Back to Journals » Cancer Management and Research » Volume 13
Predictive Value of Pretreatment Lymphocyte-to-Monocyte Ratio and Platelet-to-Lymphocyte Ratio in the Survival of Nasopharyngeal Carcinoma Patients
Authors Chen Y, Sun J, Hu D, Zhang J, Xu Y, Feng H, Chen Z, Luo Y, Lou Y, Wu H
Received 18 September 2021
Accepted for publication 10 November 2021
Published 23 November 2021 Volume 2021:13 Pages 8767—8779
DOI https://doi.org/10.2147/CMAR.S338394
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Rudolph Navari
Yibiao Chen,1,2 Jianda Sun,1,2 Dan Hu,1,2 Jian Zhang,1,2 Yuyun Xu,1,2 Huiting Feng,1,2 Zhijie Chen,1,2 Yi Luo,1,2 Yunlong Lou,2,3 Heming Wu2,4
1Department of Radiation Oncology, Meizhou People’s Hospital (Huangtang Hospital), Meizhou Academy of Medical Sciences, Meizhou, People’s Republic of China; 2Guangdong Provincial Key Laboratory of Precision Medicine and Clinical Translational Research of Hakka Population, Meizhou People’s Hospital (Huangtang Hospital), Meizhou Academy of Medical Sciences, Meizhou, People’s Republic of China; 3Department of Nuclear Medicine, Meizhou People’s Hospital (Huangtang Hospital), Meizhou Academy of Medical Sciences, Meizhou, People’s Republic of China; 4Center for Precision Medicine, Meizhou People’s Hospital (Huangtang Hospital), Meizhou Academy of Medical Sciences, Meizhou, People’s Republic of China
Correspondence: Yunlong Lou; Heming Wu
Meizhou People’s Hospital (Huangtang Hospital), No. 63 Huangtang Road, Meijiang District, Meizhou, 514031, People’s Republic of China
Tel +86 753-2131-591
Email [email protected]; [email protected]
Objective: The present study aimed to investigate the predictive value of some indexes, such as neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR), systemic inflammatory response index (SIRI), and systemic immune-inflammatory index (SII) in the survival of nasopharyngeal carcinoma (NPC) and provide reference for the treatment.
Methods: A retrospective analysis was performed on 216 patients from 2016 to 2018. The cutoff values of these indexes were determined by the receiver operating characteristic (ROC) curve. The prognostic value of the indexes was evaluated according to the rate of overall survival (OS), regional recurrence-free survival (RRFS), locoregional recurrence-free survival (LRRFS), and distant metastasis-free survival (DMFS).
Results: The survival analysis showed that NLR ≤ 2.695 (P = 0.017) and PLR ≤ 140.065 (P = 0.041) were associated with poor OS; however, the LMR and SIRI showed no significant statistical significance. NLR ≤ 2.045 (P = 0.018) and PLR ≤ 125.605 (P = 0.003) were associated with poor RRFS, LMR ≤ 2.535 (P = 0.027) and PLR ≤ 140.065 (P = 0.009) were associated with poor DMFS, NLR ≤ 2.125 (P = 0.018) and PLR ≤ 132.645 (P = 0.026) were associated with poor LRRFS, respectively. Logistic regression analysis showed that low LMR (≤ 2.535) was significantly inferior in OS (HR 23.085, 95% CI 3.425– 155.622, P = 0.001) and DMFS (HR 22.839, 95% CI 4.096– 127.343, P < 0.001). Moreover, low PLR (≤ 140.065) remained significantly related to worse OS (HR 11.908, 95% CI 1.295– 109.517, P = 0.029) and DMFS (HR 9.556, 95% CI 1.448– 63.088, P = 0.019).
Conclusion: The index LMR and PLR can be used for predicting survival in NPC patients.
Keywords: nasopharyngeal carcinoma, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, systemic inflammatory response index, systemic immune-inflammatory index
Introduction
Nasopharyngeal carcinoma (NPC) is an epithelial carcinoma arises from the nasopharyngeal mucosal lining. In the nasopharynx, the tumour often occur at the pharyngeal recess.1 There is a low incidence in the white population and Northern China, nevertheless the malignancy shows a high incidence in the Southern part of China. According to the International Agency for Research on Cancer, in 2018, there were about 129,000 new cases of NPC, and >70% of new cases were in Southeast Asia, with an age-standardised rate of 3.0 per 100,000 in China.2 At present, radiotherapy is the main treatment for NPC, and with the promotion of comprehensive treatment, the treatment effect and prognosis of NPC patients have been significantly improved. However, due to individual patient differences, recurrence and metastasis remain the main causes of treatment failure for NPC.3
To date, the prognosis of tumors, including NPC, is largely dependent on TNM stage. However, TNM staging is distinguished based on different anatomical structures and does not represent biological heterogeneity in NPC patients.4 For NPC, it is necessary to find some meaningful prognostic biomarkers. Inflammatory response leads to chronic oxidative stress and production of oxygen-free radicals, which is closely related to the occurrence and development of cancer.5 The detection of inflammatory markers in blood is convenient and low cost, which can be a prognostic reference index of NPC. At present, many inflammatory markers such as neutrophils, lymphocytes, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR) have been found to be associated with cancer prognosis.6–8 In addition, systemic inflammatory response index (SIRI) and systemic immune-inflammatory index (SII) are essential factors in predicting the survival of cancer patients.9,10 However, inflammatory markers are not tumor-specific indicators, and the relationship between inflammatory markers and NPC prognosis needs to be further confirmed.
The complex interactions between tumor cells and tumor microenvironment (TME) may play an important role in patient prognosis.11 The host immune response to the tumor is largely dependent on lymphocytes.12 Neutrophils are a major source of circulating angiogenesis regulating chemokines, growth factors and proteases, and are involved in tumor angiogenesis.13 Platelets can secrete inflammatory cytokines, chemokines, and vascular endothelial growth factors, promote the migration of inflammatory cells, and promote tumor angiogenesis and stroma formation.14,15 Macrophages and monocytes secrete tumor necrosis factor-alpha (TNF-α), vascular endothelial growth factor and epidermal growth factor, and promote tumgenesis and angiogenesis.16 Combined analysis of lymphocyte and monocyte levels (such as LMR) may be a prognostic factor for cancer patients.17 In recent years, the systemic inflammation response index (SIRI), an inflammation-based biomarker combining peripheral monocytes, neutrophils, and lymphocytes count, showed good prognostic ability in some solid tumors.18–20 A pooled analysis showed that NLR, PLR, and LMR are related to NPC survival outcomes in Asian populations, but there were some studies on the relationship between inflammatory markers and NPC prognosis have shown inconsistent results, which need to be further confirmed.21 In this study, the clinical stages, classification, hematological indicators and treatment methods before treatment were analyzed to evaluate the prognostic value of these indicators for NPC.
Materials and Methods
Subjects and Samples Collection
We retrospectively analyzed NPC patients treated at the Meizhou People’s Hospital between January 2016 and December 2018. The inclusion criteria were as follows: (1) The pathological diagnosis was nasopharyngeal carcinoma; (2) Computed tomography (CT) or magnetic resonance imaging (MRI) examination of nasopharynx and neck were performed before radiotherapy; (3) Complete blood biological test sheet before treatment. The exclusion criteria were as follows: (1) Patients with distant metastasis prior to treatment; (2) Patients with serious liver, kidney or heart diseases; (3) Patients with infection, or hematologic disorder; (4) NPC patients with concurrent other tumors. The study was conducted on the basis of the Declaration of Helsinki, and was supported by the Ethics Committee of the Meizhou People’s Hospital (Huangtang Hospital) (Clearance No.: 2016-A-50).
A total of 216 NPC patients were included in this study. Baseline data, including the general information, related medical history, hematological parameters, staging (AJCC 8th edition staging), and survival were collected. The blood samples were collected at admission and 2–3 days before treatment. 2 mL blood sample was taken via venipuncture of an antecubital vein from each subject and collected in tube with ethylenediaminetetraacetic acid (EDTA) as anticoagulant. Erythrocyte correlative indices were detected by Sysmex XE-2100 blood analyzer (Sysmex Corporation, Japan) according to the standard operating procedures (SOP).
Treatment: Radiotherapy and Chemotherapy
Radiotherapy is an important part of most NPC treatments. Radiotherapy equipment and technology all adopted Elekta Synergy S linear accelerator, image guidance in radiotherapy (IGRT) technology and Kilovoltage cone beam computed tomography (kV-CBCT) scanning. The process of IGRT treatment as follows: (1) Patients are fixed with thermoplastic body film, positioned under CT simulation, and then the scanned images are transmitted to the treatment planning system through local area network. (2) At least one attending physician and one deputy chief physician shall outline the target area and organs at risk. According to the guidance of the International Commission on Radiation Units and Measurements (ICRU) 50/62, gross tumor volume (GTV) and clinical target volume (CTV) in adult shall be delineated respectively. (3) The physicist performs field setting, parameter setting and dose calculation to optimize the treatment plan, transmitted to the Elekta Synergy S linear accelerator. Volumetric modulated arc therapy (VMAT) has been widely used in RT for NPC. Patients underwent a CT-scan with a 3 mm slice thickness. CTV were delineated based on EORTC 1219 head and neck study protocol. A margin of 3 mm was used to generate the PTV. All patients were evaluated weekly for treatment response and toxicity during chemoradiotherapy and radiotherapy. After completion of radiotherapy, the first follow-up was done within a month using an indirect fiberoptic nasopharyngoscope and physical examination.
Patients in stage I received radiotherapy alone, patients in stage II received concurrent chemoradiotherapy, patients in stage III and IVa received neoadjuvant chemotherapy combined with concurrent chemoradiotherapy, and patients in T4 and N3 received neoadjuvant chemotherapy combined with concurrent chemoradiotherapy followed by adjuvant chemotherapy. Neoadjuvant chemotherapy uses platinum-based two-drug or three-drug combination regimens for 1–3 courses. Concurrent chemoradiotherapy was performed with platinum, 1–2 courses of treatment. Adjuvant chemotherapy used platinum-based two-drug combination regimen, 1–3 courses; or fluorouracil oral chemotherapy drugs, 6–8 courses. Patients in stage III and IVa received targeted therapy with Nimotuzumab once a week during radiotherapy.
Follow-Up
After the completion of treatment, patients received regular examinations at our outpatient clinics every 3 months during the first 2 years, every 6–9 months in the 3rd to 5th years, and annually thereafter. Salvage treatments such as neck nodal dissection, re-radiotherapy or systemic chemotherapy were provided to patients with confirmed local-regional relapse or distant metastatic event. Endpoints included overall survival (OS), regional recurrence-free survival (RRFS), locoregional recurrence-free survival (LRRFS), and distant metastasis-free survival (DMFS).
In this study, OS is defined as time from the date of the completion of treatment to death from any cause. RRFS is defined as time from the date of the completion of treatment to recurrence of regional lymph nodes. LRRFS is defined as time from the date of the completion of treatment to recurrence of local or regional lymph nodes. DMFS is defined as time from the date of the completion of treatment to the date of distant metastasis.
Data Processing
Blood routine results were collected before the treatment and calculated the inflammation index according to the following formula: SII = platelet×neutrophil/lymphocyte, SIRI = monocyte×neutrophil/lymphocyte, NLR=neutrophil/lymphocyte, PLR=platelet/lymphocyte, LMR = lymphocyte/monocyte.
Statistical Analysis
Determination of Cutoff Values
The optimal cutoff values of NLR, LMR, PLR, SIRI, and SII for the predicting survival were determined by receiver operating characteristic (ROC) curve analysis. The optimal cut-off points with the highest Youden’s index of NLR, LMR, PLR, SIRI, and SII for OS, RRFS, DMFS and LRRFS analysis were selected by ROC curve analysis.
Survival Analysis
Survival curves were plotted using the Kaplan–Meier method, and the Log rank test was applied to assess the differences between survival rates. Survival outcomes in NPC patients were analyzed. Stage I-II and stage III-IV NPC patients were analyzed, respectively. The prognostic value of NLR, LMR, PLR, SIRI, SII, and other variables was assessed by multivariate Cox regression analysis. P < 0.05 indicated statistically significant difference. All the analyses were carried out using SPSS version 21.0.
Results
Patient Characteristics
A total of 216 NPC patients were included in this study, including 142 (65.7%) male patients and 74 (34.3%) female patients. One hundred and sixteen (53.7%) cases were 50 years old or younger, and 100 (46.3%) cases were older than 50 years old. The tumor stages of these patients were mainly in the middle and late stages. There were 122 (56.5%) patients in the T3-T4 stage, 119 (55.1%) patients in the N2-N3 stage. There were 38 (17.6%) patients in stageI-II, and 178 (82.4%) patients in stage III-IV (Table 1). The baseline hematologic markers of 216 NPC patients are shown in Table 2. There were no statistically significant differences in neutrophil, monocyte, lymphocyte counts, NLR, LMR, PLR, SII, and SIRI between patients aged under 50 and those aged over 50. There were statistically significant differences in monocyte count (P = 0.003) and LMR (P = 0.033) between male and female patients, while there were no statistically significant differences in neutrophil, lymphocyte counts, NLR, PLR, SII and SIRI. There was statistically significant difference in SII (P = 0.044) between T1-T2 and T3-T4 patients, while there were no statistically significant differences in neutrophil, monocyte, lymphocyte counts, NLR, LMR, PLR, and SIRI. There was statistically significant difference in monocyte count (P = 0.046) between N0-N1 and N2-N3 patients, while there were no statistically significant differences in neutrophil, lymphocyte counts, NLR, LMR, PLR, SII, and SIRI.
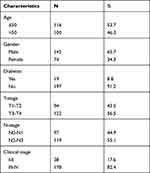 |
Table 1 Baseline Characteristics of 216 Patients with Nasopharyngeal Carcinoma |
 |
Table 2 Baseline Hematologic Markers of 216 Patients with Nasopharyngeal Carcinoma |
Cut-off Values for NLR, LMR, PLR, SII, and SIRI
The optimal cut-off value for the survival prediction was determined by ROC curve analysis. When OS was taken as the endpoint of NLR, LMR, PLR, SII, and SIRI, the critical value of NLR was 2.695 (sensitivity 65.5%, specificity 100%), the LMR cutoff value was 2.535 (sensitivity 70.9%, specificity 54.5%), the PLR cutoff value was 140.065 (sensitivity 50.2%, specificity 81.8%), and the SIRI cutoff value was 1.825 (sensitivity 73.4%, specificity 90.9%) (Figure 1A). When RRFS was used as the endpoint, the NLR cutoff value was 2.045 (sensitivity 58.4%, specificity 100%), the PLR cutoff value was 125.605 (sensitivity 65.1%, specificity 100%), and the SIRI cutoff value was 1.025 (sensitivity 61.2%, specificity 80.0%) (Figure 1B). When DMFS was used as the endpoint, the NLR cutoff value was 2.695 (sensitivity 59.1%, specificity 63.6%), the LMR cutoff value was 2.535 (sensitivity 71.4%, specificity 63.6%), the PLR cutoff value was 140.065 (sensitivity 50.2%, specificity 81.8%), and the SIRI cutoff value was 1.795 (sensitivity 72.4%, specificity 81.8%) (Figure 1C). When LRRFS was used as the endpoint, the NLR cutoff value was 2.125 (sensitivity 54.9%, specificity 87.5%), the LMR cutoff value was 3.065 (sensitivity 59.7%, specificity 50.0%), the PLR cutoff value was 132.645 (sensitivity 58.3%, specificity 75.0%), and the SIRI cutoff value was 1.280 (sensitivity 47.6%, specificity 75.0%) (Figure 1D).
Kaplan–Meier Curves
The Kaplan–Meier survival curves showed that NLR ≤2.695 (P = 0.017) (Figure 2A) and PLR ≤140.065 (P = 0.041) (Figure 2C) were associated with poor OS; however, the K-M curve of LMR (Figure 2B) and SIRI (Figure 2D) showed no significant statistical significance (P > 0.05). The same results were seen in stage III-IV NPC patients (Supplemental Figure 1).
 |
Figure 2 The Kaplan–Meier survival curves of NLR (A), LMR (B), PLR (C), SIRI (D) based on the overall survival (OS) in NPC patients. |
The Kaplan–Meier survival curves showed that NLR ≤2.045 (P = 0.018) (Figure 3A) and PLR ≤125.605 (P = 0.003) (Figure 3C) were associated with poor RRFS; however, the K-M curve of LMR (Figure 3B) and SIRI (Figure 3D) showed no significant statistical significance (P > 0.05). The same results were seen in stage III-IV NPC patients (Supplemental Figure 2).
 |
Figure 3 The Kaplan–Meier survival curves of NLR (A), LMR (B), PLR (C), SIRI (D) based on the regional recurrence-free survival (RRFS) in NPC patients. |
The Kaplan–Meier survival curves showed that LMR ≤2.535 (P = 0.027) (Figure 4B) and PLR ≤140.065 (P = 0.009) (Figure 4C) were associated with poor DMFS; however, the K-M curve of NLR (Figure 4A) and SIRI (Figure 4D) showed no significant statistical significance (P > 0.05). The same results were seen in stage III-IV NPC patients (Supplemental Figure 3).
 |
Figure 4 The Kaplan–Meier survival curves of NLR (A), LMR (B), PLR (C), SIRI (D) based on the distant metastasis-free survival (DMFS) in NPC patients. |
The Kaplan–Meier survival curves showed that NLR ≤2.125 (P = 0.018) (Figure 5A) and PLR ≤132.645 (P = 0.026) (Figure 5C) were associated with poor LRRFS; however, the K-M curve of LMR (Figure 5B) and SIRI (Figure 5D) showed no significant statistical significance (P > 0.05). The K-M curve of NLR, LMR, PLR, and SIRI showed no significant statistical significance (P > 0.05) (Supplemental Figure 4).
 |
Figure 5 The Kaplan–Meier survival curves of NLR (A), LMR (B), PLR (C), SIRI (D) based on the locoregional recurrence-free survival (LRRFS) in NPC patients. |
Logistic Regression Analysis for Survival of NPC Patients
Logistic multivariate analysis model was used to obtain the independent predictive factors of survival of NPC. After adjustment for some potential confounders, such as age, gender, TNM stage, low LMR (≤2.535) was significantly inferior in OS (HR 23.085, 95% CI 3.425–155.622, P = 0.001) and DMFS (HR 22.839, 95% CI 4.096–127.343, P < 0.001). Moreover, low PLR (≤140.065) remained significantly related to worse OS (HR 11.908, 95% CI 1.295–109.517, P = 0.029) and DMFS (HR 9.556, 95% CI 1.448–63.088, P = 0.019) (Table 3).
 |
Table 3 Multivariate Analysis of OS, RRFS, LRRFS, and DMFS |
Discussion
NPC has distinct geographical distribution characteristics, and its incidence is mainly concentrated in Southeast Asia.22 With the progress of medical treatment, the survival rate of patients has been greatly improved. To better improve the survival rate and quality of life of patients, individual medical care has attracted more and more attention previous studies have analyzed the influence of prognostic factors on NPC patients.23 The analysis of influencing factors of prognosis helps doctors to improve patients’ treatment plans further, effectively improve the survival rate and quality of life of patients, and also help to promote individualized medical treatment and find a more suitable plan for patients.
Due to its special anatomical location and sensitivity to radiation, radiotherapy, especially intensity modulated radiotherapy (IMRT), has become the standard treatment for NPC.24 In recent years, targeted therapy has received more and more attention.25 In terms of prognosis, TNM staging system is the gold standard for predicting the prognosis of NPC. However, this system cannot reflect the biological heterogeneity among tumor individuals. Reliable biomarkers are of great significance in determining and improving the prognosis of NPC patients, and can be an important supplement to the existing TNM staging system.
The prognosis of tumor is not only related to the local characteristics of tumor, but also closely related to the immune and inflammatory responses of the body itself.26 The host immune response to the tumor is largely dependent on lymphocytes.12 Neutrophils are a major source of circulating angiogenesis regulating chemokines, growth factors and proteases, and are involved in tumor angiogenesis.13 Platelets also release a variety of growth factors and promote angiogenesis.27 At present, several inflammatory markers such as neutrophils, lymphocytes, NLR, and PLR have been found to be associated with NPC prognosis.21 The detection of blood inflammatory markers is convenient and low cost, which can be further promoted as a prognostic indicator of NPC. Previous studies on the relationship between inflammatory markers and NPC prognosis have shown inconsistent results, which need to be further confirmed.21
A study performed by Chua et al showed that no relationship between NLR and prognosis of NPC patients.28 The study analyzed two published randomized controlled trials, including 221 patients with nasopharyngeal cancer in the SQNP01 study and 172 in the NCC0901 study, which used 3.0 as the cut-off for NLR and showed that high NLR was associated with T stage, N stage, and overall TNM stage, but there was no statistical association with OS, DFS, DMFS, and LRFS. In fact, SQNP01 study and NCC0901 study included significantly different patients and treatment regiments, as well as different radiotherapy methods, which may be the main reason for this negative result. Although clinically standardized treatment is carried out according to clinical stages, differences in the treatment process of different patients may affect the prognosis, such as differences in treatment methods and courses. These factors are not included in the analysis of many studies, including this one, which is one of the deficiencies of our study.
The threshold determination method is also very important in the study of the prognostic value of NLR and PLR, and the specific values used in several published studies are inconsistent. ROC curve analysis was used to determine the cut-off value of NLR. Lu et al defined NLR > 3.73 as the group with high NLR,29 while another study used 2.28 as the critical value of NLR.30 In our study, the results of ROC curve analysis showed that for OS, RRFS, DMFS, and LRRFS, the AUC of NLR was 0.554, 0.780, 0.559 and 0.646, respectively. Regression analysis showed that there was no relationship between NLR and OS, RRFS, DMFS, and LRRFS. This suggests that NLR is not an independent prognostic factor in NPC patients.
The decrease of LMR indicates the increase of neutrophils and monocytes or the relative or absolute decrease of lymphocyte count. Neutrophils can secrete vascular endothelial-derived growth factor (VEGF) to provide sufficient microenvironment for tumor development.31 Monocytes can also promote tumor growth and angiogenesis by releasing VEGF, epidermal growth factor (EGF) and tumor necrosis factor-alpha (TNF-α) from megakaryocytes.32 At the same time, in vitro culture experiments showed that neutrophils could inhibit NK cell activity, and the inhibition degree was proportional to the number of neutrophils.33 Study has shown that the increase of monocytes suggests poor prognosis in some tumors,34 which is consistent with the findings in this study that NPC group with poor survival has lower LMR than the NPC patients with better survival group. As an important part of the body’s immune system to defend against tumor cells, lymphocytes can inhibit the proliferation and migration of tumor cells.35 Therefore, lymphocytopenia can weaken the host’s lymphocyte-dependent anti-tumor response and increase the risk of poor prognosis.
Studies have shown that PLR has no relationship with the prognosis of patients with intensity modulated radiotherapy for NPC and other head and neck tumors, and regression analysis showed that PLR has no significant relationship with PFS, DMFS and LRFS.36,37 However, PLR has been shown to be associated with NPC.38 Platelets can not only protect cancer cells by building a partial physical barrier towards NK cells, but also interfere with the recognition of cancer cells by NK cells. Platelets can transfer “normal” MHC-class I molecules onto the surface of tumor cells, which makes them unrecognizable as foreign cells and impairs cytotoxicity and IFN-γ production by NK cells. Moreover, platelet-derived TGF-β diminishes NK cell activity by NKG2D downregulation and inhibition of NK cell antitumor reactivity. TGF-β from platelets not only weakens NK cell function, but also increases tumor cell survival by activating the TGF-β/Smad and NF-κB pathways, which induce an EMT-like phenotype in cancer cells and increase metastases in vivo, and increases proliferation of cancer cells.39 Moreover, responses of the adaptive immune system and T-helper cell 17 differentiation are also regulated by platelets. It represents a double-edged sword in cancer progression, as these cells propagate angiogenesis and immunosuppressive activities but are also involved in recruiting immune cells into tumors and stimulating effector CD8+ T cells.40 The results of this study suggest that low PLR is a factor of poor survival in NPC. This may be related to the disorder of platelets, and the specific mechanism needs further study. Since this study was a relatively small retrospective study, it could not represent the all NPC patients, and the follow-up time was short, which had certain limitations. Therefore, a multi-center, large-sample prospective study is needed for further confirmation.
Conclusion
The results of this study indicated that LMR and PLR levels before treatment are associated with the prognosis of NPC patients, and may be independent risk factors affecting the prognosis of NPC patients. The detection of LMR and PLR in blood is convenient and low cost, which can be a prognostic reference index of NPC and can be used as a beneficial supplement for the evaluation of NPC patients.
Acknowledgments
The author would like to thank other colleagues who were not listed in the authorship of Center for Precision Medicine, Meizhou People’s Hospital (Huangtang Hospital) for their helpful comments on the manuscript.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Guangdong Provincial Key Laboratory of Precision Medicine and Clinical Translation Research of Hakka Population (Grant No.: 2018B030322003); the Science and Technology Program of Meizhou (Grant No.: 2019B0202001).
Disclosure
The authors declare that they have no competing interests.
References
1. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80. doi:10.1016/S0140-6736(19)30956-0
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
3. Lee VH, Lam KO, Chang AT, et al. Management of nasopharyngeal carcinoma: Is adjuvant therapy needed? J Oncol Pract. 2018;14(10):594–602. doi:10.1200/JOP.18.00219
4. Huang SH, O’Sullivan B. Overview of the 8th edition TNM classification for head and neck cancer. Curr Treat Options Oncol. 2017;18(7):40. doi:10.1007/s11864-017-0484-y
5. Khandia R, Munjal A. Interplay between inflammation and cancer. Adv Protein Chem Struct Biol. 2020;119:199–245. doi:10.1016/bs.apcsb.2019.09.004
6. Mano Y, Shirabe K, Yamashita Y, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg. 2013;258(2):301–305. doi:10.1097/SLA.0b013e318297ad6b
7. Yang HJ, Jiang JH, Liu QA, et al. Preoperative platelet-to-lymphocyte ratio is a valuable prognostic biomarker in patients with hepatocellular carcinoma undergoing curative liver resection. Tumour Biol. 2017;39(6):1010428317707375. doi:10.1177/1010428317707375
8. Ma JY, Liu Q. Clinicopathological and prognostic significance of lymphocyte to monocyte ratio in patients with gastric cancer: a meta-analysis. Int J Surg. 2018;50:67–71. doi:10.1016/j.ijsu.2018.01.002
9. Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. doi:10.1158/1078-0432.CCR-14-0442
10. Xu L, Yu S, Zhuang L, et al. Systemic inflammation response index (SIRI) predicts prognosis in hepatocellular carcinoma patients. Oncotarget. 2017;8(21):34954–34960. doi:10.18632/oncotarget.16865
11. Roma-Rodrigues C, Mendes R, Baptista PV. Targeting tumor microenvironment for cancer therapy. Int J Mol Sci. 2019;20(4):840. doi:10.3390/ijms20040840
12. Mansuri N, Birkman EM, Heuser VD, et al. Association of tumor-infiltrating T lymphocytes with intestinal-type gastric cancer molecular subtypes and outcome. Virchows Arch. 2021;478(4):707–717. doi:10.1007/s00428-020-02932-3
13. Liang W, Ferrara N. The complex role of neutrophils in tumor angiogenesis and metastasis. Cancer Immunol Res. 2016;4(2):83–91. doi:10.1158/2326-6066.CIR-15-0313
14. Cen RX, Li YG. Platelet-to-lymphocyte ratio as a potential prognostic factor in nasopharyngeal carcinoma: a meta-analysis. Medicine. 2019;98(38):e17176. doi:10.1097/MD.0000000000017176
15. Chen YP, Zhao BC, Chen C, et al. Pretreatment platelet count improves the prognostic performance of the TNM staging system and aids in planning therapeutic regimens for nasopharyngeal carcinoma: a single-institutional study of 2626 patients. Chin J Cancer. 2015;34(3):137–146. doi:10.1186/s40880-015-0006-x
16. Olingy CE, Dinh HQ, Hedrick CC. Monocyte heterogeneity and functions in cancer. J Leukoc Biol. 2019;106(2):309–322. doi:10.1002/JLB.4RI0818-311R
17. Teng JJ, Zhang J, Zhang TY, Zhang S, Li BS. Prognostic value of peripheral blood lymphocyte-to-monocyte ratio in patients with solid tumors: a meta-analysis. Onco Targets Ther. 2016;9:37–47. doi:10.2147/OTT.S94458
18. Qi Q, Zhuang L, Shen Y, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. 2016;122(14):2158–2167. doi:10.1002/cncr.30057
19. Hua X, Long ZQ, Huang X, et al. The preoperative systemic inflammation response index (SIRI) independently predicts survival in postmenopausal women with breast cancer. Curr Probl Cancer. 2020;44(4):100560. doi:10.1016/j.currproblcancer.2020.100560
20. Valero C, Pardo L, Sansa A, et al. Prognostic capacity of Systemic Inflammation Response Index (SIRI) in patients with head and neck squamous cell carcinoma. Head Neck. 2020;42(2):336–343. doi:10.1002/hed.26010
21. Yang S, Zhao K, Ding X, Jiang H, Lu H. Prognostic significance of hematological markers for patients with nasopharyngeal carcinoma: a meta-analysis. J Cancer. 2019;10(11):2568–2577. doi:10.7150/jca.26770
22. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
23. Scott JG, Berglund A, Schell MJ, et al. A genome-based model for adjusting radiotherapy dose (GARD): a retrospective, cohort-based study. Lancet Oncol. 2017;18(2):202–211. doi:10.1016/S1470-2045(16)30648-9
24. Yao JJ, Qi ZY, Liu ZG, et al. Clinical features and survival outcomes between ascending and descending types of nasopharyngeal carcinoma in the intensity-modulated radiotherapy era: a big-data intelligence platform-based analysis. Radiother Oncol. 2019;137:137–144. doi:10.1016/j.radonc.2019.04.025
25. Kang Y, He W, Ren C. Advances in targeted therapy mainly based on signal pathways for nasopharyngeal carcinoma. Signal Transduct Target Ther. 2020;5(1):245. doi:10.1038/s41392-020-00340-2
26. Ramadan S, Saka B, Yarikkaya E, Bilici A, Oncel M. The potential prognostic role of peritumoral eosinophils within whole tumor-associated inflammatory cells and stromal histological characteristics in colorectal cancer. Pol J Pathol. 2020;71(3):207–220. doi:10.5114/pjp.2020.99787
27. Wojtukiewicz MZ, Sierko E, Hempel D, Tucker SC, Honn KV. Platelets and cancer angiogenesis nexus. Cancer Metastasis Rev. 2017;36(2):249–262. doi:10.1007/s10555-017-9673-1
28. Chua ML, Tan SH, Kusumawidjaja G, et al. Neutrophil-to-lymphocyte ratio as a prognostic marker in locally advanced nasopharyngeal carcinoma: a pooled analysis of two randomised controlled trials. Eur J Cancer. 2016;67:119–129. doi:10.1016/j.ejca.2016.08.006
29. Lu A, Li H, Zheng Y, et al. Prognostic significance of neutrophil to lymphocyte ratio, lymphocyte to monocyte ratio, and platelet to lymphocyte ratio in patients with nasopharyngeal carcinoma. Biomed Res Int. 2017;2017:3047802. doi:10.1155/2017/3047802
30. Jin Y, Ye X, He C, Zhang B, Zhang Y. Pretreatment neutrophil-to-lymphocyte ratio as predictor of survival for patients with metastatic nasopharyngeal carcinoma. Head Neck. 2015;37(1):69–75. doi:10.1002/hed.23565
31. Itatani Y, Yamamoto T, Zhong C. Suppressing neutrophil-dependent angiogenesis abrogates resistance to anti-VEGF antibody in a genetic model of colorectal cancer. Proc Natl Acad Sci U S A. 2020;117(35):21598–21608. doi:10.1073/pnas.2008112117
32. Yang JG, Wang LL, Ma DC. Effects of vascular endothelial growth factors and their receptors on megakaryocytes and platelets and related diseases. Br J Haematol. 2018;180(3):321–334. doi:10.1111/bjh.15000
33. Spiegel A, Brooks MW, Houshyar S, et al. Neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov. 2016;6(6):630–649. doi:10.1158/2159-8290.CD-15-1157
34. Deng Q, He B, Liu X, et al. Prognostic value of pre-operative inflammatory response biomarkers in gastric cancer patients and the construction of a predictive model. J Transl Med. 2015;13(1):66. doi:10.1186/s12967-015-0409-0
35. He J, Lv P, Yang X, Chen Y, Liu C, Qiu X. Pretreatment lymphocyte to monocyte ratio as a predictor of prognosis in patients with early-stage triple-negative breast cancer. Tumour Biol. 2016;37(7):9037–9043. doi:10.1007/s13277-016-4793-8
36. Farhan-Alanie OM, McMahon J, McMillan DC. Systemic inflammatory response and survival in patients undergoing curative resection of oral squamous cell carcinoma. Br J Oral Maxillofac Surg. 2015;53(2):126–131. doi:10.1016/j.bjoms.2014.10.007
37. Selzer E, Grah A, Heiduschka G, Kornek G, Thurnher D. Primary radiotherapy or postoperative radiotherapy in patients with head and neck cancer: comparative analysis of inflammation-based prognostic scoring systems. Strahlenther Onkol. 2015;191(6):486–494. doi:10.1007/s00066-014-0803-1
38. Peng RR, Liang ZG, Chen KH, Li L, Qu S, Zhu XD. Nomogram based on Lactate Dehydrogenase-to-Albumin Ratio (LAR) and Platelet-to-Lymphocyte Ratio (PLR) for predicting survival in nasopharyngeal carcinoma. J Inflamm Res. 2021;14:4019–4033. doi:10.2147/JIR.S322475
39. Haemmerle M, Stone RL, Menter DG, Afshar-Kharghan V, Sood AK. The platelet lifeline to cancer: challenges and opportunities. Cancer Cell. 2018;33(6):965–983. doi:10.1016/j.ccell.2018.03.002
40. Stoiber D, Assinger A. Platelet-leukocyte interplay in cancer development and progression. Cells. 2020;9(4):855. doi:10.3390/cells9040855
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

