Back to Journals » Cancer Management and Research » Volume 13
Predictive Value of Pretreatment Peripheral Neutrophil-to-Lymphocyte Ratio for Response to Neoadjuvant Chemotherapy and Breast Cancer Prognosis
Authors Li X , Tan Q , Li H , Yang X
Received 27 March 2021
Accepted for publication 16 July 2021
Published 28 July 2021 Volume 2021:13 Pages 5889—5898
DOI https://doi.org/10.2147/CMAR.S313123
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Xueqiong Zhu
Xiaomin Li,* Qiuwen Tan,* Hongjiang Li, Xiaoqin Yang
Department of Breast Surgery, West China Hospital, Sichuan University, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaoqin Yang
Department of Breast Surgery, West China Hospital, Sichuan University, No. 37 Guoxue Alley, Wuhou District, Chengdu City, 610041, Sichuan Province, People’s Republic of China
Tel +86-18980605792
Email [email protected]
Background: Neutrophil-to-lymphocyte ratio (NLR) is connected with the response to neoadjuvant chemotherapy (NAC) and prognosis. In addition, residual lymph node burden after NAC is likely important for prognosis. However, most studies have focused on the predictive value of NLR for NAC pathological complete response (pCR) rate. The relationship between NLR and post-operative residual lymph node ratio (LNR), and their prognostic values remain to be determined.
Methods: We retrospectively studied 282 patients with breast cancer who underwent curative surgery after NAC from 2008 to 2018. We collected pretreatment NLR in peripheral blood, the response to NAC, and the amount of axillary lymph nodes (positive and total) from patients who received axillary lymph node dissection (ALND). We followed up all patients from 2 to 116 months, with an average of 63 months. We analyzed the predictive value of pretherapeutic NLR in peripheral blood on the response of NAC, including pCR rate and postoperative LNR. The prognostic value of NLR and LNR was also analyzed.
Results: A pCR was achieved in 20 (27.0%) of 74 patients with low NLR, and 34 (16.3%) of 208 with high NLR (P = 0.045). In luminal A and luminal B tumors, patients with high NLR tended to have elevated LNR (LNR> 0.5; P=0.041). In Kaplan–Meier analysis, overall survival of patients with low NLR (NLR < 1.8; P = 0.033) was longer than that of patients with high NLR (NLR ≥ 1.8). Moreover, by multivariable analysis, LNR was negatively correlated with overall survival (P < 0.05) and disease-free survival (DFS) (P < 0.05).
Conclusion: pCR rate, post-operative remaining lymph node involvement and overall survival in all patients who received NAC may be predicted by NLR. Low NLR and LNR may suggest favorable outcomes.
Keywords: positive lymph node ratio, pathological complete response, lymph node ratio, positive lymph node ratio categories
Introduction
Great progresses have been made in neoadjuvant chemotherapy (NAC) for breast cancer.1,2 The function of NAC has been evolving, from debulking tumor burden in local advanced breast cancer and increasing breast conservation rate in relatively large tumors, to prediction of better prognosis in patients who achieved pathological complete response (pCR), especially in triple negative and HER2-positive diseases.3–7 Patients with residual tumors after NAC should receive treatments such as administration of capecitabine or TD-M1 to improve the prognosis.8,9 Therefore, to schedule treatment individually, it is important to find predictive markers for NAC.
Cancer-related inflammation has a critical effect on cancer development and progression. In tumor patients, abnormalities occur in circulating white blood cells, with a main change in the proportions of neutrophils, lymphocytes, and monocytes.10 Neutrophils release pro-inflammatory factors that promote cancer progression. In contrast to neutrophils, lymphocytes are a tumor-inhibiting factor.11,12 Thus, the neutrophil-to-lymphocyte ratio may be associated with systemic inflammatory responses in tumor patients. In a variety of cancers, including breast cancer, a high NLR correlated with poor prognosis.13–18 Furthermore, NLR had predictive value for chemosensitivity.19,20
Lymph node staging of breast cancer, on the basis of the 7th edition of the American Joint Committee on Cancer (AJCC) and International Union Against Cancer (UICC) TNM staging system,21,22 is based on positive lymph node count but not the entire number of lymph nodes dissected or the negative lymph node count. Numerous studies have indicated that lymph node ratio (LNR) has prognostic value in breast cancer.23–25 As total lymph nodes by axillary lymph node dissection (ALND) is comprised of positive and negative lymph nodes, it is essential to bring the negative lymph node count into consideration for predicting breast cancer prognosis. We have found that a combination of the negative lymph node count with LNR could be a substitute for positive lymph node count alone in predicting postoperative breast cancer survival.26 Besides, concerning the optimal cutoff of LNR for predicting the prognosis of breast cancer patients, previous studies have still not reached a consensus.27–29 Therefore, the prognostic value of LNR after NAC still needs to be determined.
In this study, we assessed the predictive value of NLR for NAC pCR rate and the prognostic value of both NLR and LNR after NAC. We also evaluated the correlation between NLR and LNR.
Methods
Patients
We retrospectively reviewed the medical records of 282 patients who were diagnosed with primary breast cancer and who received NAC in hospital from March 2008 to March 2018. The inclusion criteria were given below: (1) diagnosed as primary breast cancer by core needle biopsy before NAC; (2) female with clinical stage I–III; (3) obtained NAC and subsequent mastectomy or breast-conserving surgery. We excluded those patients with inflammatory breast cancer, stage IV disease, and pregnancy-associated breast cancer. This study was conducted in accordance with the Declaration of Helsinki. The Ethics Commission of West China Hospital of Sichuan University approved this research, and every participator signed informed consent.30
We collected information on age, pretreatment tumor size (T), tumor grade, lymph node status (N), status of metastasis (M), NAC regimen, surgical intervention, and outcomes (pathology results, response to NAC). Tumor stage was defined according to the TNM Classification of Malignant Tumors, UICC Seventh Edition.22 Subgroup analysis was performed for three subtypes: luminal-HER2-negative breast cancer (hormone receptor (estrogen receptor (ER) and/or progesterone receptor (PR)) positive and HER2 negative), HER2-positive breast cancer (HER2 overexpressed and amplified with any ER/PR status), and triple-negative breast cancer (TNBC) (ER negative, PR negative, and HER2 not overexpressed nor amplified). Laboratory results included absolute neutrophil count and absolute lymphocyte count from peripheral blood obtained at the time of diagnosis, before NAC. Neutrophil-to-lymphocyte ratio (NLR) was defined as the ratio of neutrophil count to lymphocyte count from the peripheral blood sample.
Treatment
Every single patient got a standard protocol of NAC that comprised of 6 courses of TAC (75 mg/m2 docetaxel, 60 mg/m2 epirubicin, and 500 mg/m2 cyclophosphamide) every three weeks, or four courses of EC (90 mg/m2 epirubicin and 600 mg/m2 cyclophosphamide) every two weeks followed by 4 courses of 175 mg/m2 paclitaxel administered every two weeks or four courses of 100 mg/m2 docetaxel administered every three weeks. Patients with HER2-positive breast cancer got 6 mg/kg of trastuzumab every three weeks at the time of docetaxel treatment as well.30
The response to NAC was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria (version 1.1).31 After NAC, all 282 patients underwent mastectomy or breast-conserving surgery. Among them, 258 patients went through ALND. Pathological complete response (pCR) was defined as the complete disappearance of the invasive compartment of the lesion with or without intraductal components, including in the lymph nodes.7 Positive lymph node ratio (LNR) was worked out by dividing the number of involved lymph nodes by the number of dissected lymph nodes. In accordance with former studies,32–34 we grouped LNR values into LNR categories (LNRC: 0, 0.01–0.20, 0.21–0.65, >0.65), or by single LNR value 0.5 (high LNR: >0.5, low LNR: ≤0.5). The treatment (including NAC, surgery, radiotherapy, and endocrine therapy) of each patient was given in line with National Comprehensive Cancer Network (NCCN) guidelines.35
Follow-Up
After surgery, all the patients were assessed every three months for two years, and then every six months after two years. The median follow-up was 63 (range 2–116) months. Physical examination and ultrasound for breast and locoregional lymph nodes were performed at every visit to monitor tumor recurrence. Mammography and chest CT scans were performed every year.
Statistical Analysis
Statistical analysis was performed through the SPSS version 25.0 (IBM, Armonk, NY, USA). NLR cut-off points were analyzed using the Kaplan–Meier analysis. The Log rank test was used to compare the difference of variables between the two groups. We used a chi-square test to assess the association between NLR and clinical-pathological parameters as well as LNR. Overall survival and disease-free survival were evaluated using the Kaplan–Meier method (Log rank test) and Cox proportional hazard model as univariate and multivariate analysis. A value of P < 0.05 was considered to be statistically significant.
Results
Optimal Cutoff Value of NLR
ROC analysis (Figure 1) revealed the optimal cutoff of NLR was 1.8, and the corresponding area under the ROC curve as 0.591 (P=0.043). We divided the patients into high NLR (> 1.8) and low NLR (≤ 1.8) groups.
 |
Figure 1 Receiver operating curve for response to neoadjuvant chemotherapy was plotted to determine the optimum cut-off for neutrophil-to-lymphocyte ratio (NLR). |
Patient Features
We analyzed 282 patients, 208 with a high NLR and 74 with low NLR. Table 1 presents the baseline characteristics of the patients, showing the association of NLR with clinicopathological parameters. Low NLR was significantly correlated with pCR (P = 0.045, Pearson chi-square test) and decreased death events (P = 0.033, Pearson chi-square test). A pCR was achieved in 20 (27.0%) of 74 patients with low NLR and 34 (16.3%) of 208 patients with high NLR (P = 0.045; Figure 2).
 |
Table 1 Correlation Between Clinicopathological Parameters and NLR |
 |
Figure 2 The response to neoadjuvant chemotherapy in neutrophil-to-lymphocyte ratio (NLR) high and low groups. |
Association Between NLR and LNR
After NAC, 258 patients experienced ALND. Our analysis showed an association between NLR and LNR for these patients according to the molecular subtyping (Table 2). A notable difference existed in luminal A and luminal B tumors (P = 0.041, Pearson test) and no statistical significance in HER2-enriched and TNBC as well as total patients (Fisher’s Exact test, Fisher’s Exact test and Pearson's test, respectively).
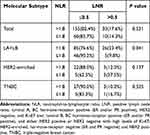 |
Table 2 Correlation Between NLR and LNR in Various Subtypes |
The Prognostic Value of NLR and LNR
Using Kaplan–Meier analysis (Log rank test), we found a lower NLR associated with longer overall survival (P = 0.033) but not with disease-free survival (P = 0.413; Figure 3). Moreover, patients with more residual lymph node involvement (high LNR) showed shorter overall survival (P < 0.001) and disease-free survival (P < 0.001; Figure 4). However, there was no significant difference in overall survival and disease-free survival between LNR=0 and NLR= 0.01–0.2 (P > 0.05; Supplementary Figure 1).
 |
Figure 3 The prognostic value of NLR for overall survival (A) and disease-free survival (B). |
Univariate analysis for prognosis indicated that, either by LNRC or single LNR value 0.5, the rise in LNR was associated with less overall survival (P < 0.001) and disease-free survival (P < 0.001; Table 3); High NLR was related to unfavorable overall survival (P=0.042). Moreover, multivariate analysis showed that rising LNR was independently correlated to severe prognosis by LNRC (Table 4).
 |
Table 3 Univariate Analysis of Factors Affecting Overall Survival and Disease-Free Survival |
 |
Table 4 Multivariate Analysis of Factors Affecting Overall Survival and Disease-Free Survival |
Discussion
In this study, we analyzed the association between NLR and prognosis (including the efficacy of NAC and survival outcomes) in breast cancer patients who received NAC. We found that low pretreatment NLR was a favorable prognostic predictor, which indicated that lower NLR (<1.8) was significantly associated with higher pCR rate and longer overall survival; these findings agreed with earlier reports.7,18,36,37 However, our study did not show any significant correlation between pretreatment NLR and disease-free survival (P = 0.413). Therefore, additional prospective and multicenter research is needed to affirm the relationship between NLR and survival outcomes of breast cancer patients who receive NAC.
Among circulating immune cells, neutrophils secrete many factors that promote cancer development,11,38,39 whereas lymphocytes exert a key function in the immune reaction against neoplasm. A high NLR in the peripheral blood, reflecting systemic inflammatory response to tumors, was reported as a poor prognostic factor in breast cancer.7,18,36,37 For patients who received NAC, we obtained a similar conclusion, ie, a high NLR was predictive for the inadequate reaction to NAC and poor survival. Our findings may have resulted from impaired host immune responses to malignancy.
Currently, axillary nodal status after NAC is based only on residual positive lymph node count and evaluated by TNM staging, according to the American Joint Committee on Cancer (AJCC). Axillary nodal status does not include the number of negative lymph node count. Rarely has the prognostic value of the negative lymph node count been reported. Wang et al.40 found that patients with a high negative lymph node count (≥10) had better survival after NAC. In our study, we assessed the prognosis of patients who went through ALND after NAC by LNR. We found that the increase in LNR, which suggested more lymph node residue and less negative lymph nodes, was markedly correlated with poor prognosis. Besides, we found LNR by LNRC could be an independent prognostic factor. Thus, more negative lymph nodes after NAC may indicate stronger host lymphatic response to malignancy after treatment and better prognosis.
Few studies have emphasized on the association between pretreatment NLR and post-NAC axillary nodal residue in breast cancer patients. In our study, we found that NLR was a predictive factor for lymph node involvement in luminal A and luminal B patients after NAC. Patients with high NLR showed higher LNR. Thus, we combined immune response in peripheral blood and in axillary lymph nodes. We found that both measurements were parameters for superior clinical outcomes of patients who received NAC.
Our investigation was a single-center, retrospective study, and the strength of our conclusion was weakened. Therefore, large-scale prospective clinical studies are needed to validate our results.
Conclusion
In summary, we have shown that decreased pretreatment NLR in peripheral blood of breast cancer patients predicted higher pCR rate after NAC and better overall survival. We reported for the first time that NLR was positively correlated with positive lymph node ratio in patients who went through ALND after NAC.
Abbreviations
NLR, neutrophil-to-lymphocyte ratio; NAC, neoadjuvant chemotherapy; pCR, pathological complete response; LNR, lymph node ratio; AJCC, the American Joint Committee on Cancer; UICC, International Union Against Cancer; LNRC, positive lymph node ratio categories; ALND, axillary lymph node dissection.
Funding
This study was funded by the Science and Technology Department of Sichuan Province (No.2019YFS0377).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bonadonna G, Veronesi U, Brambilla C, et al. Primary chemotherapy to avoid mastectomy in tumors with diameters of three centimeters or more. J Natl Cancer Inst. 1990;82(19):1539–1545. doi:10.1093/jnci/82.19.1539
2. Untch M, Konecny GE, Paepke S, von Minckwitz G. Current and future role of neoadjuvant therapy for breast cancer. Breast. 2014;23(5):526–537. doi:10.1016/j.breast.2014.06.004
3. Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from national surgical adjuvant breast and bowel project B-18. J Natl Cancer Inst Monogr. 2001;2001(30):96–102. doi:10.1093/oxfordjournals.jncimonographs.a003469
4. van der Hage JA, van de Velde CJH, Julien J-P, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for research and treatment of cancer trial 10902. J Clin Oncol. 2001;19(22):4224–4237. doi:10.1200/JCO.2001.19.22.4224
5. Mayer EL, Carey LA, Burstein HJ. Clinical trial update: implications and management of residual disease after neoadjuvant therapy for breast cancer. Breast Cancer Res. 2007;9(5):110. doi:10.1186/bcr1755
6. Sachelarie I, Grossbard ML, Chadha M, Feldman S, Ghesani M, Blum RH. Primary systemic therapy of breast cancer. Oncologist. 2006;11(6):574–589. doi:10.1634/theoncologist.11-6-574
7. Asano Y, Kashiwagi S, Onoda N, et al. Predictive value of neutrophil/lymphocyte ratio for efficacy of preoperative chemotherapy in triple-negative breast cancer. Ann Surg Oncol. 2016;23(4):1104–1110. doi:10.1245/s10434-015-4934-0
8. Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147–2159. doi:10.1056/NEJMoa1612645
9. von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–628. doi:10.1056/NEJMoa1814017
10. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi:10.1038/nature01322
11. McCourt M, Wang JH, Sookhai S, Redmond HP. Activated human neutrophils release hepatocyte growth factor/scatter factor. Eur J Surg Oncol. 2001;27(4):396–403. doi:10.1053/ejso.2001.1133
12. Mahmoud SM, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29(15):1949–1955. doi:10.1200/JCO.2010.30.5037
13. Tomita M, Shimizu T, Ayabe T, Yonei A, Onitsuka T. Preoperative neutrophil to lymphocyte ratio as a prognostic predictor after curative resection for non-small cell lung cancer. Anticancer Res. 2011;31(9):2995–2998.
14. Kishi Y, Kopetz S, Chun YS, Palavecino M, Abdalla EK, Vauthey JN. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. Ann Surg Oncol. 2009;16(3):614–622. doi:10.1245/s10434-008-0267-6
15. Aizawa M, Gotohda N, Takahashi S, Konishi M, Kinoshita T. Predictive value of baseline neutrophil/lymphocyte ratio for T4 disease in wall-penetrating gastric cancer. World J Surg. 2011;35(12):2717–2722. doi:10.1007/s00268-011-1269-2
16. Halazun KJ, Hardy MA, Rana AA, et al. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg. 2009;250(1):141–151. doi:10.1097/SLA.0b013e3181a77e59
17. Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg. 2010;200(2):197–203. doi:10.1016/j.amjsurg.2009.08.041
18. Chen Y, Chen K, Xiao X, et al. Pretreatment neutrophil-to-lymphocyte ratio is correlated with response to neoadjuvant chemotherapy as an independent prognostic indicator in breast cancer patients: a retrospective study. BMC Cancer. 2016;16:320. doi:10.1186/s12885-016-2352-8
19. Rossi L, Santoni M, Crabb SJ, et al. High neutrophil-to-lymphocyte ratio persistent during first-line chemotherapy predicts poor clinical outcome in patients with advanced urothelial cancer. Ann Surg Oncol. 2015;22(4):1377–1384. doi:10.1245/s10434-014-4097-4
20. Luo G, Guo M, Liu Z, et al. Blood neutrophil-lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Ann Surg Oncol. 2015;22(2):670–676. doi:10.1245/s10434-014-4021-y
21. Stephen BE, Compton C. The American Joint Committee on cancer: the 7th edition of the AJCC. Ann Surg Oncol. 2010;17:1471–1474. doi:10.1245/s10434-010-0985-4
22. SobinLH, WittekindC. International Union Against Cancer (UICC) TNM Classification of Malignant Tumours. 7th ed. NewYork: Wiley-Liss; 2011: 164–173.
23. Hatoum HA, Jamali FR, El-Saghir NS, et al. Ratio between positive lymph nodes and total excised axillary lymph nodes as an independent prognostic factor for overall survival in patients with nonmetastatic lymph node-positive breast cancer. Indian J Surg Oncol. 2010;1(4):305–312. doi:10.1007/s13193-011-0062-x
24. Van Belle V, Van Calster B, Wildiers H, Van Huffel S, Neven P. Lymph node ratio better predicts disease-free survival in node-positive breast cancer than the number of positive lymph nodes. J Clin Oncol. 2009;27(30):
25. Ibrahim EM, Elkhodary TR, Zekri JM, et al. Prognostic value of lymph node ratio in poor prognosis node-positive breast cancer patients in Saudi Arabia. Asia Pac J Clin Oncol. 2010;6(2):130–137. doi:10.1111/j.1743-7563.2010.01288.x
26. Yang J, Long Q, Li H, Lv Q, Tan Q, Yang X. The value of positive lymph nodes ratio combined with negative lymph node count in prediction of breast cancer survival. J Thorac Dis. 2017;9(6):1531–1537. doi:10.21037/jtd.2017.05.30
27. Truong PT, Vinh-Hung V, Cserni G, Woodward WA, Tai P, Vlastos G; Member of the International Nodal Ratio Working Group. The number of positive nodes and the ratio of positive to excised nodes are significant predictors of survival in women with micrometastatic node-positive breast cancer. Eur J Cancer. 2008;44(12):1670–1677. doi:10.1016/j.ejca.2008.05.011
28. Keam B, Im SA, Kim HJ, et al. Clinical significance of axillary nodal ratio in stage II/III breast cancer treated with neoadjuvant chemotherapy. Breast Cancer Res Treat. 2009;116(1):153–160. doi:10.1007/s10549-008-0160-9
29. Cho DH, Bae SY, You JY, et al. Lymph node ratio as an alternative to pN staging for predicting prognosis after neoadjuvant chemotherapy in breast cancer. Kaohsiung J Med Sci. 2018;34(6):341–347. doi:10.1016/j.kjms.2017.12.015
30. Li X, Tan Q, Li H, Yang X. Predictive value of tumor-infiltrating lymphocytes for response to neoadjuvant chemotherapy and breast cancer prognosis. J Surg Oncol. 2021;123(1):89–95. doi:10.1002/jso.26252
31. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
32. Tsai J, Bertoni D, Hernandez-Boussard T, Telli ML, Wapnir IL. Lymph node ratio analysis after neoadjuvant chemotherapy is prognostic in hormone receptor-positive and triple-negative breast cancer. Ann Surg Oncol. 2016;23(10):3310–3316. doi:10.1245/s10434-016-5319-8
33. Voordeckers M, Vinh-Hung V, Van de Steene J, Lamote J, Storme G. The lymph node ratio as prognostic factor in node-positive breast cancer. Radiother Oncol. 2004;70(3):225–230. doi:10.1016/j.radonc.2003.10.015
34. Danko ME, Bennett KM, Zhai J, Marks JR, Olson JA
35. N.C.C.N. (NCCN). Clinical practice guidelines in oncology. v.1.2012. Breast Cancer; 2012. Available from: http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf.
36. Marin Hernandez C, Pinero Madrona A, Gil Vazquez PJ, et al. Usefulness of lymphocyte-to-monocyte, neutrophil-to-monocyte and neutrophil-to-lymphocyte ratios as prognostic markers in breast cancer patients treated with neoadjuvant chemotherapy. Clin Transl Oncol. 2018;20(4):476–483. doi:10.1007/s12094-017-1732-0
37. Xu J, Ni C, Ma C, et al. Association of neutrophil/lymphocyte ratio and platelet/lymphocyte ratio with ER and PR in breast cancer patients and their changes after neoadjuvant chemotherapy. Clin Transl Oncol. 2017;19(8):989–996. doi:10.1007/s12094-017-1630-5
38. McCourt M, Wang JH, Sookhai S, Redmond HP. Proinflammatory mediators stimulate neutrophil-directed angiogenesis. Arch Surg. 1999;134(12):
39. Jablonska E, Kiluk M, Markiewicz W, Piotrowski L, Grabowska Z, Jablonski J. TNF-alpha, IL-6 and their soluble receptor serum levels and secretion by neutrophils in cancer patients. Arch Immunol Ther Exp. 2001;49(1):63–69.
40. Wang X, Yin Z, Wang D, et al. Greater negative lymph node count predicts favorable survival of patients with breast cancer in the setting of neoadjuvant chemotherapy and mastectomy. Future Oncol. 2019;15(32):3701–3709. doi:10.2217/fon-2019-0439
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

