. [Homepage on the internet] Pandemic notification. Available from: https://wsjkw.sh.gov.cn/xwfb/20220514/5680eabd2c7741118cddb443e3e671ce.html.
19. Doyen D, Dupland P, Morand L, et al. Characteristics of cardiac injury in critically ill patients with Coronavirus Disease 2019. Chest. 2021;159(5):1974–1985. doi:10.1016/j.chest.2020.10.056
20. De Michieli L, Ola O, Knott JD, et al. High-sensitivity cardiac troponin T for the detection of myocardial injury and risk stratification in COVID-19. Clin Chem. 2021;67(8):1080–1089. doi:10.1093/clinchem/hvab062
21. Roh JD, Kitchen RR, Guseh JS, et al. Plasma proteomics of COVID-19-associated cardiovascular complications: implications for pathophysiology and therapeutics. JACC Basic Transl Sci. 2022;7:425–441. doi:10.1016/j.jacbts.2022.01.013
22. Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63(3):390–391. doi:10.1016/j.pcad.2020.03.001
23. Nishihira K, Yoshioka G, Kuriyama N, et al. Impact of frailty on outcomes in elderly patients with acute myocardial infarction who undergo percutaneous coronary intervention. Eur Heart J Qual Care Clin Outcomes. 2021;7(2):189–197. doi:10.1093/ehjqcco/qcaa018
24. Montone RA, Iannaccone G, Meucci MC, Gurgoglione F, Niccoli G. Myocardial and microvascular injury due to Coronavirus Disease 2019. Eur Cardiol. 2020;15:e52. doi:10.15420/ecr.2020.22
25. Maloberti A, Giannattasio C, Rebora P, et al. Incident atrial fibrillation and In-hospital mortality in SARS-CoV-2 patients incident atrial fibrillation and In-hospital mortality in SARS-CoV-2 patients. Biomedicines. 2022;10(8):1940. doi:10.3390/biomedicines10081940
26. Monneret D, Gellerstedt M, Bonnefont-Rousselot D. Determination of age- and sex-specific 99th percentiles for high-sensitive troponin T from patients: an analytical imprecision- and partitioning-based approach. Clin Chem Lab Med. 2018;56(5):818–829. doi:10.1515/cclm-2017-0256
27. Gore MO, Seliger SL, Defilippi CR, et al. Age- and sex-dependent upper reference limits for the high-sensitivity cardiac troponin T assay. J Am Coll Cardiol. 2014;63(14):1441–1448. doi:10.1016/j.jacc.2013.12.032
28. Chapman AR, Bularga A, Mills NL. High-sensitivity cardiac troponin can be an ally in the fight against COVID-19. Circulation. 2020;141(22):1733–1735. doi:10.1161/CIRCULATIONAHA.120.047008
29. Vu VH, Nguyen TC, Pham QDD, Pham DN, Le LB, Le KM. Prevalence and impact of myocardial injury among patients hospitalized with COVID-19. Front Cardiovasc Med. 2023;10:1202332. doi:10.3389/fcvm.2023.1202332
30. Rinaldi R, Basile M, Salzillo C, et al.; Of The Gemelli Against Covid Group. Myocardial injury portends a higher risk of mortality and long-term cardiovascular sequelae after hospital discharge in COVID-19 survivors. J Clin Med. 2022;11(19):5964. doi:10.3390/jcm11195964
31. Wang F, Nie J, Wang H, et al. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. 2020;221(11):1762–1769. doi:10.1093/infdis/jiaa150
32. Li X, Liu C, Mao Z, et al. Predictive values of neutrophil-to-lymphocyte ratio on disease severity and mortality in COVID-19 patients: a systematic review and meta-analysis. Critical Care. 2020;24(1):647. doi:10.1186/s13054-020-03374-8
33. Violi F, Cangemi R, Romiti GF, et al. Is albumin predictor of mortality in COVID-19? Antioxid Redox Signal. 2021;35(2):139–142. doi:10.1089/ars.2020.8142
34. Arques S. Human serum albumin in cardiovascular diseases. Eur J Intern Med. 2018;52:8–12. doi:10.1016/j.ejim.2018.04.014
35. Arroyo V, García-Martinez R, Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol. 2014;61(2):396–407. doi:10.1016/j.jhep.2014.04.012
36. Lam FW, Cruz MA, Leung HC, Parikh KS, Smith CW, Rumbaut RE. Histone induced platelet aggregation is inhibited by normal albumin. Thromb Res. 2013;132(1):69–76. doi:10.1016/j.thromres.2013.04.018
37. Arques S, Ambrosi P. Human serum albumin in the clinical syndrome of heart failure. J Card Fail. 2011;17(6):451–458. doi:10.1016/j.cardfail.2011.02.010
38. Eckart A, Struja T, Kutz A, et al. Relationship of nutritional status, inflammation, and serum albumin levels during acute illness: a prospective study. Am J Med. 2020;133(6):713–722. doi:10.1016/j.amjmed.2019.10.031
39. Caraceni P, Domenicali M, Tovoli A, et al. Clinical indications for the albumin use: still a controversial issue. Eur J Intern Med. 2013;24(8):721–728. doi:10.1016/j.ejim.2013.05.015
40. Fan E, Stewart TE. Albumin in critical care: SAFE, but worth its salt? Crit Care. 2004;8(5):297–299. doi:10.1186/cc2943
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.