Back to Journals » Journal of Inflammation Research » Volume 16
Predictive Value of Glycemic Variability and HDL-C for Secondary Persistent Inflammatory Immunosuppressed Catabolic Syndrome in Patients with Sepsis
Authors Yao J, Chen K, Tong H, Liu R
Received 4 August 2023
Accepted for publication 7 November 2023
Published 16 November 2023 Volume 2023:16 Pages 5299—5307
DOI https://doi.org/10.2147/JIR.S433895
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Jiali Yao, Kun Chen, Hongjie Tong, Ruixin Liu
Department of Critical Care Medicine, Jinhua Hospital Affiliated to Zhejiang University, Zhejiang, People’s Republic of China
Correspondence: Kun Chen; Hongjie Tong, Department of Critical Care Medicine, Jinhua Hospital Affiliated to Zhejiang University, 365 Renmin East Road, Wucheng District, Zhejiang, 321000, People’s Republic of China, Email [email protected]; [email protected]
Purpose: Sepsis patients with persistent inflammation, immunosuppression, and catabolism syndrome (PICS) have a poor prognosis, and early detection of biomarkers to predict its prognosis is particularly important. The purpose of this study is to investigate the predictive value of glycemic variability (GV) and high density lipid-cholesterol (HDL-C) for secondary PICS in patients with sepsis.
Patients and Methods: One hundred and sixty-five patients with sepsis were enrolled in the retrospective study and divided into sepsis secondary PICS and non-PICS group. The daily blood glucose levels from 1 to 7 days after the diagnosis of sepsis were recorded, and GV was calculated. Logistic regression was used to analyze the risk factors of sepsis with PICS, receiver operating characteristic (ROC) curve was drawn to evaluate the predictive value of GV, and HDL-C for the prognosis of sepsis with PICS.
Results: In a study of 165 patients, PICS group tended to have higher GV and lower HDL-C levels than those in the non-PICS group. Logistic regression analysis identified GV and HDL-C as independent risk factors for the secondary PICS of sepsis. The results of ROC curve showed that GV and HDL-C had a certain predictive value for the secondary PICS of sepsis, the sensitivity of GV was 77.5%, specificity of 81.8%, the sensitivity of HDL-C was 76.6%, and specificity of 74.8%.
Conclusion: GV and HDL are risk factors for PICS secondary to sepsis and have predictive value for patients’ prognosis.
Keywords: glycemic variability, high density lipid-cholesterol, sepsis, persistent inflammatory immunosuppressed catabolic syndrome
Introduction
Sepsis is the dysregulated immune response to infection that leads to life-threatening organ dysfunction and may cause or contribute to up to 5.3 million deaths worldwide per annum.1 The estimated incidence of ICU-treated sepsis was 58 per 100,000 person years,2 of which 41.9% had a higher risk of worse outcome or died prior to hospital discharge. The remaining survivors are presenting with chronic immunosuppressive and inflammatory states and chronically critical conditions.3 This is called persistent inflammation immunosuppression and catabolic syndrome (PICS), which explains the pathophysiological process of chronic critical illness (CCI),4 and is characterized by persistent inflammation, reduced host immunity, ongoing organ injury, cachexia, metabolic derangements, and myeloid dysfunction.5 Gentile et al coined the term PICS in 2012,6 and they propose that PICS is initiated by an early genomic and cytokine storm in response to microbial invasion during the early phase of sepsis.7 However, early identification, pathogenesis, and targeted interventions for PICS remain challenges faced by clinicians.
GV refers to fluctuations in blood glucose levels throughout the day as well as on different days but at the same time and is a risk factor for mortality among patients with sepsis that has been studied extensively in recent years. Lukana et al reported that sepsis patients with SOFA scores ≥9 had higher GV and long-term mortality rates than those with SOFA scores <9, and that sepsis severity was correlated with GV.8 HbA1c only represents the average blood glucose level in the past 1–3 months, whereas GV reflects the blood glucose changes in a shorter period of time, which can better reflect the drastic pathophysiological changes caused by sepsis.
High-density lipid-cholesterol (HDL-C) plays an important role in modulating the septic inflammatory response and characterization of lipid dysregulation and chronic inflammation during sepsis will aid mortality risk stratification, detection of sepsis, and inform individualized pharmacologic therapies.9 Lower HDL levels are closely associated with a poor prognosis in patients with sepsis. In one study, HDL levels independently predicted adverse outcomes and organ failure in patients with sepsis, and therefore, we speculate that lipids and their mediators may play an important role in patients with PICS secondary to sepsis.10 However, little has been reported regarding the predictive value of GV and HDL-C levels in PICS secondary to sepsis. To improve prognosis, it is important to explore effective diagnostic tools to assess the development of PICS in patients with sepsis. Therefore, this study investigated the predictive value of GV and HDL-C for secondary PICS in patients with sepsis, which could provide better guidance for clinical diagnosis and treatment.
Materials and Methods
In this study, G*Power 3.1.9.7 was used to calculate the minimum sample size, and t-tests were used, α=0.05, 1-β=0.8. This study included 165 patients with sepsis who were admitted to the ICU of Jinhua Hospital Affiliated to Zhejiang University from July 2020 to August 2022. Overall cohort inclusion criteria were (i) age ≥18 years, (ii) clinical diagnosis of sepsis as defined by the 2016 consensus guidelines,11 and (iii) the acute physiology and chronic health evaluation II (APACHE II) score ≥15 points. Exclusion criteria included any of the following: (i) refractory shock, ICU stay < 14 days or readmission or pre-admission expected life span <3 months; (ii) diagnosed of diabetic ketoacidosis, hyperosmolar hyperglycemia syndrome, or other hyperglycemic emergencies; (iii) receiving chronic corticosteroids or immunosuppressive agents; (iv) pregnancy; (v) severe fatty liver disease, dyslipidemia, or long-term use of lipid-lowering drugs; (vi) malignant tumor and chemotherapy or radiotherapy within 30 days; (vii) severe liver insufficiency and coagulation dysfunction; (viii) basic diseases of immune abnormality, such as autoimmune diseases; and (ix) inability to obtain informed consent or lack of clinical data. Following the application of the aforementioned inclusion and exclusion criteria, a total of 165 patients were ultimately enrolled in the study. This study followed the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Jinhua Hospital Affiliated to Zhejiang University (approval number 2022–101). Written informed consent to participate was waived for the use of clinical samples as retrospective samples were used in this study.
Blood Collection and Measurements
As patient characteristics, the following were extracted and evaluated: age, sex, APACHE II score on admission, length of ICU stay, and infection site (respiratory system, urinary system, bloodstream, abdomen, and others). Total white blood cells (WBCs) count, neutrophil count, lymphocyte (LYM) count, C-reactive protein (CRP), and serum albumin (ALB) levels were measured on the day of sepsis diagnosis and the 14th day of the course of the disease. At the same time, the levels of procalcitonin (PCT), lactic acid (Lac), interleukin-6 (IL-6), IL-8, IL-10, HDL-C, low-density lipoprotein cholesterol (LDL-C), cholesterol (TC), and glycated hemoglobin (HbA1c) were detected.
After admission, all patients underwent peripheral blood glucose monitoring every 2 hours. If the blood sugar level is greater than 180 mg/dl, continuous intravenous insulin is administered to maintain the blood sugar level between 100 and 180 mg/dL. In this study, patients were not treated with any other hypoglycemic therapy other than intravenous insulin infusion. During the ICU stay, fasting blood glucose levels of sepsis patients were monitored at the same time on days 1 to 7, and the glucose average (GLUave) within 7 days was calculated. GV refers to fluctuations in blood glucose levels throughout the day as well as on different days, but at same time, however, there remains no generally accepted gold standard or recommended value for assessing glucose variability. Among the most commonly used methods is long-term variability of the fasting blood glucose can be used to assess long-term variability in serum glucose levels. GV is the percentage of the standard deviation of all blood glucose measurements to the mean for each patient over a 7-day period, and GV = standard deviation/mean ×100% (GV=S / 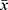 ×100%), and the standard deviation is calculated as the square root of the mean square variance of a single blood glucose value and the mean blood glucose. APACHE II score was scored according to the second version of APACHE II.
×100%), and the standard deviation is calculated as the square root of the mean square variance of a single blood glucose value and the mean blood glucose. APACHE II score was scored according to the second version of APACHE II.
Classification of PICS, Non-PICS
Following the application of the aforementioned inclusion and exclusion criteria, a total of 165 patients were ultimately enrolled in the study. According to the PICS criteria, the patients were divided into a non-PICS group and a PICS group. Subsequently, patients meet PICS criteria if residing in the ICU ≥14 days, CRP > 1.5 mg/L, serum ALB concentrations < 30 g/L and LYM counts of <0.80×109/ L on day 14.6 Patients who do not meet the above criteria were categorized as “non-PICS”.
Statistical Analysis
Statistical analyses were performed with IBM SPSS version 25 (IBM Corporation, New York City, NY, USA). Continuous variables were presented as mean ± standard deviation or median (interquartile range). The Student’s t-test or Mann‒Whitney U-test was used accordingly for the data analysis. Categorical patient data were presented as frequency and percentage and performed with the Fisher's exact test. Pearson correlation coefficient analysis was performed for the correlation analysis. Logistic regression was used to analyze the risk factors of secondary PICS in sepsis patients. Receiver operating characteristic curve (ROC curve) was used to evaluate the predictive value of related indicators for secondary PICS in sepsis patients. All significance tests were 2-sided, with p-value < 0.05 considered statistically significant.
Results
Baseline Characteristics
Of the 165 enrolled patients, 13 patients (14.8%) died within 28 days in non-PICS group, and 24 patients (31.2%) died within 28 days in PICS group. Age, ICU stay, APACHE II, HbA1c, GV, GLUave, IL-6, IL-8 and IL-10 were significantly higher, and LYM, 14d LYM, 14d ALB, HDL-C was lower in PICS group than in non-PICS group. There were no significant differences in gender, Infection site, WBCs, neutrophil, CRP, Lac, PCT, 14d WBCs, 14d Neutrophil, 14d CRP, LDL-C, TC in two groups (Table 1).
 |
Table 1 Baseline Patient Demographics and Characteristics |
The Column Chart Analysis
The significant variables in Table 1 were included in the column chart for prognostic analysis. According to the median of each variable, they were divided into two groups with high and low levels, and the influence of different levels of variables on the mortality of PICS patients with secondary sepsis was investigated. The results showed that different levels of APACHE II, GV, GLUave, IL-6 and IL-10, and HDL-C affect the mortality of patients with sepsis secondary PICS, while age, ICU stay, HbA1c, IL-8, 1d LYM, and 14d ALB had no statistical difference (Figure 1).
Characteristic Analysis of Patients Receiving Insulin Therapy
Sepsis patients who were treated with insulin and those who were not were divided into two groups, and statistical differences in GV, HDL-C, and PICS were compared. Patients were not treated with any other hypoglycemic therapy other than intravenous insulin infusion therapy. There were no significant differences in GV, HDL-C, and PICS in two groups (Table 2).
 |
Table 2 Characteristic Analysis of Patients Receiving Insulin Therapy |
Results from Logistic Regression and ROC Curve Analysis
Logistic regression analysis was performed based on the occurrence of PICS (defined “no” = 0, “yes” = 1) as the dependent variable, adjusting for confounding factors of age and insulin treatment therapy, and the results indicated that HDL-C, GV, and APACHEII were independent risk factors for PICS in patients with septic (OR: 0.013, 95% Cl: 0.001~0.141; OR: 1.089, 95% Cl: 1.030~1.151; OR: 1.036, 95% Cl: 1.008~1.066). Detailed results are indicated in Table 3.
 |
Table 3 Binary Logistic Regression Analysis of Secondary PICS Risk Factors in Patients with Sepsis |
Receiver operator characteristic analysis was performed on HDL-C and GV to assess the sensitivity/specificity predictive value of each for the development of PICS in patients with septic. High-density lipoprotein cholesterol was the best predictor of both the development of PICS (area under the curve [AUC] = 0.780, Youden index threshold = 0.514, sensitivity = 76.6%, specificity = 74.8%), and GV (AUC = 0.760, Youden index threshold = 0.593, sensitivity = 77.5%, specificity = 81.8%) (Table 4, Figure 2).
 |
Table 4 ROC Curve Parameter |
 |
Figure 2 ROC curve of the predictive value of GV and HDL-C for secondary PICS in sepsis patients. Abbreviations: GV, glycemic variability; HDL-C, high density lipid-cholesterol. |
Discussion
In this study, we aimed to analyze the relationship between GV, HDL-C level, and the development of PICS in patients with sepsis. We demonstrated that the PICS group had higher GV, HbA1c, and mortality rate, and lower HDL-C levels than the non-PICS group. We also found that GV and HDL-C levels were associated with PICS development in patients with sepsis. GV, HDL-C may be independent risk factors affecting the secondary PICS of sepsis, and the sensitivity of GV was 77.5%, the specificity of 81.8%, and the sensitivity of HDL-C was 76.6%, the specificity of 74.8%. The sensitivity and specificity of HDL-C are low to be good markers as well as GV, a good marker needs to be 85% and more whether diagnostic or prognostic, but these findings highlight the potential clinical utility of GV and HDL-C as predictors of secondary PICS in patients with sepsis.
Sepsis has three general trajectories: early death, rapid recovery, and chronic critical illness (CCI). Patients who survive the acute phase of sepsis can progress to PICS, which describes the pathobiology of this subset of CCI patients.12 While an increasing number of ICU patients survive their acute illness, they commonly remain hospitalized for extended periods of time, with complicated clinical courses and evidence of PICS.13 This study demonstrates that patients who developed PICS had markedly higher plasma IL-6, IL-8 and IL-10 levels and decreased lymphocyte counts compared with non-PICS individuals. This finding is consistent with the study by Julie A et al that demonstrated a more robust early inflammatory response (IL-6, IL-8, IL-10 were high), persistent immunosuppression (absolute lymphocyte count was low), and catabolism in PICS patients. Not only is their long-term survival dismal, but they also have significantly decreased physical function and health-related quality of life compared to rapid recovery patients.
GV is now recognized as an important index of glycemic control.14 Stress hyperglycemia is a common clinical phenomenon in sepsis at early stage, but high GV at late stage may reflect an overall response to treatment. Recent data suggest that hyperglycemia may potentiate pro-inflammatory response and play a potential modulation role in immunoinflammatory responses.15 Glucose is a powerful pro-inflammatory mediator that stimulates cytokine production and exacerbating oxidative stress response. High levels of anti-regulatory hormones such as catecholamines and glucocorticoids are released, leading to increased hepatic gluconeogenesis and insulin resistance. Compared with persistently high glucose levels, intermittent high glucose levels stimulate reactive oxygen species overproduction and increase cellular apoptosis, leading to further impaired endothelial function.16 Non-diabetic patients with sepsis have higher mean daily glucose levels and GV values than healthy subjects, and GV is associated with sepsis severity.17 In addition, Xiao F et al found that glycosylated serum protein combined with glycemic variability could effectively predict secondary PICS in elderly patients.18 In the present study, we found significant differences in GV and HbA1c when PICS individuals between patients with and without PICS. The PICS group had higher GV and HbA1c levels, and the APACHE II scores, mortality rate, and ICU stays were higher in the PICS group than in the non-PICS group. GV levels are associated with the development of PICS in patients with sepsis.
HDL-C plays an important role in regulating the immune response by clearing bacterial toxins, inhibiting endothelial cell apoptosis, reducing the monocyte inflammatory response, and inhibiting the expression of endothelial cell adhesion molecules.19 Among all lipoproteins, HDLs have the greatest affinity for binding pathogen-associated lipids, which mediate excessive immune activation in sepsis. In 3222 participants who experienced sepsis, there was a significant inverse association between continuous HDL-C levels (but not LDL-C or triglycerides) and 28-day mortality.20 A study demonstrated that plasma HDL-C level was greatly decrease in patients with sepsis and was the best prognostic marker for adverse outcomes in sepsis cohort, suggesting that the concept of increasing HDL-C levels in patients with certain infectious diseases, such as sepsis, may be a viable therapeutic target.21 Our study showed that PICS patients had lower HDL-C levels than the control group, and lower HDL-C levels in patients with sepsis were associated with development of PICS. Therefore, HDL levels play an important role in guiding clinical treatment and are a powerful parameter of disease severity in critically ill patients.
As expected, GV and HDL-C may be independent risk factors and predictors for the development of PICS in sepsis patients. The study found that septic patients with a higher acute GV had a significantly increased mortality risk.22 Hui Li et al study found that plasma HDL is downregulated in sepsis, which may facilitate inflammatory reaction and activate SOCS1 signaling to regulate the severity and affect prognosis of sepsis.23 Inhibiting cholesterylester transfer protein (CETP) can preserve high-density lipoprotein levels and decrease mortality in animal models of sepsis relative to placebo treatment.24 It has also been shown that the early stress-metabolic pattern in sepsis, characterized by a high glucose-low HDL combination, persists for the first 3 days and is associated with the acute-phase CD64 expression on neutrophils.25 HDL-C and GV levels may therefore provide valuable and easily accessible prognostic information for clinicians when encountering septic patients with suspected of developing PICS and facilitate more rapid intervention for those at highest risk for progression to PICS. The literature regarding the relationship of GV and HDL-C levels in the development of PICS has revealed a paucity of relevant studies that need further research in the future.
We note certain limitations in our study, including a relatively small sample size and the retrospective study. In addition, the data were collected from single center, and a larger sample with multi-centers is required for more accurate results.
Conclusion
Plasma GV is high and HDL-C is low in sepsis with PICS patients, these two indicators were closely related to PICS. GV and HDL-C may be independent risk factors and important predictors for the development of PICS in sepsis patients.
Ethics Approval and Consent to Participate
This study followed the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Jinhua Hospital Affiliated to Zhejiang University (approval number 2022–101). Written informed consent to participate was waived for the use of the clinical samples as retrospective samples were used in this study.
Acknowledgments
The authors gratefully acknowledge the support from Jinhua Hospital Affiliated to Zhejiang University.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by the Jinhua Hospital Affiliated to Zhejiang University in 2022 (JY2022-2-05), and the Jinhua Science and Technology Research Program in 2023 (grant number 2023-4-074).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193(3):259–272. doi:10.1164/rccm.201504-0781OC
2. Fleischmann-Struzek C, Mellhammar L, Rose N, et al. Incidence and mortality of hospital- and ICU-treated sepsis: results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. 2020;46(8):1552–1562. doi:10.1007/s00134-020-06151-x
3. Hotchkiss RS, Moldawer LL, Opal SM, et al. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2(1):16045. doi:10.1038/nrdp.2016.45
4. Hawkins RB, Raymond SL, Stortz JA, et al. Chronic critical illness and the persistent inflammation, immunosuppression, and catabolism syndrome. Front Immunol. 2018;9:1511. doi:10.3389/fimmu.2018.01511
5. Meegan A, Rosielle DA. Chronic critical illness in adults #343. J Palliat Med. 2018;21(1):99–100. doi:10.1089/jpm.2017.0546
6. Gentile LF, Cuenca AG, Efron PA, et al. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72(6):1491–1501. doi:10.1097/TA.0b013e318256e000
7. Bergmann CB, Beckmann N, Salyer CE, et al. Lymphocyte immunosuppression and dysfunction contributing to persistent inflammation, immunosuppression, and catabolism syndrome (pics). Shock. 2021;55(6):723–741. doi:10.1097/SHK.0000000000001675
8. Preechasuk L, Suwansaksri N, Ipichart N, et al. Hyperglycemia and glycemic variability are associated with the severity of sepsis in nondiabetic subjects. J Crit Care. 2017;38:319–323. doi:10.1016/j.jcrc.2016.12.005
9. Barker G, Leeuwenburgh C, Brusko T, et al. Lipid and lipoprotein dysregulation in sepsis: clinical and mechanistic insights into chronic critical illness. J Clin Med. 2021;10(8):1693. doi:10.3390/jcm10081693
10. Cirstea M, Walley KR, Russell JA, et al. Decreased high-density lipoprotein cholesterol level is an early prognostic marker for organ dysfunction and death in patients with suspected sepsis. J Crit Care. 2017;38:289–294. doi:10.1016/j.jcrc.2016.11.041
11. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
12. Mira JC, Brakenridge SC, Moldawer LL, et al. Persistent inflammation, immunosuppression and catabolism syndrome. Crit Care Clin. 2017;33(2):245–258. doi:10.1016/j.ccc.2016.12.001
13. Mira JC, Gentile LF, Mathias BJ, et al. Sepsis pathophysiology, chronic critical illness, and persistent inflammation-immunosuppression and catabolism syndrome. Crit Care Med. 2017;45(2):253–262. doi:10.1097/CCM.0000000000002074
14. Kusunoki Y, Konishi K, Tsunoda T, et al. Significance of glycemic variability in diabetes mellitus. Intern Med. 2022;61(3):281–290. doi:10.2169/internalmedicine.8424-21
15. Yu WK, Li WQ, Li N, et al. Influence of acute hyperglycemia in human sepsis on inflammatory cytokine and counterregulatory hormone concentrations. World J Gastroenterol. 2003;9(8):1824–1827. doi:10.3748/wjg.v9.i8.1824
16. Plummer MP, Deane AM. Dysglycemia and glucose control during sepsis. Clin Chest Med. 2016;37(2):309–319. doi:10.1016/j.ccm.2016.01.010
17. Lin S, Lai D, He W. Association between hyperglycemia and adverse clinical outcomes of sepsis patients with diabetes. Front Endocrinol (Lausanne). 2022;13:1046736. doi:10.3389/fendo.2022.1046736
18. Xiao F, Wang Y, Lin H, et al. predictive value of glycosylated serum protein combined with glycemic variability on secondary persistent inflammatory immunosuppressed catabolic syndrome prediction in elderly septic patients. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2018;30(11):1051–1055. doi:10.3760/cma.j.issn.2095-4352.2018.011.008
19. Barker G, Winer JR, Guirgis FW, et al. HDL and persistent inflammation immunosuppression and catabolism syndrome. Curr Opin Lipidol. 2021;32(5):315–322. doi:10.1097/MOL.0000000000000782
20. Aleman L, Guerrero J. sepsis hyperglycemia in the ICU: from the mechanism to the clinic. Rev Med Chil. 2018;146(4):502–510. doi:10.4067/s0034-98872018000400502
21. Trinder M, Walley KR, Boyd JH, et al. Causal inference for genetically determined levels of high-density lipoprotein cholesterol and risk of infectious disease. Arterioscler Thromb Vasc Biol. 2020;40(1):267–278. doi:10.1161/ATVBAHA.119.313381
22. Li X, Zhang D, Chen Y, et al. Acute glycemic variability and risk of mortality in patients with sepsis: a meta-analysis. Diabetol Metab Syndr. 2022;14(1):59. doi:10.1186/s13098-022-00819-8
23. Li H, Liu W, Su W, et al. Changes in plasma HDL and its subcomponents hdl2b and hdl3 regulate inflammatory response by modulating socs1 signaling to affect severity degree and prognosis of sepsis. Infect Genet Evol. 2021;91:104804. doi:10.1016/j.meegid.2021.104804
24. Trinder M, Wang Y, Madsen CM, et al. Inhibition of cholesteryl ester transfer protein preserves high-density lipoprotein cholesterol and improves survival in sepsis. Circulation. 2021;143(9):921–934. doi:10.1161/CIRCULATIONAHA.120.048568
25. Fitrolaki DM, Dimitriou H, Kalmanti M, et al. Cd64-neutrophil expression and stress metabolic patterns in early sepsis and severe traumatic brain injury in children. BMC Pediatr. 2013;13:31. doi:10.1186/1471-2431-13-31
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

