Back to Journals » Patient Related Outcome Measures » Volume 13
Predictive Value of the (Quick)DASH Tool for Upper Extremity Dysfunction Following Percutaneous Coronary Intervention
Authors Zwaan E, Cheung E , IJsselmuiden A, Holtzer C, Schreuders T, Kofflard M, Alings M, Coert JH
Received 14 December 2021
Accepted for publication 8 April 2022
Published 27 June 2022 Volume 2022:13 Pages 145—155
DOI https://doi.org/10.2147/PROM.S353895
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lynne Nemeth
Eva Zwaan,1,* Elena Cheung,1,2,* Alexander IJsselmuiden,2 Carlo Holtzer,3 Ton Schreuders,4 Marcel Kofflard,5 Marco Alings,2 J Henk Coert1
1Department of Plastic and Reconstructive Surgery, University Medical Center Utrecht, Utrecht, the Netherlands; 2Department of Cardiology, Amphia Hospital, Breda, the Netherlands; 3Department of Plastic and Reconstructive Surgery, Albert Schweitzer Hospital, Dordrecht, the Netherlands; 4Department of Plastic, Reconstructive and Hand Surgery, Erasmus Medical Center, Rotterdam, the Netherlands; 5Department of Cardiology, Albert Schweitzer Hospital, Dordrecht, the Netherlands
*These authors contributed equally to this work
Correspondence: Elena Cheung, Department of Plastic and Reconstructive Surgery, University Medical Center Utrecht, Heidelberglaan 100, Utrecht, 3584 CX, the Netherlands, Tel +31 88 755 6954, Email [email protected]
Purpose: The use of the Disability of the Arm, Shoulder and Hand (DASH) questionnaire and its shortened version, the QuickDASH, have been used to assess upper extremity function following a transradial percutaneous coronary intervention (TR-PCI). However, the use of these scores has not yet been validated for TR-PCI induced complications in the upper extremity. The aim of this study was to establish the validity of the DASH and the QuickDASH, for the assessment of upper extremity dysfunction following a transradial percutaneous coronary intervention (TR-PCI).
Patients and Methods: This study was a diagnostic retrospective analysis of the ARCUS study, of whom 440 underwent TR-PCI and 62 control patients were treated via the transfemoral approach. All participants completed the DASH and QuickDASH questionnaire prior to the procedure and at each follow-up visit up to six months of follow-up. Receiver operating characteristics (ROC) were constructed to determine the validity of the questionnaires, using physical examinations to determine the occurrence of upper extremity dysfunction, according to the ARCUS study.
Results: At each follow-up moment, the area under the curve (AUC) showed a poor ability of the DASH and QuickDASH to discriminate between patients with and without upper extremity dysfunction (AUC: 0.565– 0.586). There was no significant difference between the questionnaires (p > 0.05).
Conclusion: The DASH and QuickDASH questionnaires are both equally incapable of discriminating between patients with and without upper extremity dysfunction following a TR-PCI. Study results suggest that the DASH and QuickDASH questionnaires are incapable of discerning changes in upper extremity function as a result of procedural complications following a TR-PCI vs cardiac induced activity cessation.
Keywords: patient reported outcome measures, PROM, coronary angiography, upper extremity, patient outcomes, transradial access
Introduction
The Disability of the Arm, Shoulder and Hand (DASH) questionnaire is a 30-item, patient-reported questionnaire that measures physical function and symptoms in people with musculoskeletal disorders of the upper extremity.1–3 The shortened version, the QuickDASH, an 11-item questionnaire, was designed to improve practicality and item redundancy.4,5 A few examples of conditions where the QuickDASH can be used with similar precision as the DASH are as follows: Carpal Tunnel Syndrome, ganglion- and shoulder disorders.6,7
Transradial Percutaneous Coronary Intervention (TR-PCI) has become the gold standard in treating coronary artery stenosis.8 Transfemoral approach is an alternative access route when a patient presents with (relative) contra-indications for TR-PCI: eg previously failed transradial approach or radial artery occlusion. Compared to femoral access, in patients with ST-Segment Elevation Myocardial Infarction (STEMI), radial access caused 73% less major access bleeding, with a trend towards reduction of mortality and ischemic events.9
The QuickDASH has been used to assess the upper extremity function following TR-PCI.10–12 The use of these patient-reported outcome measures (PROMs), however, has not yet been validated for TR-PCI induced complications in the upper extremity. The objective of this analysis was to establish the validity of the DASH and QuickDASH questionnaires for the assessment of upper extremity dysfunction (UED) following TR-PCI. Furthermore, the validity and interchangeability of these PROMs were assessed. We hypothesized that patients undergoing TR-PCI without UED would report similar (Quick)DASH scores when compared to patients with UED.
Materials and Methods
This study is a retrospective sub-analysis of the Effects of trAnsRadial perCUtaneouS coronary intervention on upper extremity function (ARCUS) study, a multicenter prospective cohort study performed in the Netherlands, providing insight into access-site morbidity and UED following TR-PCI.13,14 The upper extremity function following a TR-PCI was assessed using a combination of objective measurements (eg strength and sensibility measurements) and the DASH.14
Patients were included in the ARCUS study, if they presented for a percutaneous coronary intervention, had a palpable radial artery, and if Doppler ultrasound examination confirmed a non-occluded flow. Patients underwent clinical assessments of the upper extremity and completed two self-administered questionnaires (the DASH and Numeric Rating Scale for Pain (NRSP) prior to the index procedure. Procedures using the contralateral radial artery or femoral artery after previous unsuccessful attempts were also included in this study.13
Exclusion criteria were 1) absence of informed consent, 2) an absent signal of the radial artery objectified by Doppler ultrasound, 3) illiteracy, and 4) incapability to accomplish measurements due to comorbidities.
Questionnaires
The NRSP and the DASH were evaluated on the day of the procedure, and at 2-week, 1-month, and 6-months of follow-up. The NRSP has an 11-point scale, from “0: no pain at all” to “10: worst imaginable pain”.15 The DASH questionnaire consists of 30-items: 24 items to assess function/disability and six items to measure symptoms.16 Each item is scored on a five-point scale. The total of the item-scores are used to calculate the DASH score ranging from 0 to 100, with a higher DASH score indicating a poorer level of function.3
The 11-items that comprise the QuickDASH were extracted from the DASH questionnaire and had the same five-point scale per item.4,5 The QuickDASH has eight function/disability items and three symptom items.4
Reference Test for Upper Extremity Function
The physical status of the upper extremity was examined by assessing tactile sensation using the Weinstein Enhanced Sensory Test (WEST), with results presented on a five-point scale (1: 0.05 gr; 2: 0.20 gr; 3: 2.00 gr; 4: 4.00 gr; 5: 300.0 gr).17 The palmar grip and key-pinch strength measurements were measured using a Jamar hydraulic hand dynamometer and Jamar hydraulic pinch gauge (Jamar, Performance Health, Warrenville, Illinois, United States).18 Isometric strength of flexion and extension of the elbow and wrist were assessed using the microFET2 digital handheld dynamometer (microFET2, Hogan Scientific, Salt Lake City, Utah, United States).19 For both arms, the palmar grip, key-pinch and isometric strength were documented as the mean of three repeated measurements. Additionally, the circumference of the hand was measured using a figure-of-eight method, whereas circumference of the forearm was measured circumferentially at eight cm distal of the medial epicondyle of the humerus, both noted in centimeters.20,21
All examinations were performed by a dedicated study member, trained and certified by a hand rehabilitation specialist. Clinical information and (Quick)DASH results were available to the investigators who performed the clinical assessment of the upper extremity.
A precise and published definition of upper extremity function does not exist, therefore hand experts tried to capture a definition for upper extremity function with the help of several examinations, performed according to the American Society of Hand Therapist (ASHT).22 A binary score, absence or presence of UED, was composed out of eight pre-specified criteria. The presence of the UED is defined as ≥2 criteria present as specified in Table 1.14
 |
Table 1 Upper Extremity Dysfunction Criteria |
Procedure
All patients were treated with conventional 6 French introducer sheaths and catheters, after which non-patent hemostasis of the radial artery was achieved by using a compression device (Terumo Medical Corporation, Japan). For the transfemoral group, an implantable device (Angio-Seal) was used to achieve hemostasis. Complications were noted during the procedure and clinical outpatient visits, and when necessary, patients were referred to a hand specialist.
Handling of Incomplete Responses
For this sub-analysis, only patients with complete baseline data for the questionnaires and clinical assessments were included. A minimum of, respectively, 27 or 10 completed items of the DASH and QuickDASH was required to consider the questionnaires complete.7 An incomplete questionnaire resulted in exclusion from the analysis.
Data Analysis
Descriptive statistics were provided for all variables. Results were presented as mean with standard deviation (SD), counts, and/or percentages. Non-normally distributed results were presented as median with interquartile range (IQR). The baseline and follow-up scores for the (Quick)DASH were calculated for the transradial and transfemoral approach group. Between-group differences of dichotomous variables were analyzed using Chi-square test with Yates continuity correction. Categorical variables with at least two categories were analyzed with the Pearson’s chi-squared test. For normally distributed variables, an independent sample t-test was used, and for continuous variables that were not normally distributed, the Mann–Whitney U-test was applied. A Spearman rank test was performed to evaluate the correlation between changing (Quick)DASH scores and the severity of UED.
To study the validity of the scoring of the (Quick)DASH, the extracted scores were correlated to the upper extremity function as was found during the physical examination.
Receiver operating characteristics (ROC) were constructed using change in (Quick)DASH scores (baseline vs follow-up) as the test variable and patients’ UED status (“UED” and “no UED”) as the classifying variable (Table 1).23 The difference in the areas under the ROC curves (AUC) for the two questionnaires was compared for each consecutive follow-up moment. An AUC of approximately (0.5), suggested no discrimination between patients with and without UED, 0.7–0.8 was considered acceptable, 0.8–0.9 was considered excellent, and an AUC exceeding 0.9 was considered outstanding.23
The minimal clinically important difference (MCID) score that corresponds to a clinically relevant decrease in upper extremity function was an increase exceeding 15 points for the DASH questionnaire and eight points for the QuickDASH questionnaire, respectively.24–26 Differences in the occurrence of a score greater than the MCID thresholds between the UED group and non-UED group and between the referral and no referral group were analyzed with Pearson’s chi-squared test.
All the tests were two-sided, with a statistical significance level set at 5%. Statistical analyses were performed with SPSS for Apple version 26 (IBM Corp, Armonk, NY) and R Statistics for Apple version 3.6.2 (The R Foundation, Austria).
The ARCUS study was conducted in accordance with the Declaration of Helsinki and was approved by the regional ethics committee “Toetsingscommissie Wetenschappelijk Onderzoek” (TWOR) (Rotterdam, the Netherlands). The TWOR has been merged with the regional ethics committee “Medical Research Ethics Committees United” (MEC-U) in 2019 (Nieuwegein, the Netherlands) (NL45613.101.13). All TWOR research files have been taken over by the MEC-U. The ARCUS study was registered at ClinicalTrials.gov (NCT02204423).
For this sub-analysis, permission was obtained to utilize data gathered through the ARCUS study. Written informed consent was obtained before study participation, including consent to be published. All patient data were anonymised and stored for at least 15 years at the study site.
Results
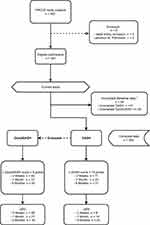
All procedures were performed between January 2014 and July 2018. Five hundred and two patients were included in the ARCUS study of whom 440 underwent an elective intervention via the transradial approach (TRA) and 62 via the transfemoral approach (TFA). Five patients were considered ineligible because they did not meet the inclusion criteria. Figure 1 shows the patient enrollment flowchart. Complete baseline data on the DASH and QuickDASH questionnaires were obtained from 463 patients. In 34 (6.8%) patients, baseline questionnaire data were incomplete. Incomplete or absent questionnaire data was caused by subject withdrawal or lost to follow-up (Figure 1). The transradial and transfemoral groups were similar in all baseline characteristics except for family history of heart disease (Table 2).
 |
Table 2 Baseline Characteristics of the Study Sample |
Upper Extremity Dysfunction
A total of 96 (32.7%) patients in the transradial group developed UED after 2-week follow-up vs five patients (13.9%) in the transfemoral group (p = 0.03). This evolved to 99 patients (32.0%) at 1-month follow-up in the transradial group and 14 patients (31.8%) in the transfemoral group (p = 1.00). At 6-months follow-up 134 patients (44.4%) in the transradial group and 24 patients (57.1%) in the transfemoral group developed UED (p = 0.16).
Questionnaire Scores
The median DASH score at baseline in the total study population was 0.83 (IQR 7.28). The median baseline QuickDASH score was 0.00 (IQR 9.09). The median DASH score at baseline and at 2-week follow-up was significantly higher in the radial group vs the transfemoral control group (p < 0.05) (Table 3). Similar results were observed when the baseline QuickDASH score was compared between the radial- and transfemoral control groups (p < 0.01) (Table 3). A Mann–Whitney U-test revealed a significant difference for both DASH and QuickDASH scores at two weeks, at the disadvantage of the transradial group, as they had higher scores for disability (Table 3).
 |
Table 3 DASH and QuickDASH Score Between the Transradial Group and Transfemoral Group |
The correlation coefficient between the change in DASH or QuickDASH score (difference baseline-follow-up) and UED severity was 0.14 (p = 0.02) and 0.16 (p = 0.006), respectively, at 2-week follow-up. At 1-month follow-up, a correlation coefficient of 0.12 (p = 0.03) and 0.14 (p = 0.01) was found for the DASH and QuickDASH scores, respectively. At 6-months follow-up, the correlation coefficient was 0.13 (p = 0.02) and 0.12 (p = 0.02) for the DASH and QuickDASH, respectively. All correlation coefficients indicate a very weak association between increasing DASH and QuickDASH scores with UED severity.
There was a significant difference between the UED and non-UED group, in the occurrence of a DASH score increase (baseline – follow-up) exceeding the MCID threshold (>15 points) at 1-month (11.2% vs 2.8%, respectively, p = 0.01) and 6-months follow-up (12.2% vs 4.1%, respectively, p = 0.01) (Table 4). For the QuickDASH, a significant difference between the UED and non-UED groups in a score exceeding the MCID threshold (>8 points) was found at 2-week and 6-months follow-up (p < 0.001) (Table 4).
 |
Table 4 Difference in the Occurrence of a Score Greater Than the MCID* Threshold Between the UED Group and Non-UED Group |
Referred Patients
The referral rate to a hand specialist during the total follow-up period was 18%: four subjects at day of procedure, 24 subjects at 2-week follow-up, 23 subjects at 1-month follow-up and 28 subjects at 6-months follow-up.
When comparing the referred group to the non-referred group, a significant difference in DASH score increase (baseline – follow-up) exceeding the MCID (>15 points), was found at 1-month follow-up (36.7% vs 4.6%, respectively, p < 0.001) and 6-months follow-up (28.6% vs 6.8%, respectively, p = 0.002). For the QuickDASH, a significant difference in the occurrence of a MCID (>8 points) between the referred- and the non-referred group was found at every executive follow-up moment: 2-week follow-up (50.0% vs 11.8%, respectively, p < 0.001), 1-month follow-up (47.4% vs 10.1%, respectively, p < 0.001) and 6-months follow-up (56.5% vs 13.5%, respectively, p = 0.002).
Diagnostic Ability of the DASH and QuickDASH
The AUC of the DASH and QuickDASH for UED was 0.566 and 0.586, respectively, at 2-week follow-up (Figure 2). These scores indicate a poor ability to discriminate between patients with- and without UED (Figure 2). The AUC was 0.565 and 0.565 at 1-month follow-up and 0.578 and 0.576 at 6-months of follow-up, for the DASH and QuickDASH, respectively.
The difference in the AUC between the DASH and QuickDASH at 2-week follow-up was 0.02 (p = 0.73), indicating that both questionnaires were equally incapable of discriminating between patients with- and without UED (Figure 2). At 1-month follow-up, the difference in the AUC for the DASH and QuickDASH was 0.00 (p = 1.00) and the difference in the AUC at 6-months follow-up was −0.002 (p = 0.96) between the DASH and QuickDASH (Figure 2).
Discussion
This study investigated the validity of the DASH and QuickDASH in the evaluation of post-PCI upper extremity dysfunction, controlled by objective functional measurements. Both objective measurements and self-reported questionnaires indicated that PCI does affect upper extremity function.
In contrast to previously published literature, the UED at two weeks following TR-PCI as measured in our study increased significantly over time, and showed a significant difference in occurrence of UED between the transradial and transfemoral approaches.10,11 Furthermore, referral to a hand specialist was arranged in 18% patients during follow-up, indicating clinically relevant complaints. The DASH questionnaire was chosen because the individual’s quality of life and ability to do activities regardless of which upper extremity they use is also of interest.1
The considerable number of patients with complaints in the control group can be explained by an increased focus and awareness in study subjects regarding the upper extremity function. This could lead to excessive referral, which is most likely not a result of TR-PCI.
The high number of patients with complaints in both groups could also be explained by the initial reaction to anxiety following a myocardial infarct. Previous studies consistently report that almost one-fifth of patients experience clinical levels of anxiety and depression symptoms shortly following the myocardial infarct.26–28 Patients with distress are more likely to reduce their everyday activities and physical exercise up to one year after the myocardial infarct.29 Additionally, this distress and anxiety, in combination with delayed access (>4 weeks) to a cardiac rehabilitation program, can result in decreased physical activity, resulting in detraining of the (upper extremity) muscles.30
The DASH and QuickDASH are upper extremity-specific PROMs. A previous study that investigated whether the DASH questionnaire exclusively measured disability associated with upper extremity injuries found that the DASH questionnaire also measured disability in patients with injuries to the lower limb.31 A possible explanation was the healing process following the lower limb injury that interfered with the mobility and stability of the upper limb, for example, by using crutches.31 It was hypothesized that the increased scores of the DASH and the QuickDASH in the transfemoral group represent the overall disability and inactivity in patients with a cardiovascular condition as the primary impairment instead of upper extremity-specific disability. The function items on the QuickDASH refer to heavier tasks requiring greater strength than the function items of the DASH, possibly giving a distorted representation in cardiac patients with impaired exercise capacity. Angst et al demonstrated that the QuickDASH underestimates symptoms but overestimates disability and does not measure the same symptoms content as the DASH.32 The QuickDASH outcome measure is a cut-down version of the DASH, and this shortening raises the question whether the QuickDASH is non-inferior to the DASH and whether excluding 19 items loses too much information.
The increase in DASH and QuickDASH mean scores exceeding the MCID threshold occurred significantly more in referred patients. Similar results were found when comparing UED and non-UED patients. However, according to the MCID, deterioration of upper extremity function was not of clinical relevance in most cases (Table 4).
Additionally, the properties of the shortened QuickDASH as compared to the full-length DASH specifically for UED following PCI were examined. The ROC curves showed that both the DASH and QuickDASH were incapable of discriminating between patients with- and without UED. This suggests that despite a significant increase in scores for patients with UED and patients who were referred, the clinical relevance of this increase is debatable
Care must therefore be taken when attributing disability measured by the DASH and QuickDASH scores to injuries of the upper extremity when myocardial problems are also present. Its inability to discern changes in specific functional aspects of the upper extremity as a result of local complication or physical activity cessation post-PCI must be considered when using this instrument in clinical practice or research.
Limitations
No standard definition of UED exists, therefore a comprehensive definition was designed including all relevant aspects of upper extremity function (eg strength, sensibility, pain symptoms). The definition of UED in this study was designed by hand specialists based on examinations performed according to the ASHT.22 This definition of UED has not been validated.
In our study sample, the DASH and QuickDASH were calculated based on a single set of responses, using the DASH questionnaires proforma. Therefore, intra-observer reproducibility or test–retest reliability is not observed. Furthermore, the response rate was low, creating a risk of unverifiable selection bias. The patients in the present study were only followed for 6 months post intervention, and their DASH scores may not represent final outcomes due to treatment of disease and progressive recovery. However, the methodology of this study was appropriate to fulfill the specific objectives of this study.
Finally, the investigators in this study were not blinded. Information bias was minimized by using standardized physical tests for questionnaire validation. These tests were minimally influenced by the performing investigator.
Conclusion
The use of the DASH and QuickDASH questionnaires for identifying UED after a TR-PCI has been shown to be invalid. Our study results suggest that both PROMS are equally incapable of discerning UED as a result of local complication or physical activity cessation post PCI, and more likely measures post-cardiac intervention induced impaired condition.
The DASH and QuickDASH should not be used for the assessment of access-site complications in the upper extremity following a TR-PCI. It remains important to actively inquire after symptoms and complications following a TR-PCI, especially with occupations depending on manual labor and dexterity influencing patients’ functional independence and quality of life.
Abbreviations
DASH, Disability of the Arm, Shoulder and Hand; PCI, percutaneous coronary intervention; PROM, patient reported outcome measures; ROC, receiver-operating-characteristic; STEMI, segment elevation myocardial infarction; TR-PCI, transradial percutaneous coronary intervention.
Disclosure
M. Alings reports research grants or personal fees from Sanofi, outside the submitted work. All other authors report no conflicts of interest in this work.
References
1. Institute for Work & Health. Development and testing of the DASH and Quick-DASH Outcome Measure Instruments and the DASH User’s Manual. Available from: http://dash.iwh.on.ca.
2. Beaton DE, Katz JN, Fossel AH, Wright JG, Tarasuk V, Bombardier C. Measuring the whole or the parts? Validity, reliability, and responsiveness of the disabilities of the arm, shoulder and hand outcome measure in different regions of the upper extremity. J Hand Ther. 2001;14(2):128–146. doi:10.1016/S0894-1130(01)80043-0
3. Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am J Ind Med. 1996;29(6):602–608. doi:10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L
4. Beaton DE, Wright JG, Katz JN; for Upper Extremity Collaborative Group. Development of the QuickDASH: comparison of three item-reduction approaches. J Bone Joint Surg Am. 2005;87(5):1038–1046. doi:10.2106/JBJS.D.02060
5. Gummesson C, Ward MM, Atroshi I. The shortened disabilities of the arm, shoulder and hand questionnaire (QuickDASH): validity and reliability based on responses within the full-length DASH. BMC Musculoskeletal Disord. 2006;7:7–44.
6. Macdermid JC, Khadilkar L, Birmingham TB, Athwal GS. Validity of the QuickDASH in patients with shoulder-related disorders undergoing surgery. J Orthop Sports Phys Ther. 2015;45(1):25–36. doi:10.2519/jospt.2015.5033
7. Hobby JL. Comparative responsiveness of the Hand 20 and the DASH-JSSH to clinical changes after carpal tunnel release. J Hand Surg. 2014;39(2):152–154. doi:10.1177/1753193413493733
8. Caputo RP, Tremmel JA, Rao S, et al. Transradial arterial access for coronary and peripheral procedures: executive summary by the transradial committee of the SCAI. Catheter Cardiovasc Interv. 2011;78(6):823–839. doi:10.1002/ccd.23052
9. Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am Heart J. 2009;157(1):132–140. doi:10.1016/j.ahj.2008.08.023
10. van Leeuwen MAH, van Mieghem NM, Lenzen MJ, et al. The effect of transradial coronary catheterization on upper limb function. JACC Cardiovasc Interv. 2015;8(4):515–523. doi:10.1016/j.jcin.2014.10.025
11. van Leeuwen MAH, van der Heijden DJ, Hermie J, et al. The long-term effect of transradial coronary catheterisation on upper limb function. EuroIntervention. 2017;12(14):1766–1772. doi:10.4244/EIJ-D-15-00395
12. Amoroso G, Van der Brug S, Franx V, et al. Slender versus conventional approach reduces post-procedural upper extremity dysfunction after transradial coronary procedures. Catheter Cardiovasc Interv. 2020;96(1):66–73. doi:10.1002/ccd.28758
13. Zwaan EM, IJsselmuiden AJJ, van Rosmalen J, et al. Rationale and design of the ARCUS: effects of trAnsRadial perCUtaneouS coronary intervention on upper extremity function. Catheter Cardiovasc Interv. 2016;88(7):1036–1043. doi:10.1002/ccd.26525
14. IJsselmuiden AJJ, Holtzer CAJ, Zwaan EM, et al. Upper extremity function after transradial percutaneous coronary intervention: short-term interim results of the ARCUS study. J Heart Cardiol. 2017;3(2):1–6.
15. Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27(1):117–126. doi:10.1016/0304-3959(86)90228-9
16. McConnell S, Beaton DE, Bombardier C. The DASH Outcome Measure: A User’s Manual. Toronto: Institute for Work and Health; 1999.
17. Weinstein S. Fifty years of somatosensory research: from the Semmes-Weinstein monofilaments to the Weinstein Enhanced Sensory Test. J Hand Ther. 1993;6(1):11–50. doi:10.1016/S0894-1130(12)80176-1
18. Garner NC. Clinical Assessment Recommendations.
19. Douma RK, Soer R, Krijnen WP, Reneman M, van der Schans CP. Reference values for isometric muscle force among workers for the Netherlands: a comparison of reference values. BMC Sports Sci Med Rehabil. 2014;6(1):10. doi:10.1186/2052-1847-6-10
20. Leard JS, Breglio L, Fraga L, et al. Reliability and concurrent validity of the figure-of-eight method of measuring hand size in patients with hand pathology. J Orthop Sports Phys Ther. 2004;34(6):335–340. doi:10.2519/jospt.2004.34.6.335
21. Boland R, Adams R. Development and evaluation of a precision forearm and hand volumeter and measuring cylinder. J Hand Ther. 1996;9(4):349–358. doi:10.1016/S0894-1130(96)80041-X
22. Mac Dermi J, Solomon G, Valsdes K. ASHT Clinical Assessment: Impairment -Based Conditions Recommendations.
23. Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Onco. 2010;5(9):1315–1316. doi:10.1097/JTO.0b013e3181ec173d
24. Beaton DE, Davis AM, Hudak P, Mcconnell S. The DASH (Disabilities of the Arm, Shoulder and Hand) outcome measure: what do we know about it now? Hand Ther. 2001b;6(4):109–118. doi:10.1177/175899830100600401
25. Beaton DE, van Eerd D, Smith P, et al. Minimal change is sensitive, less specific to recovery: a diagnostic testing approach to interpretability. J Clin Epidemiol. 2011;64(5):487–496. doi:10.1016/j.jclinepi.2010.07.012
26. Mintken PE, Glynn P, Cleland JA. Psychometric properties of the shortened disabilities of the Arm, Shoulder, and Hand Questionnaire (QuickDASH) and numeric pain rating scale in patients with shoulder pain. Shoulder Elbow Surg. 2009;18(6):920–926. doi:10.1016/j.jse.2008.12.015
27. Smolderen KG, Buchanan DM, Gosch K, et al. Depression treatment and 1-year mortality after acute myocardial infarction: insights from the TRIUMPH registry (Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status). Circulation. 2017;135(18):1681–1689. doi:10.1161/CIRCULATIONAHA.116.025140
28. Larsen KK. Depression following myocardial infarction-an overseen complication with prognostic importance. Dan Med J. 2013;60(8):B4689.
29. Mayou RA, Gill D, Thompson DR, et al. Depression and anxiety as predictors of outcome after myocardial infarction. Psychosom Med. 2000;62(2):212–219. doi:10.1097/00006842-200003000-00011
30. Popadic Gacesa JZ, Dusko KB, Grujic NG. Triceps brachii strength and regional body composition changes after detraining quantified by MRI. J Magn Reson Imaging. 2011;33(5):1114–1120. doi:10.1002/jmri.22548
31. Dowrick AS, Gabbe BJ, Williamson OC, Cameron PA. Does the disabilities of the arm, shoulder and hand (DASH) scoring system only measure disability due to injuries to the upper limb? J Bone Joint Surg Br. 2006;88(4):524–527. doi:10.1302/0301-620X.88B4.17223
32. Angst F, Goldhahn J, Drerup S, Flury M, Schwyzer HK, Simmen BR. How sharp is the short QuickDASH? A refined content and validity analysis of the short form of the disabilities of the shoulder, arm and hand questionnaire in the strata of symptoms and function and specific joint conditions. Qual Life Res. 2009;18(8):1043–1051. doi:10.1007/s11136-009-9529-4
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.