Back to Journals » International Journal of General Medicine » Volume 15
Predictive Value of Red Blood Cell Distribution Width for 1-Year All-Cause Mortality in Critically Ill Patients with Acute Myocardial Infarction
Authors Chen M , Liao L , Yan J, Lin FQ
Received 10 November 2021
Accepted for publication 16 December 2021
Published 11 January 2022 Volume 2022:15 Pages 465—471
DOI https://doi.org/10.2147/IJGM.S345109
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Min Chen, Lin Liao, Jie Yan, Fa-Quan Lin
Department of Clinical Laboratory, The First Affiliated Hospital of Guangxi Medical University, Nanning, People’s Republic of China
Correspondence: Fa-Quan Lin Email [email protected]
Objective: Red blood cell distribution width (RDW) on admission is a prognostic factor in cardiovascular disease. This study investigated the prognostic value of the RDW measured within 24 hours before discharge (24h dRDW) on 1-year all-cause mortality in critically ill patients with acute myocardial infarction (AMI), and compared the effect of 24h dRDW in anemia and non-anemia patients.
Materials and Methods: Altogether, 4088 patients with AMI were studied retrospectively. Data from the MIMIC-III database were collected and analyzed. The Kaplan–Meier method, Cox regression models, and receiver operating characteristic (ROC) analysis were used to assess the impact of 24h dRDW on all-cause mortality in AMI patients, and a stratified analysis was performed to investigate the prognostic value of 24h dRDW in anemia and non-anemia patients.
Results: Of the 4088 patients, there were 704 non-survivors (17.2%). The non-survivors had a higher RDW than the survivors (p< 0.001). Cox regression analysis showed that 24h dRDW had a significant independent association with 1‐year all-cause mortality in critically ill patients with AMI (quintile 5 vs quintile 1, HR, 95% CI: 2.847, 2.014– 4.023). The area under the ROC curve of 24h dRDW was 0.710 (95% CI, 0.689– 0.730). In the stratified analysis, a significant prognostic value of 24h dRDW was found in anemia patients for 1-year all-cause mortality, but not in non-anemia patients.
Conclusion: Elevated 24h dRDW values are significantly associated with increased hazards of all‐cause mortality in critically ill patients with AMI. Significant prognostic value of 24h dRDW was found in AMI patients with anemia, but not in those without anemia.
Keywords: acute myocardial infarction, anemia, prognostic factors, red blood cell distribution width, survival rate
Introduction
Red blood cell distribution width (RDW) is an indicator of the heterogeneity of red blood cell volume in the peripheral blood and is usually reported as part of the standard complete blood cell count. Combined with other parameters of the complete blood cell count, RDW is used to identify hematological diseases. However, increasing numbers of studies have shown that high RDW also is an independent predictor of a poor prognosis in many diseases, including coronary heart disease,1,2 heart failure,3,4 ischemic stroke,5 cardiogenic shock,6 and acute kidney injury.7 In addition, two recent reports have demonstrated the effectiveness of RDW in predicting postoperative damage to the central nervous system.8,9 Acute myocardial infarction (AMI) is one of the most serious cardiovascular events and the main cause of hospitalization and death worldwide.10 Therefore, identifying sensitive risk factors that indicate a poor prognosis before discharge can help improve the risk stratification of patients who have been hospitalized and treated for an AMI, and better understand the high-risk population about to be discharged. This will ensure that those patients who have a high mortality risk after AMI will have a close follow-up and additional home care after discharge in an effort to reduce the mortality of AMI. However, previous studies focused on the prognosis of baseline RDW at admission.11 After hospitalization, whether RDW before discharge can be used to predict the mid-term prognosis of AMI patients and the efficiency of RDW in anemia and non-anemia patients merit further investigation.
Therefore, the purpose of this study was to investigate the relationship between the RDW measured within 24 hours before discharge and the 1-year all-cause mortality in critically ill patients with AMI, and to compare the effect of RDW therein between anemia and non-anemia patients.
Materials and Methods
Data Collection
Data were extracted from the Medical Information Mart for Intensive Care (MIMIC-III). This public database on intensive care contains the health-related data of 53,423 patients treated in the intensive care unit (ICU) at the Beth Israel Deaconess Medical Center in Boston, MS, USA, from 2001 to 2012, including demographics, laboratory tests, vital signs, and survival data. After removing patient identification information, all researchers had free access to the data and the data were available from the PhysioNet website (http://www.physionet.org).12 The use of the database in this study was approved by the Institutional Ethics Committees of the Beth Israel Deaconess Medical Center and Massachusetts Institute of Technology (certification number 30165505). Requirement for individual patient consent was waived because the project did not impact clinical care and all protected health information was deidentified. This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University.
Case Inclusion Criteria
We restricted the search to adult patients (aged 18 years or above) with AMI using International Classification of Diseases- (ICD-) 9 code (codes from 410.00 to 410.92). Patients who met one or more of the following criteria were excluded from this study: (1) RDW not measured within 24 hours before discharge or more than 5% of personal data missing; (2) blood diseases, such as leukemia and myelodysplastic syndrome; and (3) death during hospitalization or within 1 day after discharge.
Data Extraction
We used the MIMIC-III structured query option to extract the following parameters: age, sex, race, type of admission, marital status, body mass index, hospital days, length of ICU stay, complications, laboratory parameters, and patients’ scores for the Sequential Organ Failure Assessment (SOFA) and Simplified Acute Physiology Score II (SAPS II). The comorbidities included hypertension, diabetes, congestive heart failure, coagulopathy, liver disease, renal failure, obesity, alcohol abuse, drug abuse, mechanical ventilation, and sepsis. Laboratory parameters included white blood cell (WBC), platelet, hemoglobin, blood glucose, sodium, chloride, and potassium. We recorded the RDW measured within 24 hours before discharge (24h dRDW) in AMI patients, and categorized in quintiles as <13.3%, 13.3%–14.0%, 14.0%–14.8%, 14.8%–15.9%, and ≥15.9%.
Statistical Analysis
Continuous variables were reported as mean ± standard deviation (SD) or median (interquartile range [IQR]) and compared using the analysis of variance or the Kruskal–Wallis test. Categorical variables were reported as numbers and percentages and compared using chi-square test. We used the Kaplan-Meier method for survival analysis and the Log rank test to compare the survival rate among the groups. The relationship between the 24h dRDW before discharge and 1-year all-cause mortality of patients was investigated with Cox regression models. The results were expressed as the hazard ratio (HR) with the 95% confidence interval (CI). We adjusted for age, sex, ethnicity, congestive heart failure, coagulopathy, renal failure, obesity, sepsis, platelet, hemoglobin, sodium, chloride, and potassium, SOFA and SAPS II, hospital length of stay (LOS), and ICU LOS. These confounding factors were selected based on their correlation with the all-cause mortality of 24h dRDW or a change in effect estimate of >10%.13 The receiver operating characteristic (ROC) curve analysis was performed to investigate the prognostic value of the 24h dRDW. The prognostic value of the 24h dRDW was compared with the SOFA and SAPS II scores with the DeLong test. Because the RDW value can be affected by anemia, a stratified analysis was further performed to detect whether the value of 24h dRDW for survival prediction was different in patients with and without anemia. Anemia was defined by Hb <13 g/dL for men and Hb <12 g/dL for women, in accordance with the World Health Organization criteria.14 P < 0.05 was considered statistically significant. IBM SPSS Statistics for Windows, version 25.0 (IBM Corp., Armonk, NY, USA) was used for the statistical analysis, and graphs were generated with Prism, version 8.0 (GraphPad Software, San Diego, CA, USA).
Results
Patient Characteristics
A total of 4088 AMI patients were studied, including 1526 (37.3%) females and 2562 (62.7%) males. The median age was 70.7 years (IQR: 60.0–80.5 years), and the majority of patients were Caucasian (69.0%). The total number of deaths was 704 (17.2%). The most common comorbidities were hypertension (64.3%), congestive heart failure (46.8%) and mechanical ventilation (46.6%). The 24h dRDW of non-survivors was higher than that of survivors (median 15.5%; IQR 14.4%-16.8%, vs median 14.2%; IQR 13.4%-15.3%, p < 0.001). The characteristics of patients stratified according to 24h dRDW levels are shown in Table 1. Factors that significantly differed between the stratified groups included age, sex, congestive heart failure, coagulopathy, liver disease, renal failure, alcohol abuse, mechanical ventilation, sepsis, WBC, hemoglobin, Sodium, SOFA, SAPSII, hospital LOS, ICU LOS, and 1-year mortality.
 |
Table 1 Comparison of Clinical Characteristics Among Patients with Different 24h dRDW Levels |
Relationship Between 24-Hour RDW Before Discharge and 1-Year All-Cause Mortality in Patients with AMI
The Kaplan-Meier curve analysis showed that the 1-year all-cause mortality increases with the increase in the 24h dRDW quintiles (Log rank test, P < 0.001) (Figure 1). The results of the Cox regression analysis of the relationship between 24h dRDW and 1-year all-cause mortality are shown in Table 2. After adjusting for age, sex, ethnicity, congestive heart failure, coagulation disorder, renal failure, obesity, sepsis, platelet, hemoglobin, sodium, chloride, potassium, SOFA, SAPSII, hospital LOS, ICU LOS, and the 24h dRDW was an independent predictor of 1-year all-cause mortality, the HRs and 95% CIs (quintile 4 and quintile 5 vs quintile 1) were 1.874 (1.326, 2.649) and 2.847 (2.014, 4.023), respectively.
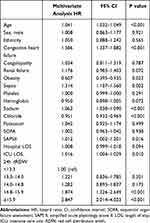 |
Table 2 HRs (95% CIs) for All-Cause Mortality Among Patient Groups Stratified by the 24h dRDW Levels |
 |
Figure 1 Kaplan-Meier curve for 1-year all-cause mortality of critically ill patients with acute myocardial infarction (AMI) according to quintile of 24h dRDW. |
Mortality Prediction
The ROC curves were generated using indicator variables (24h dRDW and SOFA and SAPS II scores) to predict 1-year all-cause mortality, as shown in Figure 2. The area under the curve (AUC) of the 24h dRDW, SOFA, and SAPS II were 0.710, 0.639, and 0.703, respectively. The AUC of the 24h dRDW was significantly larger than that of the SOFA score (DeLong test, P < 0.001). No significant difference was found between the AUC of the 24h dRDW and that of the SAPS II score. The optimal cutoff value of 24h dRDW was 14.65%, with a sensitivity of 71.3% and a specificity of 62.1%.
 |
Figure 2 Receiver operating curve (ROC) analyses of predictors of critically ill patients with acute myocardial infarction (AMI). |
Stratified Analysis for Anemia
In the stratified analysis, the 24h dRDW was significantly higher in patients with anemia than in patients without anemia (median 14.6%; IQR 13.6–15.7, vs median 13.5%; IQR 13.0–14.2, P < 0.001). A significant prognostic value of 24h dRDW was found in the anemia patients for 1-year all-cause mortality, but not in the non-anemia patients. (Table 3) The anemia patients with a higher 24h dRDW had a higher mortality rate than those with a lower 24h dRDW; the adjusted HRs and 95% CIs (quintile 4 and quintile 5 vs quintile 1) were 2.243 (1.732, 2.905) and 4.070 (3.203, 5.171), respectively (Table 3).
 |
Table 3 Adjusted HRs (95% CIs) for All-Cause Mortality Among Patient Groups Stratified by the 24h dRDW Levels and Presence of Concomitant Anemia |
Discussion
We found that the 24h dRDW of critically ill patients with AMI who died within 1 year after ICU discharge was significantly higher than that of survivors. A higher 24h dRDW was associated with increased 1-year all-cause mortality in these patients. After adjusting for confounding factors, 24h dRDW remained an independent predictor of 1-year all-cause mortality in critically ill patients with AMI. In the stratified analysis, a significant prognostic value of 24h dRDW was found in the anemia patients for 1-year all-cause mortality, but not in the non-anemia patients. The anemia patients with a higher 24h dRDW had a higher mortality rate than those with a lower 24h dRDW.
The RDW is a parameter that is commonly used to evaluate anemia and inflammation in the clinic. In recent years, increasing attention has been paid to the relationship between RDW and cardiovascular disease. In the current study, we retrospectively analyzed the relationship between the 24h dRDW and 1-year all-cause mortality in critically ill patients with AMI. Different from previous studies focusing on the baseline RDW on admission, the RDW we studied was measured within 24 hours before discharge; thus, patients with a high risk of subsequent adverse outcomes may benefit from more intensive follow-up regimens in the outpatient clinic and at rehabilitation. Previous studies on the relationship between baseline RDW on admission and patients with ST-elevation myocardial infarction showed that RDW is a marker with a very low prognostic accuracy and does not seem to be clinically helpful.11 Different from previous studies, The predictive ability of 24h dRDW was found to be significantly better than the traditional SOFA scoring system in our study. This inconsistency may be due to the differences in sample size and selection of subjects on the one hand, and the timing of RDW measurement on the other hand. The timing of the RDW measurement may affect the prognostic value of the RDW in AMI patients and its practical application in risk stratification. The importance of the timing of measurements was also acknowledged in a previous study on the relationship between hemoglobin and prognosis.15 The controversy regarding whether low hemoglobin presents a risk factor for a poor prognosis in AMI patients stems from the different times at which hemoglobin levels were measured in studies. Therefore, if the RDW is to be used to predict mortality, the exact time when the RDW has to be measured should be defined.
Anemia is an independent predictor of adverse cardiovascular events, bleeding, and mortality in patients with acute heart disease.16–18 In our study, we found that the association between 24h dRDW and 1-year all-cause mortality was influenced by the presence or absence of anemia, as evidenced by the significant statistical interaction term on multivariable Cox regression. Anemia patients with a higher 24h dRDW had a higher mortality rate than those with a lower 24h dRDW, implying an association between myocardial dysfunction and these hematologic abnormalities. Moreover, anemia is common among acutely ill cardiac patients.19 Our findings emphasize the importance of easily-available hematologic laboratory data for the risk stratification of anemic AMI patients, and highlight the interactions between hematologic abnormalities and acute cardiovascular disease outcomes.
Previous studies have found that RDW is associated with a poor prognosis of cardiovascular diseases, metabolic diseases, and acute and chronic diseases,3,20,21 but the underlying mechanism linking RDW with poor prognosis is not well understood. We believe that the RDW can be used to predict the prognosis of AMI patients because it reflects the level of inflammation. An inflammatory reaction has been shown to play an important role in the occurrence and development of AMI.22 When an inflammatory reaction occurs, iron metabolism, bone marrow function, and red blood cell proliferation and maturation are impaired, red blood cell production is ineffective, and the RDW is increased.23 When RDW levels exceed 14%, the deformability of red blood cells in microvessels24 and, therewith, perfusion decreases, resulting in the disturbance of the microcirculation.25,26 However, further studies are needed to better describe the mechanisms underlying the association between a high RDW and a poor prognosis of AMI patients as well as the clinical impact of using RDW measurements. One example in this context is whether RDW has any therapeutic value in addition to its prognostic value.
Our results should be interpreted within the limitations of this study. First of all, this was a single-center retrospective cohort study, which is easily affected by a bias in the study population selection. Second, due to the lack of data on some prognostic factors in the MIMIC-III database, for example, cardiac troponin, we could not assess whether the prognostic value of the 24h dRDW is independent of these established prognostic factors. Third, this study only studied the 24h dRDW. We hypothesize that if the RDW is dynamically monitored during the entire hospitalization period, it may prove to be an even more significant parameter. Lastly, it is worth noting that this study only included AMI patients who were treated in the ICU, and it is not clear whether our findings can be extended to AMI patients in cardiology or emergency departments. Therefore, a multicenter prospective study is needed to determine the relationship between the 24h dRDW and mortality in a larger population regardless of ICU treatment.
Conclusions
Elevated 24h dRDW values are significantly associated with increased hazards of 1-year all-cause mortality in the anemic AMI patients, but not in the non-anemic AMI patients. This result indicates that 24h dRDW is a readily available and inexpensive laboratory biomarker, and may be a promising prognostic clinical variable for risk stratification in anemic AMI patients. However, more studies are needed to confirm the present findings in different and larger AMI cohorts.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Health and Family Planning Commission of Guangxi Zhuang Autonomous Region [grant numbers Z20180963], and the National Natural Science Foundation of China [grant number 81800130].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Osadnik T, Strzelczyk J, Hawranek M, et al. Red cell distribution width is associated with long-term prognosis in patients with stable coronary artery disease. BMC Cardiovasc Disord. 2013;13(1):113. doi:10.1186/1471-2261-13-113
2. Parizadeh SM, Jafarzadeh-Esfehani R, Bahreyni A, et al. The diagnostic and prognostic value of red cell distribution width in cardiovascular disease; current status and prospective. Biofactors. 2019;45(4):507–516. doi:10.1002/biof.1518
3. Uemura Y, Shibata R, Takemoto K, et al. Elevation of red blood cell distribution width during hospitalization predicts mortality in patients with acute decompensated heart failure. J Cardiol. 2016;67(3):268–273. doi:10.1016/j.jjcc.2015.05.011
4. Huang YL, Hu ZD, Liu SJ, et al. Prognostic value of red blood cell distribution width for patients with heart failure: a systematic review and meta-analysis of cohort studies. PLoS One. 2014;9(8):e104861. doi:10.1371/journal.pone.0104861
5. Turcato G, Cappellari M, Follador L, et al. Red blood cell distribution width is an independent predictor of outcome in patients undergoing thrombolysis for ischemic stroke. Semin Thromb Hemost. 2017;43(1):30–35. doi:10.1055/s-0036-1592165
6. Wang B, Aihemaiti G, Cheng B, et al. Red blood cell distribution width is associated with all-cause mortality in critically ill patients with cardiogenic shock. Med Sci Monit. 2019;25:7005–7015. doi:10.12659/MSM.917436
7. Jia L, Cui S, Yang J, et al. Red blood cell distribution width predicts long-term mortality in critically ill patients with acute kidney injury: a retrospective database study. Sci Rep. 2020;10(1):4563. doi:10.1038/s41598-020-61516-y
8. Duchnowski P, Hryniewiecki T, Kuśmierczyk M, et al. Red cell distribution width as a predictor of multiple organ dysfunction syndrome in patients undergoing heart valve surgery. Biol Open. 2018;7:10.
9. Duchnowski P, Hryniewiecki T, Kuśmierczyk M, et al. The usefulness of selected biomarkers in aortic regurgitation. Cardiol J. 2019;26(5):477–482. doi:10.5603/CJ.a2018.0108
10. Moran AE, Tzong KY, Forouzanfar MH, et al. Variations in ischemic heart disease burden by age, country, and income: the global burden of diseases, injuries, and risk factors 2010 study. Glob Heart. 2014;9(1):91–99. doi:10.1016/j.gheart.2013.12.007
11. Sun XP, Chen WM, Sun ZJ, et al. Impact of red blood cell distribution width on long-term mortality in patients with ST-elevation myocardial infarction. Cardiology. 2014;128(4):343–348. doi:10.1159/000359994
12. Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3(1):160035. doi:10.1038/sdata.2016.35
13. Agoritsas T, Merglen A, Shah ND, et al. Adjusted analyses in studies addressing therapy and harm: users’ guides to the medical literature. JAMA. 2017;317(7):748–759. doi:10.1001/jama.2016.20029
14. Cappellini MD, Motta I. Anemia in clinical practice-definition and classification: does hemoglobin change with aging? Semin Hematol. 2015;52(4):261–269. doi:10.1053/j.seminhematol.2015.07.006
15. Zhang L, Yin P, Lv H, et al. Anemia on admission is an independent predictor of long-term mortality in hip fracture population: a prospective study with 2-year follow-up. Medicine. 2016;95(5):e2469. doi:10.1097/MD.0000000000002469
16. Backhaus T, Fach A, Schmucker J, et al. Management and predictors of outcome in unselected patients with cardiogenic shock complicating acute ST-segment elevation myocardial infarction: results from the Bremen STEMI Registry. Clin Res Cardiol. 2018;107(5):371–379. doi:10.1007/s00392-017-1192-0
17. Kwok CS, Tiong D, Pradhan A, et al. Meta-analysis of the prognostic impact of anemia in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2016;118(4):610–620. doi:10.1016/j.amjcard.2016.05.059
18. Brener SJ, Mehran R, Dangas GD, et al. Relation of baseline hemoglobin levels and adverse events in patients with acute coronary syndromes (from the acute catheterization and urgent intervention triage strategY and harmonizing outcomes with RevasculariZatiON and stents in acute myocardial infarction trials). Am J Cardiol. 2017;119(11):1710–1716. doi:10.1016/j.amjcard.2017.02.052
19. Lawler PR, Filion KB, Dourian T, et al. Anemia and mortality in acute coronary syndromes: a systematic review and meta-analysis. Am Heart J. 2013;165(2):143–53.e5. doi:10.1016/j.ahj.2012.10.024
20. Wang B, Gong Y, Ying B, et al. Relation between red cell distribution width and mortality in critically ill patients with acute respiratory distress syndrome. Biomed Res Int. 2019;2019:1942078. doi:10.1155/2019/1942078
21. Xanthopoulos A, Giamouzis G, Melidonis A, et al. Red blood cell distribution width as a prognostic marker in patients with heart failure and diabetes mellitus. Cardiovasc Diabetol. 2017;16(1):81. doi:10.1186/s12933-017-0563-1
22. Fang L, Moore XL, Dart AM, et al. Systemic inflammatory response following acute myocardial infarction. J Geriatr Cardiol. 2015;12(3):305–312. doi:10.11909/j.issn.1671-5411.2015.03.020
23. Arbel Y, Birati EY, Finkelstein A, et al. Red blood cell distribution width and 3-year outcome in patients undergoing cardiac catheterization. J Thromb Thrombolysis. 2014;37(4):469–474. doi:10.1007/s11239-013-0964-2
24. Patel KV, Mohanty JG, Kanapuru B, et al. Association of the red cell distribution width with red blood cell deformability. Adv Exp Med Biol. 2013;765:211–216.
25. Reinhart WH, Piety NZ, Goede JS, et al. Effect of osmolality on erythrocyte rheology and perfusion of an artificial microvascular network. Microvasc Res. 2015;98:102–107. doi:10.1016/j.mvr.2015.01.010
26. den Uil CA, Lagrand WK, van der Ent M, et al. Impaired microcirculation predicts poor outcome of patients with acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2010;31(24):3032–3039. doi:10.1093/eurheartj/ehq324
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
