Back to Journals » Journal of Inflammation Research » Volume 16
Predictive Value of Serum Heat Shock Protein 90α on the Prognosis of Patients with Lung Adenocarcinoma
Authors Fang Y , Yuan Z , Zhang H, Wang P, Hao J
Received 14 December 2022
Accepted for publication 22 February 2023
Published 16 March 2023 Volume 2023:16 Pages 1183—1193
DOI https://doi.org/10.2147/JIR.S401444
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Yue Fang,1,2 Zhichao Yuan,1 Hao Zhang,1 Peng Wang,3 Jiqing Hao1
1Department of Oncology, First Affiliated Hospital of Anhui Medical University, Hefei, People’s Republic of China; 2Intern of Oncology, Hefei Cancer Hospital, Chinese Academy of Sciences (CAS), Hefei, People’s Republic of China; 3Department of Anesthesiology, First Affiliated Hospital of Anhui Medical University, Hefei, People’s Republic of China
Correspondence: Jiqing Hao, Email [email protected]
Purpose: Heat shock protein 90α (HSP90α) is highly expressed in tumors, and predicts tumor progression. This study analyzed the correlation between the expression level of HSP90α in the serum and the prognosis of patients with lung adenocarcinoma.
Patients and methods: The medical records of patients with 228 lung adenocarcinoma from September 2015 to December 2021 were analyzed. HSP90α expression in the patients’ serum was detected by ELISA and the cut-off value (93.76 ng/mL) was determined according to the ROC curve, then the patients were divided into high- and low-level groups. The differences in the medical records of the two groups were compared using the X2 test, and Univariate and multivariate Cox regression analyses showed that serum HSP90α level were independent risk factors for both PFS and OS (P < 0.05).
Results: HSP90α was positively correlated with TNM staging (P < 0.01) by One-way analysis of variance. The results of the correlation analysis and the Kaplan–Meier survival curve showed that the expression levels of HSP90α and CEA of patients were positively correlated (R=0.54, P < 0.001), and patients with high HSP90α and CEA levels had the worst OS (P < 0.001).
Conclusion: HSP90α expression is negatively correlated with the prognosis of patients with lung adenocarcinoma and is a potential prognostic marker.
Keywords: heat shock protein 90α, lung adenocarcinoma, prognosis, CEA
Introduction
Lung cancer is the leading malignant tumor and cause of cancer-related deaths worldwide.1 Annually, there are over 2 million new cases and 1.7 million deaths due to lung cancer worldwide, and non-small cell lung cancer (NSCLC) accounts for approximately 85% of the total deaths.1,2 Lung adenocarcinoma (LUAD) is the most common non-small cell lung cancer type. The complex pathogenesis of LUAD makes it highly heterogeneous. Traditional treatment methods are not effective for the treatment of LUAD, and the 5-year survival rate of patients with LUAD is approximately 15%.2,3 Exploring new and effective biological targets is of great value for treating and prognosis patients with LUAD.
Heat shock proteins (HSPs) are a large class of functional proteins involved in protein folding and maturation, and their expression is upregulated when the body is overstressed. They are divided into subfamilies, including HSP27, HSP70, HSP90, and large HSP, according to their molecular weight.4 HSP90s are the most widely distributed proteins in the human body, with different levels of expression in various tissues and organs, and their expression is elevated in various malignant tumor tissues.5–7 HSP90s are related to tumorigenesis. Previous studies have confirmed that HSP90, as an upstream target, can be activated by a variety of oncogenic factors and promote the transformation of normal cells into malignant cells by binding to a series of client proteins, such as matrix metallopeptidase 9, hypoxia-inducible factor 1α, and epidermal growth factor receptor, to promote cancer.8
HSP90s include five isoforms encoded by HSPC1-5. HSP90α is encoded and expressed by HSPC1 (also known as HSP90AA1), and its expression is increased in the cytoplasm of lung cancer, breast cancer, multiple myeloma, and other tumor cells. It can promote tumor progression by promoting cell invasion and metastasis and inhibiting apoptosis.4,9–11 By comparing the expression levels of HSP90α in the serum of healthy individuals and patients with lung cancer, Wang et al12 found that the expression level of HSP90α in the serum of patients with lung cancer was higher than that of healthy individuals, suggesting that HSP90α can be used as a new biomarker for the diagnosis of lung cancer. Zhou et al13 found that HSP90α in serum is consistent with tumor markers, such as carcinoembryonic antigen (CEA), and has a high diagnostic effect on patients with NSCLC.
The diagnostic effect of HSP90α in patients with lung cancer has been previously confirmed. However, studies on its importance as a prognostic marker for patients with LUAD remain significantly limited. In this study, 228 patients with LUAD were included, and the correlation between their clinical characteristics, tumor markers, and HSP90α expression levels and prognosis were retrospectively analyzed to evaluate the possibility of using HSP90α as a prognostic predictor.
Methods
Study Subjects
This study retrospectively analyzed patients with LUAD who first visited the First Affiliated Hospital of Anhui Medical University from September 2015 to November 2021. The inclusion criteria were as follows: (1) patients with complete clinical data and histopathologically confirmed primary LUAD, (2) patients not receiving any antitumor therapy, and (3) patients with no severe liver and kidney functions or autoimmune diseases. The exclusion criteria were as follows: (1) patients with other primary tumors at the same time and (2) patients with LUAD combined with severe infection. Informed consent was obtained from all patients or their families, and this study was approved by the Ethics Review Committee of the First Affiliated Hospital of Anhui Medical University [Ethics Approval number: Quick-PJ 2022–05-35].
Study Method
This was a retrospective study, the baseline clinical characteristics of 228 patients with LUAD were collected, including age, sex, smoking history, tumor differentiation level, and tumor-node-metastasis (TNM) stage. Serological index test results were obtained within 2 weeks after the first diagnosis, and before receiving antitumor therapy, including CEA, neuron-specific enolase (NSE), cytokeratin-19 fragments in serum (CYFA21-1), and HSP90α. CEA, NSE, and CYFA21-1 values were determined by chemiluminescence, and the HSP90α value was determined by enzyme-linked immunosorbent assay (ELISA test). The cut-off HSP90α value was 93.76 ng/mL, which was obtained by the ROC curve, and all patients were divided into high- and low-level groups. The normal ranges of CEA, NSE, and CYFA21-1 were 0–5 ng/mL, 0–15 ng/mL, and 0–4 ng/mL, respectively.
Follow-Up
Patients’ survival was monitored through inpatient, outpatient, and telephone follow-ups until death from any cause or the study cut-off date (November 30, 2021). The median follow-up duration was 25 (range, 2.13–69.83) months. Progression-free survival (PFS) is defined as the time from entry into the trial to the first disease progression or death, and overall survival (OS) is defined as the time from the first diagnosis to the time of death from any cause.
Statistical Analyses
SPSS version 22.0 and GraphPad Prism (Waltham, MA, United States of America) version 8 were used for data analysis and graphing. The X2 or Fisher’s exact test, and one-way analysis of variance (ANOVA) test, were used to analyze and compare the data between the two, and multiple groups. Survival curves were plotted as Kaplan–Meier (K–M) curves and compared between the groups using the Log-rank test. The Cox risk regression models were used to identify independent risk factors affecting PFS and OS. P < 0.05 was set as statistical significance (α=0.05).
Results
Clinical Features of Patients
A total of 228 (154 [67.5%] women and 74 [34.5%] men) patients with LUAD were included in this study, with an average age of 62.04±9.55 years, and 76 (33.3%) patients had a smoking history. Moreover, 140 (61.4%) and 88 (38.6%) patients had poorly differentiated/undifferentiated and well-differentiated/moderately-differentiated adenocarcinomas, respectively. In terms of staging, 6.1%, 27.2%, 36.0%, and 30.7% of the patients were in stages I, II, III, and IV, respectively. Among them, 7%, 43.0%, and 50% of the patients were in the T1, T2, T3, and T4 stages, respectively. Lymph node invasion (N1, N2, N3) and distant metastasis (M1) were observed in 69.3% and 29.2% of the patients, respectively. Analysis of serum tumor marker levels of 228 patients showed that 92.1% of the patients had CEA levels greater than 5 ng/mL, 84.2% of the patients had NSE levels greater than 15 ng/mL, and 85.5% of the patients had CYFA21-1 levels greater than 4 ng/mL. According to the HSP90α cut-off value, the patients were divided into the high- and low-level groups, of which 92 and 136 patients were in the high- and low-level groups, respectively. The clinical characteristics and tumor marker levels of the two groups of patients were compared, and the results showed that there were significant differences in sex; smoking history; T, N, and M staging; TNM staging; and CEA, NSE, and CYFA21-1 levels between the two groups (P < 0.05, Table 1).
 |
Table 1 Patient Baseline Characteristics and Correlations with HSP90α (n=228) |
Correlation Analysis Between Heat Shock Protein 90α (HSP90α) and Survival Period
Univariate Cox regression analysis showed that sex, smoking history, T staging, N staging, M staging, TNM staging, degree of differentiation, NSE level, CYFA21-1 level, and HSP90α level were associated with PFS (Table 2). Then the Multivariate Cox regression analysis found that T staging, TNM staging, degree of differentiation, and HSP90α level were independent risk factors for PFS (Table 3). And the patients with high HSP90a levels were 1.418 times more likely to experience disease progression than those with low HSP90a levels (HR=1.418; 95% CI, 0.501–0.922; P = 0.015).
 |
Table 2 Comparative Univariate Survival Analyses of 228 Patients with LUAD |
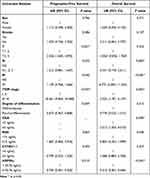 |
Table 3 Comparative Multivariate Survival Analyses of 228 Patients with LUAD |
Also, the univariate Cox regression analysis showed that sex, smoking history, T staging, N staging, M staging, TNM staging, degree of differentiation, CEA level, NSE level, CYFA21-1 level, and HSP90α level were associated with OS (Table 2). Then the Multivariate Cox regression analysis confirmed that N staging, M staging, TNM staging, CEA level, and HSP90α level were independent risk factors for OS (Table 3). And the patients with high HSP90a levels were 3.205 times more likely to experience disease-related death than those with low HSP90a levels (HR=3.205; 95% CI, 0.201–0.484; P < 0.001).
Survival Analysis
The overall mean follow-up period in this study was 28.87 (range, 2.13–69.83) months, and 132 patients died during the follow-up period. The median PFS and OS periods of all patients were 18.89 (range, 0.01–52.01) and 24.74 (range, 2.13–69.83) months, respectively. The median PFS period of the patients in the low-level group was shorter by 16.51 months than that of the patients in the high-level group (22.35 [range, 0.13–52.01] months vs 5.84 [range, 0.01–29.02] months). Moreover, the OS period of the patients in the low-level group was shorter by 13.95 months than that of the patients in the high-level group (35.40 [range, 13.13–69.83] months vs 21.45 [range, 2.13–60.02] months). In addition, the K–M curve and the Log-rank test confirmed that the PFS and OS periods of the patients in the high-level group were lower than those of patients in the low-level group (P < 0.001, Figure 1).
Correlation Between HSP90α and Tumor-Node-Metastasis Staging in Patients with LUAD
Multivariate Cox regression analysis suggested that TNM staging was an independent risk factor for PFS and OS in patients with LUAD. To explore the correlation between TNM staging and HSP90α expression level, this study analyzed the HSP90α expression levels in patients with different stages. The results showed that the mean HSP90α values in patients with stages I, II, III, and IV were 42.00±8.85, 86.15±38.63, 90.20±44.48, and 104.25±50.70 ng/mL, respectively. One-way ANOVA was used to analyze the differences among the four groups. The results showed that there were differences among the four groups (Figure 2, F=13.32, P<0.001), suggesting that HSP90α is positively correlated with the TNM staging of patients with LUAD.
 |
Figure 2 Relationship between HSP90α and TNM stage in 228 LUAD patients. |
Prognosis of HSP90α Combined with Carcinoembryonic Antigen in Patients with LUAD
Multivariate Cox regression analysis showed that HSP90α and CEA levels were independent risk factors for OS in patients with LUAD. Correlation analysis between the expression levels of HSP90α and CEA showed that the expression level of CEA in the serum of patients with LUAD was positively correlated with HSP90α level (R=0.54, P<0.001) (Figure 3A). Based on the HSP90α and CEA cut-off values, 228 patients were divided into the high-level HSP90α+high-level CEA group (Hhigh+Chigh), low-level HSP90α+low-level CEA group (Hlow+Clow), low-level HSP90α+high-level CEA group (Hlow+Chigh), and low-level HSP90α+high-level CEA group (Hlow+Chigh). The median OS periods of the Hhigh+Chigh, Hlow+Clow, Hlow+Chigh, and Hlow+Chigh groups were 19.6 (range, 2.13–47.21), 43.67 (range, 36.10–55.40), 37.01 (range, 25.30–60.04), and 28.05 (range, 10.00–69.83) months, respectively. The patients in the Hhigh+Chigh group had the worst OS. The K–M curve and Log rank test also confirmed that the Hhigh+Chigh group had the worst OS (P<0.001, Figure 3B). The above results suggest that when HSP90α is ≥93.76 ng/mL and CEA is ≥5 ng/mL in the serum of patients with LUAD, the prognosis of the patients is worst.
Discussion
HSP90α has been the central target of the progression of various malignant tumors and is an efficient biomarker for predicting the prognosis of patients with breast cancer, melanoma, and other malignant tumors.14,15 The correlation between HSP90α level and the prognosis of patients with LUAD remains to be explored.
In this study, the clinical characteristics of CEA, NSE, CYEA21-1, and HSP90α in 228 patients with LUAD were compared with the prognosis of patients. The results showed that T staging, lymph node metastasis, TNM staging, and HSP90α level were the independent risk factors for PFS, whereas the lymph node metastasis, distant metastasis, TNM staging, and CEA and HSP90α levels were independent risk factors for OS in patients with LUAD. In addition, the K–M curve and Log rank test further confirmed that serum HSP90α level was negatively correlated with the prognosis of patients with LUAD.
HSP90s are stably expressed in the body. Under non-stress conditions, its expression accounts for approximately 1–2% of the total human protein, whereas under stress conditions, it is upregulated to 4–6% to maintain homeostasis.16,17 The HSP90 family includes four subtypes, HSP90α and β, glucose-regulated protein 94, and tumor necrosis factor receptor-associated protein 1, all of which have a classical protein structure composed of the N-terminal, middle section, and C-terminal and a highly consistent amino acid composition sequence. Although the similarity of the constituent amino acids is high, at the nucleotide level there are still some differences between HSP90α and the other three proteins, and this difference allows HSP90α to have more biological functions.18,19 In vitro and in vivo experiments have found that HSP90α, which can promote wound healing, regulate inflammatory responses in normal tissues, and promote cell proliferation, invasion, epithelial–mesenchymal transition, and secondary drug resistance in tumor tissues, is a key target in tumor progression.20–24
Trepel et al25 analyzed the patient information from The Cancer Genome Atlas and found that HSP90AA1, encoding HSP90α, is highly expressed in bladder cancer and other malignant tumor tissues and is negatively correlated with the prognosis of patients, suggesting that HSP90α may be a potential tumor marker. However, a series of clinical and retrospective analyses have drawn opposing conclusions on the correlation between HSP90α and the prognosis of patients with tumors. For example, Zhang et al26 found that HSP90α was highly expressed in the peripheral blood of 103 patients with gastric cancer and 83 patients with colorectal cancer, but was not related to the PFS and OS of the patients, whereas in breast cancer, serum HSP90α levels were negatively correlated with OS.27,28 Chen29 et al compared the expression level of HSP90 in the serum of 137 patients with advanced lung cancer treated with immunotherapy with the PFS and OS of the patients and found that the patients in the high-level group of HSP90α had worse PFS and OS than those in the low-level group. In a study by Zhang,30 et al. In their study, 42 melanoma patients included in the study were grouped according to differences in HSP90 expression levels, named: abnormal patients and normal patients group, and the study found that the normal patients group had greater PFS than abnormal patients (8.0 vs 3.5 months). In addition, the study also divided the patients into ORR (objective response), DC (disease control), and PD (progressive disease) groups according to the difference in recent treatment effects. The opposite was true for the PD group. All of these suggest that HSP90 levels of patients with malignant tumors before treatment are negatively correlated with the treatment effect of patients. This conclusion is also confirmed in Li et al’s study.31 In this study, we found that the expression level of HSP90 was negatively correlated with the prognosis of patients with LUAD. The difference is that the study by Chen et al32 included 137 patients with lung cancer, with only 75 patients having adenocarcinoma, and Li et al’s study comprised 231 patients with lung cancer, with only 105 patients having adenocarcinoma. The results of these two studies may be affected by the survival of patients with other pathological types of lung cancer.
Previous studies were mostly limited to exploring the correlation between TNM staging and patient prognosis but did not focus on the correlation between TNM staging and HSP90a levels.32–34 This study not only confirmed that TNM staging is correlated with patients’ survival period but also explored the correlation between serum HSP90α expression and TNM staging for the first time. By comparing the TNM staging of patients with different levels of HSP90α for LUAD, we found that there were significant differences in the TNM staging of patients at different levels. We then compared the expression levels of HSP90α in the serum of patients at different stages, and the results showed that with the progression of the patient’s stage, the expression level of HSP90α in the serum of the patients also gradually increased. The level of serum HSP90α is positively correlated with the progression of LUAD, which may be one of the potential mechanisms by which the high expression of HSP90α in the serum promotes tumor progression.
The regulatory mechanism by which HSP90α promotes the progression of LUAD remains unknown. Through in vitro and in vivo experiments, Liu et al35 found that the expression of HSP90α in lung cancer cell lines, such as A549, was significantly higher than that in bronchial epithelial cells 16-HBE, and was involved in the TRPM7/Hsp90α/uPA/MMP2 axis to improve metastasis of lung cancer cells. Moreover, when the expression of HSP90α in H157 LUAD cells is inhibited, the level of apoptosis increases, suggesting that HSP90α may promote tumor progression by inhibiting apoptosis of LUAD cells.36 Additionally, as a class of proteins of interest and relevance to tumor growth, HSP90 has been shown in previous in vitro and vivo studies to be effective in inhibiting the proliferation of lung adenocarcinoma cells with EGFR mutations or ALK-rearranged. In the study by Piotrowska et al,37 a total of 29 patients with EGFR-mutated lung adenocarcinoma were included, with an ORR of 17% and a median OS of 13 months after treatment with an HSP90 inhibitor. In a study by Sang et al,38 it was first demonstrated in vitro that hsp90 inhibitors in non-adenocarcinoma cell lines with ALK rearrangement could inhibit cell proliferation in a gradient manner and that EGFR and ALK expression levels in the cell lines were subsequently reduced. However, these studies have not identified a co-expression or upstream or downstream relationship between EGFR or ALK and HSP90. In addition, Sang et al,3 show in detail that patients with ectopic ALK-rearranged lung adenocarcinoma, who developed a secondary resistance to an ALK-TKI (crizotinib), developed multiple metastases in both lungs. However, after 3 cycles of an HSP90 inhibitor (ganetespib), the metastases in both lungs were significantly reduced in size and the patient achieved significant improvement in clinical symptoms such as coughing and hemoptysis. All of the above suggests that although the correlation between HSP90 expression levels and EGFR and ALK expression levels in lung adenocarcinoma remains uncertain, the use of HSP90 inhibitors in patients with these mutations may be beneficial. Unfortunately, there are still no studies correlating ROS1 expression levels with HSP90 treatment.
CEA, NSE, and CYFA21-1 are all commonly used tumor markers and are widely used in the initial screening of patients with tumors, monitoring of recurrence and metastasis, and evaluating the efficiency of diagnosis. The combination of their index detection can help improve sensitivity and specificity.39,40 Zhou et al13 compared the levels of HSP90 and CEA, NSE, SCC, and CYFRA21-1 in the serum of healthy individuals and patients with lung cancer and found that HSP90α and four other tumor markers have the same role in diagnosing patients with tumors, and the sensitivity and specificity were both improved after combining them with the other markers. In Shi et al’s study,41 the diagnostic effect of HSP90α combined with CEA and CYFRA21-1 in patients with lung cancer was confirmed. However, there are no relevant studies on HSP90α combined with other tumor markers to predict the prognosis of patients with tumors. This study found that serum CEA levels were negatively correlated with OS in patients with LUAD, consistent with the result of a previous study.40 Furthermore, through correlation analysis, we found that the expression levels of HSP90α and CEA in the serum of patients with LUAD were positively correlated and that patients with both HSP90α≥93.76 ng/mL and CEA≥5 ng/mL had the worst OS, suggesting that the combination of HSP90αand CEA have a higher level of efficacy in predicting the prognosis of patients.
Our study confirms the correlation between HSP90α and prognosis in patients with lung adenocarcinoma, but there are also some limitations as follows: (1) the correlation between treatment modalities and survival among the 228 patients included in this study was not analyzed, and (2) this was a single-center study, with the included patient group being single and with a certain bias.
Conclusions
This study is one of the few that analyzed the correlation between serum HSP90α levels and patient prognosis in a large number of patients with LUAD. The study found that the expression level of HSP90α in the serum of patients was negatively correlated with PFS and OS. In addition, this study also demonstrated for the first time that the expression level of HSP90α was positively correlated with the TNM staging and the expression level of CEA, and the combination of HSP90α and CEA may better predict the prognosis of patients.
Ethics Approval Statement
This study complies with the Declaration of Helsinki and was approved by the Ethics Review Committee of the First Affiliated Hospital of Anhui Medical University. [Ethics Approval number: Quick-PJ 2022-05-34].
Patient Consent Statement
Informed consent was obtained from all patients and their families.
Acknowledgments
Thanks to Jialin Meng, Department of Urology, The First Affiliated Hospital of Anhui Medical University for his guidance and help.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Anhui Clinical Medical Research Translation Special Project (No.202204295107020016) and the Anhui Hygiene and Health Research Key Project (No.AHWJ2022a001).
Disclosure
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Thai AA, Solomon BJ, Sequitur LV, et al. Lung cancer. Lancet. 2021;398(10299):535–554. doi:10.1016/S0140-6736(21)00312-3
2. Lu L, Liu LP, Zhao QQ, et al. Identification of a Ferroptosis-Related LncRNA Signature as a Novel Prognosis Model for Lung Adenocarcinoma. Front Oncol. 2021;11:675545. doi:10.3389/fonc.2021.675545
3. Spella M, Stathopoulos GT. Immune Resistance in Lung Adenocarcinoma. Cancers. 2021;13(3):384. doi:10.3390/cancers13030384
4. Wu J, Liu T, Rios Z, et al. Heat Shock Proteins and Cancer. Trends Pharmacol Sci. 2017;38(3):226–256. doi:10.1016/j.tips.2016.11.009
5. Li W, Sahu D, Tsen F. Secreted heat shock protein-90 (Hsp90) in wound healing and cancer. Biochim Biophys Acta. 2012;1823(3):730–741. doi:10.1016/j.bbamcr.2011.09.009
6. Hoter A, El-Sabban ME, Naim HY. The HSP90 Family: structure, Regulation, Function, and Implications in Health and Disease. Int J Mol Sci. 2018;19(9):2560. doi:10.3390/ijms19092560
7. Birbo B, Madu EE, Madu CO, et al. Role of HSP90 in Cancer. Int J Mol Sci. 2021;22(19):10317. doi:10.3390/ijms221910317
8. Wang X, Song X, Zhuo W, et al. The regulatory mechanism of Hsp90α secretion and its function in tumor malignancy. Proce National Academy Sci. 2009;106(50):21288–21293. doi:10.1073/pnas.0908151106
9. Cheng Q, Chang JT, Geradts J, et al. Amplification and high-level expression of heat shock protein 90 marks aggressive phenotypes of human epidermal growth factor receptor 2 negative breast cancer. Breast Cancer Res. 2012;14(2):R62. doi:10.1186/bcr3168
10. Duus J, Bahar HI, Venkataraman G, et al. Analysis of expression of heat shock protein-90 (HSP90) and the effects of HSP90 inhibitor (17-AAG) in multiple myeloma. Leuk Lymphoma. 2006;47(7):1369–1378. doi:10.1080/10428190500472123
11. Snigireva AV, Morenkov OS, Skarga YY, et al. A 2,5-Dihydroxybenzoic Acid-Gelatin Conjugate Inhibits the Basal and Hsp90-Stimulated Migration and Invasion of Tumor Cells. J Funct Biomater. 2020;11(2):39. doi:10.3390/jfb11020039
12. Wang Y, Seyed Barghi SM, Yang Y, Akhavan-Sigari R. Value of HSP90α in Lung Cancer Diagnosis and Recurrence Prediction: a Cohort Study. Oncol Res Treat. 2021;44(11):583–589. doi:10.1159/000519277
13. Zhou W, Yang Y, Wang Z, et al. Impact of HSP90α, CEA, NSE, SCC, and CYFRA21-1 on Lung Cancer Patients. J Healthc Eng. 2021;2021:6929971. doi:10.1155/2021/6929971
14. Pick E, Kluger Y, Giltnane JM, et al. High HSP90 expression is associated with decreased survival in breast cancer. Cancer Res. 2007;67(7):2932–2937. doi:10.1158/0008-5472.CAN-06-4511
15. Zhang Y, Ni L, Li Q, et al. Diagnostic, clinicopathologic, therapeutic and prognostic value of Plasma Heat Shock Protein 90 levels in patients with advanced Gastrointestinal Carcinoma. J Cancer. 2020;11(20):5890–5899. doi:10.7150/jca.46343
16. Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11(7):515–528. doi:10.1038/nrm2918
17. Finka A, Goloubinoff P. Proteomic data from human cell cultures refine mechanisms of chaperone-mediated protein homeostasis. Cell Stress Chaperones. 2013;18(10):591–605. doi:10.1007/s12192-013-0413-3
18. Zhang S, Yu J, Cheng X, et al. Regulation of human Hsp90α gene expression. FEBS Let. 1999;444:130–135. doi:10.1016/S0014-5793(99)00044-7
19. Rebbe NG, Hickman WS, Ley TJ, et al. Nucleotide sequence and regulation of a human 90-kDa heat shock protein gene. J Biol Chem. 1989;264(25):15006–15011. doi:10.1016/S0021-9258(18)63803-7
20. Eustace BK, Sakurai T, Stewart JK, et al. Functional proteomic screens reveal an essential extracellular role for hsp90 alpha in cancer cell invasiveness. Nat Cell Biol. 2004;6(6):507–514. doi:10.1038/ncb1131
21. Ma J, Yang C, Zhong H, et al. Role of HSP90α in osteoclast formation and osteoporosis development. Exp Ther Med. 2022;23(4):273. doi:10.3892/etm.2022.11199
22. Liao Y, Yang Y, Pan D, et al. HSP90α Mediates Sorafenib Resistance in Human Hepatocellular Carcinoma by Necroptosis Inhibition under Hypoxia. Cancers. 2021;13(2):243. doi:10.3390/cancers13020243
23. Tian Y, Wang C, Chen S, et al. Extracellular Hsp90α and clusterin synergistically promote breast cancer epithelial-to-mesenchymal transition and metastasis via LRP1. J Cell Sci. 2019;132(15):jcs228213. doi:10.1242/jcs.228213
24. Xu D, Dong P, Xiong Y, et al. MicroRNA-361-Mediated Inhibition of HSP90 Expression and EMT in Cervical Cancer Is Counteracted by Oncogenic lncRNA NEAT1. Cells. 2020;9(3):632. doi:10.3390/cells9030632
25. Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10(8):537–549. doi:10.1038/nrc2887
26. Zhang Y, Ni L, Li Q, et al. Diagnostic, clinicopathologic, therapeutic and prognostic value of Plasma Heat Shock Protein 90 levels in patients with advanced Gastrointestinal Carcinoma. J Cancer. 2020;11(20):5890–5899.
27. Jameel A, Law M, Coombes R, Luqmani Y. Significance of heat-shock protein-90 as a prognostic indicator in breast-cancer. Int J Oncol. 1993;2(6):1075–1080. doi:10.3892/ijo.2.6.1075
28. Dimas DT, Perlepe CD, Sergentanis TN, et al. The Prognostic Significance of Hsp70/Hsp90 Expression in Breast Cancer: a Systematic Review and Meta-analysis. Anticancer Res. 2018;38(3):1551–1562. doi:10.21873/anticanres.12384
29. Chen S, Yu Q, Zhou S. Plasmatic Levels of HSP90α at Diagnosis: a Novel Prognostic Indicator of Clinical Outcome in Advanced Lung Cancer Patients Treated With PD-1/PD-L1 Inhibitors Plus Chemotherapy. Front Oncol. 2021;11:765115. doi:10.3389/fonc.2021.765115
30. Zhang T, Li Q, Zhang Y, Wang Q, Wang H, Gu K. Diagnostic and prognostic value of heat shock protein 90α in malignant melanoma. Melanoma Res. 2021;31(2):152–161. doi:10.1097/CMR.0000000000000716
31. Li X, Tong X, Liu B, et al. Potential predictive value of plasma heat shock protein 90α in lung cancer. J Int Med Res. 2021;49(12):3000605211064393. doi:10.1177/03000605211064393
32. Bar JK, Cierpikowski P, Lis-Nawara A, et al. Comparison of p53, HSP90, E-cadherin and HPV in oral lichen planus and oral squamous cell carcinoma. Acta Otorhinolaryngol Ital. 2021;41(6):514–522. doi:10.14639/0392-100X-N1450
33. Usman M, Ilyas A, Syed B, et al. Serum HSP90-Alpha and Oral Squamous Cell Carcinoma: a Prospective Biomarker. Protein Pept Lett. 2021;28(10):1157–1163. doi:10.2174/0929866528666210616112539
34. Zhai E, Liang W, Lin Y, et al. HSP70/HSP90-Organizing Protein Contributes to Gastric Cancer Progression in an Autocrine Fashion and Predicts Poor Survival in Gastric Cancer. Cell Physiol Biochem. 2018;47(2):879–892. doi:10.1159/000490080
35. Liu K, Xu SH, Chen Z, et al. TRPM7 overexpression enhances the cancer stem cell-like and metastatic phenotypes of lung cancer through modulation of the Hsp90α/uPA/MMP2 signaling pathway. BMC Cancer. 2018;18(1):1167. doi:10.1186/s12885-018-5050-x
36. Wang Q, Sun W, Hao X, et al. Down-regulation of cellular FLICE-inhibitory protein (Long Form) contributes to apoptosis induced by Hsp90 inhibition in human lung cancer cells. Cancer Cell Int. 2012;12(1):54. doi:10.1186/1475-2867-12-54
37. Watanabe S, Goto Y, Yasuda H, et al. HSP90 inhibition overcomes EGFR amplification-induced resistance to third-generation EGFR-TKIs. Thorac Cancer. 2021;12(5):631–642. doi:10.1111/1759-7714.13839
38. Sang J, Acquaviva J, Friedland JC, et al. Targeted inhibition of the molecular chaperone Hsp90 overcomes ALK inhibitor resistance in non-small cell lung cancer. Cancer Discov. 2013;(4):430–443. doi:10.1158/2159-8290.CD-12-0440
39. Holdenrieder S, Wehnl B, Hettwer K, et al. Carcinoembryonic antigen and cytokeratin-19 fragments for assessment of therapy response in non-small cell lung cancer: a systematic review and meta-analysis. Br J Cancer. 2017;116(8):1037–1045. doi:10.1038/bjc.2017.45
40. Dal Bello MG, Filiberti RA, et al. The role of CEA, CYFRA21-1 and NSE in monitoring tumor response to Nivolumab in advanced non-small cell lung cancer (NSCLC) patients. J Transl Med. 2019;17(1):74. doi:10.1186/s12967-019-1828-0
41. Shi Y, Liu X, Lou J, et al. Plasma levels of heat shock protein 90 alpha associated with lung cancer development and treatment responses. Clin Cancer Res. 2014;20(23):6016. doi:10.1158/1078-0432.CCR-14-0174
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


