Back to Journals » Journal of Inflammation Research » Volume 16
Predictive Value of Perioperative Cardiac Troponin I in Patients Undergone Liver Transplantation: A Retrospective Cohort Study
Authors Zhang L, Guo SY, Wang G, Zheng X, Jia HM, Huang LF, Weng YB, Li WX
Received 6 May 2023
Accepted for publication 1 July 2023
Published 24 July 2023 Volume 2023:16 Pages 3135—3142
DOI https://doi.org/10.2147/JIR.S420252
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Lei Zhang,1,* Shu-Yan Guo,1,* Guan Wang,1 Xi Zheng,2 Hui-Miao Jia,2 Li-Feng Huang,2 Yi-bing Weng,1 Wen-Xiong Li2
1Department of Critical Care, Beijing Lu He Hospital, Capital Medical University, Beijing, 101120, People’s Republic of China; 2Department of Surgical Intensive Care Unit, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, 100020, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yi-bing Weng, Department of Critical Care, Beijing Luhe Hospital, Capital Medical University, 82 Xinhuanan Road, Tongzhou District, Beijing, 101199, People’s Republic of China, Tel +86 69543901-2128, Email [email protected] Wen-Xiong Li, Department of Surgical Intensive Care Unit, Beijing Chao-Yang Hospital, Capital Medical University, 8 Gongren Tiyuchuang Nanlu, Chaoyang District, Beijing, 100020, People’s Republic of China, Tel/Fax +86 1085231458, Email [email protected]
Objective: To examine the change rule and clinical significance of cardiac troponin I (cTnI) in the perioperative period of liver transplantation in adults, as well as its association with 28-day mortality.
Methods: This was a retrospective cohort study: patients who underwent elective orthotopic liver transplantation (OLT) in Beijing Chao-Yang Hospital between June 2015 and June 2020 were selected, and plasma cTnI values were collected through the electronic medical record system within 7 days after surgery. Furthermore, the baseline clinical data of these patients were collected, and the change curve of cTnI values following liver transplantation was plotted. Using univariate and multivariate logistic regression models, the relationship between the level of postoperative cTnI and short-term mortality was investigated. The primary study endpoint was mortality within 28 days after surgery.
Results: We included 414 patients who had undergone liver transplantation in this study, 48 of whom died within 28 days after surgery. cTnI, a specific marker of myocardial injury, could predict that the postoperative cardiovascular complications were higher in the death group and significantly affect the short-term prognosis of patients; however, its prognostic cut-off value was approximately 0.545 ng/mL (13×URL), indicating that a minor elevation of cTnI after liver transplantation did not significantly affect the prognosis. Moreover, a comparison of the baseline data and postoperative ICU management scores of the two groups revealed that diabetes, maximum value of cTnI > 0.545 ng/mL within 7 days, and the need for postoperative renal replacement therapy (RRT) were independent prognostic factors of death within 28 days after liver transplantation.
Conclusion: Within 7 days after surgery, an increase in cTnI to the maximum value of 0.545 ng/mL (13×URL) could have a significant impact on the short-term prognosis of patients. Diabetes and postoperative RRT were two independent prognostic factors for liver transplantation perioperative mortality.
Keywords: cardiac troponin I, liver transplantation, postoperative myocardial injury, short-term prognosis
Introduction
With the advancement of technology, liver transplantation has become one of the most effective treatments for patients with end-stage liver diseases, and the survival rate of transplant recipients has been increasing.1 However, the incidence of postoperative complications is relatively high due to the relatively long operation duration and high consumption of coagulating substances,2 specifically, the occurrence of postoperative myocardial ischemia can affect the length of stay in the intensive care unit of patients and possibly influence their prognosis.3 Cardiovascular complications are fatal complications that affect the prognosis after non-cardiac surgery,4 of which chest pain is a very common clinical symptom that is frequently masked up by postoperative pain management, such as sedation and analgesic drugs.5,6
The dynamic monitoring of cardiac troponin I (cTnI) is a primary observation index of myocardial ischemia.7 Elevation of cardiac troponin I (cTnI) has been found to be linked to postoperative myocardial infarction in non-cardiac surgeries, as reported in various studies.8 Furthermore, the elevation of cTnI has demonstrated a strong correlation with an increased risk of cardiovascular events, mortality, and graft loss in liver transplantation procedures.9 The presence of elevated preoperative cTnI levels has been associated with higher postoperative mortality rates within a 1-year follow-up for liver transplantation patients, suggesting its potential as a valuable indicator for identifying high-risk candidates.10 However, the majority of previous research has primarily focused on preoperative cTnI elevation, while the impact during the postoperative short-term period remains a subject of debate. This study aimed to investigate the prognostic factors associated with 28-day mortality and to determine if an elevated cTnI level was associated with the short-term prognosis of patients after liver transplantation.
Data and Methods
Participants
Patients who were admitted to the Surgical Intensive Care Unit (SICU) of Beijing Chao-Yang Hospital and underwent liver transplantation between June 2015 and June 2020 were enrolled in a retrospective cohort study. Inclusion criteria were (1) Age >18 years; (2) Patients with preoperative evaluation of cTnI, an electrocardiogram and transthoracic echocardiography; (3) The survival time after liver transplantation was >72 h. The following were the exclusion criteria: (1) Patients with elevated preoperative cTnI test (>0.04 ng/mL); (2) Patients undergoing multiple organ transplantations; (3) Patients with serious missing medical records. This study enrolled consecutive 414 recipients undergone liver transplantation, including living donor liver transplantation and liver transplantation from donation after cardiac death. All organs were donated voluntarily with written informed consent from the donors or their next of kin if the donors, and it was conducted in accordance with the Declaration of Istanbul. Additionally, the written informed consents were obtained from all patients. The studies were conducted in compliance with the Declaration of Helsinki and approved by the Ethics Committee of Beijing Chao-Yang Hospital, Capital Medical University (2021-Sec-55). This study was registered in the Chinese Clinical Trial Center (ChiCTR2100044257).
Data Collection and Related Definitions
Data Collection Indicators
The data of these patients were collected including demographic data (age, gender, body mass index), preoperative concomitant diseases (hypertension, diabetes, coronary atherosclerotic heart disease, chronic obstructive pulmonary disease, and chronic kidney disease), intraoperative data (operation duration, blood loss and blood transfusion volume, fluid balance, and use of vasoactive drugs) and postoperative data (including APACHE II (Acute Physiology and Chronic Health Evaluation) scores on the day of admission to SICU, Sequential Organ Failure Assessment (SOFA), and presence of concurrent sepsis). Multiple cTnI test values (cTnI D0-D7) immediate and within 7 days after surgery, liver and kidney functions (aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBiL), creatinine (Scr), prothrombin activity (PA), international standardized ratio (INR)) within 3 days after surgery, and other relevant laboratory indexes of enrolled patients were collected through the electronic medical record system.
Diagnostic Criteria for Sepsis
For patients with infections or suspected infections, the diagnosis was made according to the diagnostic criteria for Sepsis 3.0, and if the acute elevation of the sequential organ failure assessment (SOFA) score was ≥2 points.
Postoperative Anti-Infection and Immunosuppressive Treatment
Postoperative anti-infection treatment for patients was usually a third-generation cephalosporin + enzyme inhibitor, such as cefoperazone and sulbactam sodium. Considering the presence of infection in the donor or the presence of temperature increase and sepsis in the patient within 24 hours after surgery, timely addition of antifungal drugs should be considered, and timely upgrade to a broad-spectrum anti-infection treatment regimen to avoid the occurrence of septic shock. Immunotransplantation regimens usually include tacrolimus, balliximab for injection, and glucocorticoids.
Study Endpoints
The primary study endpoint of the study was patient mortality at 28 days after surgery, while the secondary endpoints included the incidence of acute myocardial infarction (MI), mechanical ventilation duration, length of SICU stay, length of hospital stay, and in-hospital mortality.
Statistical Analysis
SPSS 27.0 software was used for statistical analysis. The measurement data with a normal distribution are expressed as mean ± standard deviation (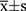 ), and t-test was used to compare between the groups. The measurement data with a non-normal distribution are expressed as median (range interquartile) [M (Q1, Q3)], and a non-parametric rank-sum test was used to compare between the groups. The enumeration data are expressed numerically, and the chi-squared test was used to compare between the groups. The relationship between the cTnI index and 28-day mortality was evaluated using Kaplan–Meier survival analysis, and its predictive value was determined using an ROC curve. Univariate logistic regression analysis was used to screen the relevant variables influencing the 28-day mortality of patients after liver transplantation. Variables with P < 0.1 were included in the multivariate logistic regression model (P < 0.05 indicated the difference was statistically significant), and odds ratios and 95% confidence intervals are also reported.
), and t-test was used to compare between the groups. The measurement data with a non-normal distribution are expressed as median (range interquartile) [M (Q1, Q3)], and a non-parametric rank-sum test was used to compare between the groups. The enumeration data are expressed numerically, and the chi-squared test was used to compare between the groups. The relationship between the cTnI index and 28-day mortality was evaluated using Kaplan–Meier survival analysis, and its predictive value was determined using an ROC curve. Univariate logistic regression analysis was used to screen the relevant variables influencing the 28-day mortality of patients after liver transplantation. Variables with P < 0.1 were included in the multivariate logistic regression model (P < 0.05 indicated the difference was statistically significant), and odds ratios and 95% confidence intervals are also reported.
Results
Clinical Characteristics of Patients
A total of 442 patients who underwent liver transplantation were initially included in the study, with 28 patients being excluded (preoperative cTnI > 0.04ng/mL) based on the data from the hospital’s electronic medical record system. The final number of included samples was 414, consisting of 293 males (89.1%) and 121 females (10.9%), with a median age of 53 years. Based on the 28-day survival status after surgery, the patients were divided into the survival group and the death group with 366 (88%) patients in the survival group and 48 (12%) patients in the death group.
Baseline Data of Patients
In Table 1, the demographic characteristics, primary diseases and complications, operation-related variables, postoperative variables, and clinical prognostic indexes are compared between the survival group and the death group. There was no significant difference between the two groups of patients in terms of age, gender, body mass index, coronary atherosclerotic heart disease, chronic obstructive pulmonary disease, cirrhosis, and other variables (P > 0.05). A total of 78 patients had diabetes, including 75 patients in the survival group and 3 patients in the death group, with the difference between the two groups being statistically significant (P < 0.05). Significantly higher rates of chronic kidney disease (16.7%) and sepsis (37.5%) were observed in the death group compared to the survival group (P < 0.05). In terms of intraoperative relevant variables, there were no significant differences between the two groups of patients regarding intraoperative RBC infusion volume, operation duration, and fluid balance (P >0.1). In terms of postoperative variables, APACHE II and SOFA scores in the death group were significantly higher than those in the survival group [13(10,18) vs 12(9,16), P < 0.10; [7(5,10) vs 9.5(6,12)], P<0.05]; The mechanical ventilation duration (M = 31.99 h) and length of hospital stay (M = 26.0 days) in the death group were significantly longer than those in the survival group (P < 0.05).
 |
Table 1 Comparison of Baseline Data Between the Survival Group and the Death Group |
Comparison of cTnI Between the Survival Group and the Death Group Within 7 Days After Surgery
In Table 2, the cTnI index within 7 days after surgery was generally higher in the death group than that in the survival group, with statistically significant differences at D1, D2, D3, D4, D6, and D7 (P < 0.05), and the median maximum value of cTnI within 7 days was significantly higher in the death group than that in the survival group (0.310 vs 0.145, P < 0.05). The ROC curve was plotted to compare the prediction value of maximum cTnI within 7 days and maximum cTnI within 3 days after surgery (Figure 1), and the AUC of maximum cTnI within 7 days was 0.600 [95% CI (0.503, 0.698) P = 0.024], which was significantly higher than the AUC of maximum cTnI within 3 days [0.571, 95% CI (0.474, 0.668) P = 0.109]. The cut-off value of maximum cTnI within 7 days was 0.545 ng/mL (13×URL). The Kaplan–Meier survival analysis curve demonstrated (Figure 2) that the survival rate of patients with cTnI > 0.545 ng/mL was significantly lower than that of patients without elevation of cTnI (Log Rank test, P < 0.05).
 |
Table 2 Intergroup Comparison of cTnI Within 7 Days After Surgery |
 |
Figure 1 ROC curve assessment predict value. |
 |
Figure 2 Kaplan-Meier survival curve. |
Analysis of Prognostic Factors
All variables were included in univariate analysis, and multivariate regression analysis was performed for variables with P < 0.1 in univariate analysis. According to the analysis results, the independent prognostic factors for 28-day mortality after liver transplantation were diabetes mellitus, maximum value of cTnI >0.545 ng/mL within 7 days, and the need for postoperative renal replacement therapy (RRT) (Table 3). The OR (95% CI) was 0.424 (0.214, 0.842) (P = 0.014) for maximum cTnI >0.545 ng/mL within 7 days.
 |
Table 3 Multivariate Logistic Regression Analysis of 28-Day Mortality |
Comparison of Liver and Kidney Function and Coagulation Function Indexes Between the Survival Group and the Death Groups
The liver and kidney function and coagulation function indexes of patients within three days of surgery were also included in this study. Compared to the relevant laboratory indexes of the survival group, the death group had higher AST and ALT values of patients. The TBil values at D0, D1, D3, and D4 were significantly higher in the death group than those in the survival group (116.30 vs 63.45,121.30 vs 69.50, 129.95 vs 54.60, 103.75 vs 48.00, P < 0.05). The death group had a higher creatinine value than the survival group, but the difference was not statistically significant. In terms of coagulation function indexes, the PA value on the second day after surgery in the death group (62.75%) was lower than that in the survival group (71.40%), P = 0.008, and the INR index on the third day after surgery in the death group was significantly higher than that in the survival group, with the difference being statistically significant (P < 0.05) (Table 4).
 |
Table 4 Inter-Group Comparison of Liver and Kidney Function and Coagulation Function Indexes |
Discussion
Multiple studies conducted in recent years on prognostic factors after liver transplantation indicate that cardiovascular events are one of the common causes of early and middle-term mortality among adult liver transplantation recipients. Koshy et al retrospectively analyzed 1,328 adult liver transplantation recipients with fatal outcomes and discovered that cardiovascular events accounted for 40% of the causes of intraoperative deaths.11 VanWagner et al conducted a retrospective analysis of 54,697 liver transplant recipients and discovered that cardiovascular events were the leading cause of death within 30 days (40.2%) after surgery.12 The reason was that analgesic sedation and other therapeutic measures were required due to mechanical ventilation, hemodynamic instability, and other reasons after the admission to the SICU post-surgery, which masked the autonomous symptoms of the patients. A multi-lead ECG could not be performed continuously due to its low sensitivity; hence, myocardial injury markers (primarily cTnI) were used for diagnosis.13 In addition, a number of factors complicate the diagnosis of postoperative acute myocardial infarction, including the unique incision of liver transplantation, the need for intraoperative blocking of arteriovenous vessels, ischemia-reperfusion injury, acute stress state, and inflammation.
In liver transplantation, the cardiac troponin concentration level has been found to be associated with all-cause mortality after liver transplantation.14 Park et al measured high sensitivity cTnI in 487 patients who underwent living donor liver transplantation (LDLT) and discovered that mortality was higher in patients with elevated cTnI levels before surgery (P < 0.001).9 Huang et al discovered that cTnI ≥0.01 ng/mL in a liver transplantation operation was significantly correlated with 30-day mortality.15 Park et al discovered that approximately 50.5% of recipients had elevated hs-cTnI levels immediately after liver transplantation, indicating myocardial injury during LDLT.7 In a retrospective study, Canbolata et al defined cTnI >0.04 ng/mL as the threshold for myocardial injury, but it could not be used to predict mortality within 30 days and 1 year after hospitalization.16 In this study, we found that an increase in the cTnI to the maximum value of 0.545 ng/mL within 7 days after surgery could have a significant impact on the short-term prognosis of patients.
cTnI, a sensitive marker of myocardial injury, is commonly used for the diagnosis of acute myocardial injury. There are two different types of myocardial injuries. “Type 1” myocardial injury manifests either as an ST-segment myocardial infarction (STEMI) or a non-STEMI due to intraductal thrombosis. The occurrence of “type 2” myocardial injury is caused by a mismatch between coronary oxygen supply and demand, or perioperative stress response, indicating an alteration in the ST segment. Most perioperative myocardial injuries are of “type 2”, and the majority of these patients are likely to have some degree of underlying coronary artery stenosis. Therefore, most perioperative liver transplant patients require fluid therapy and vasoactive or positive inotropic drugs to maintain hemodynamic stability. Meanwhile, it is justifiable to consider the management with known beneficial secondary prophylactic cardiac interventions like aspirin, beta blocker and statin for patients with a perioperative troponin elevation who survived the hospital period.17
In addition, a multivariate analysis revealed that diabetes and the requirement for postoperative RRT are two independent prognostic factors for a fatal outcome within 28 days of liver transplantation. 1) Diabetes: Prior to surgery, patients with diabetes suffer from systemic microvascular damage, abnormal glomerular blood flow, and vascular permeability, which could result in the occurrence of albuminuria, thereby damaging the kidney and inducing acute kidney injury (AKI). Wang et al discovered that the glomerular filtration rate of diabetic patients with AKI after liver transplantation decreased more rapidly during the first year following surgery, and that diabetes was an independent risk factor for AKI after orthotopic liver transplantation,18 which is similar to the findings of this study. 2) The need for RRT therapy following liver transplantation is frequently indicative of AKI or acute exacerbation of chronic renal insufficiency. According to studies, patients who require long-term renal replacement therapy after major operations have a significantly reduced short-term prognosis and survival treatment in the long run. Some scholars have determined that the mortality rate of patients with AKI within 1 year after orthotopic liver transplantation is as high as 45%.19
This study has some limitations. Due to the single-center retrospective cohort design, the scope of our findings is not universal. The factors of postoperative intensive care of patients included in this study were insufficient, such as the lack of specific analysis of postoperative invasive hemodynamic monitoring, infection indexes, pathogenic microorganisms, anti-infection therapeutic regimens, and immunosuppressive drug application. Additionally, this study did not analyze the practical significance of cTnI in predicting cardiac complications after liver transplantation by comparing it with other indicators related to cardiac complications, such as BNP, ECG and cardiac ultrasound findings. Therefore, in the future, we need more detailed monitoring of the intensive care of patients after liver transplantation to further improve their prognosis.
In conclusion, the elevation of cTnI levels is relatively common in patients within 7 days of liver transplantation, and a minor elevation of cTnI after liver transplantation did not significantly affect the prognosis, whereas a significant elevation of cTnI was an independent prognostic factor for 28-day mortality. Concurrent diabetes and postoperative kidney injury were also two independent prognostic factors for patients who had undergone liver transplantation. In order to improve the prognosis of patients who have received a liver transplant, it is necessary to strengthen the supportive treatment of organ functions.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Funding
This work was supported, in part, by grants from the National Natural Science Foundation (nos. 81372042); the Scientific and Breeding Program of Beijing Municipal Hospitals (no. PX2019010) and Beijing Municipal Science & Technology Commission (No. Z191100006619032).
Disclosure
The authors declare that they have no competing interests.
References
1. Jiang L, Zhang K, Mu Y, et al. The analysis in 41 consecutive cases at a single center for long-term survival after liver transplantation. Chin J Clin. 2012;6(2):351–354.
2. Park SK, Hur M, Kim WH. Acute kidney injury after pediatric liver transplantation. J Anesth. 2017;31(6):923–924. doi:10.1007/s00540-017-2412-5
3. Kopolovic I, Simmonds K, Duggan S, et al. Elevated cardiac troponin in the early postoperative period and mortality following ruptured abdominal aortic aneurysm: a retrospective population-based cohort study. Crit Care. 2012;16(4):R147. doi:10.1186/cc11461
4. Kertai MD, Boersma E, Klein J, et al. Long-term prognostic value of asymptomatic cardiac troponin T elevations in patients after major vascular surgery. Eur J Vasc Endovasc Surg. 2004;28(1):59–66. doi:10.1016/j.ejvs.2004.02.026
5. Reed GW, Horr S, Young L, et al. Associations between cardiac troponin, mechanism of myocardial injury and long-term mortality after non-cardiac vascular surgery. J Am Heart Assoc. 2017;6(6):e005672. doi:10.1161/JAHA.117.005672
6. Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after non-cardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564–578. doi:10.1097/ALN.0000000000000113
7. Clerico A, Emdin M, Passino C. Cardiac biomarkers and risk assessment in patients undergoing major non-cardiac surgery: time to revise the guidelines. Clin Chem Lab Med. 2014;52(7):959–963. doi:10.1515/cclm-2013-0900
8. Levy M, Heels-Ansdell D, Hiralal R, et al. Prognostic value of troponin and creatine kinase muscle and brain isoenzyme measurement after noncardiac surgery: a systematic review and meta-analysis. Anesthesiology. 2011;114:796–806. doi:10.1097/ALN.0b013e31820ad503
9. Park J, Lee SH, Han S, et al. Preoperative cardiac troponin level is associated with all-cause mortality of liver transplantation recipients. PLoS One. 2017;12(5):e0177838. doi:10.1371/journal.pone.0177838
10. Coss E, Watt KD, Pedersen R, Dierkhising R, Heimbach JK, Charlton MR. Predictors of cardiovascular events after liver transplantation: a role for pretransplant serum troponin levels. Liver Transpl. 2011;17:23–31. doi:10.1002/lt.22140
11. Koshy AN, Gow PJ, Han HC, et al. Cardiovascular mortality following liver transplantation: predictors and tem- poral trends over 30 years. Eur Heart J Qual Care Clin Outcomes. 2020;6(4):243–253. doi:10.1093/ehjqcco/qcaa009
12. VanWagner LB, Lapin B, Levitsky J, et al. High early cardiovascular mortality after liver transplantation. Liver Transpl. 2014;20(11):1306–1316. doi:10.1002/lt.23950
13. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020–2035. doi:10.1161/CIR.0b013e31826e1058
14. Watt KDS, Coss E, Pedersen RA, et al. Pretransplant serum troponin levels are highly predictive of patient and graft survival following liver transplantation. Liver Transpl. 2010;16(8):990–998. doi:10.1002/lt.22102
15. Huang S, Apinyachon W, Agopian VG, et al. Myocardial injury in patients with hemodynamic derangements during and/or after liver transplantation. Clin Transplant. 2016;30(12):1552–1557. doi:10.1111/ctr.12855
16. Canbolata IP, Adali G, Akdeniz CS, et al. Postoperative myocardial injury does not predict early and 1-year mortality after living donor liver transplantation. Transplant Proc. 2019;51(7):2478–2481. doi:10.1016/j.transproceed.2019.02.046
17. Jankowski K, Trzebicki J, Bielecki M, et al. Prognostic value of perioperative assessment of plasma cardiac troponin I in patients undergoing liver transplantation. Acta Biochim Pol. 2017;64(2):331–337. doi:10.18388/abp.2016_1436
18. Wang YJ, Li JH, Guan Y, et al. Diabetes mellitus is a risk factor of acute kidney injury in liver transplantation patients. Hepatobiliary Pancreat Dis Int. 2021;20(3):215–221. doi:10.1016/j.hbpd.2021.02.006
19. Bao B, Wang W, Wang Y, et al. A prediction score model and survival analysis of acute kidney injury following orthotopic liver transplantation in adults. Ann Palliat Med. 2021;10(6):6168–6179. doi:10.21037/apm-21-842
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
