Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Predictive Value of GDF-15 and sST2 for Pulmonary Hypertension in Acute Exacerbation of Chronic Obstructive Pulmonary Disease
Authors Lv Z, Liang G, Cheng M
Received 6 July 2023
Accepted for publication 30 October 2023
Published 6 November 2023 Volume 2023:18 Pages 2431—2438
DOI https://doi.org/10.2147/COPD.S429334
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Zhigan Lv,1,2,* Guohua Liang,3,4,* Mengyu Cheng5,6
1Department of Anesthesiology, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Third Hospital of Shanxi Medical University, Taiyuan, Shanxi, People’s Republic of China; 2Department of Anesthesiology, Tongji Medical College, Tongji Hospital, Huazhong University of Science and Technology, Wuhan, Hubei, People’s Republic of China; 3Department of Intensive Care Medicine, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Third Hospital of Shanxi Medical University, Taiyuan, Shanxi, People’s Republic of China; 4Department of Intensive Care Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, People’s Republic of China; 5Department of Respiratory and Critical Care Medicine, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Third Hospital of Shanxi Medical University, Taiyuan, Shanxi, People’s Republic of China; 6Department of Respiratory and Critical Care Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mengyu Cheng, Department of Respiratory and Critical Care Medicine, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Third Hospital of Shanxi Medical University, No. 99, Longcheng Street, Taiyuan, Shanxi, 030032, People’s Republic of China, Tel +8613994237390, Email [email protected]
Objective: To confirm whether growth differentiation factor-15 (GDF-15) and soluble suppression of tumorigenicity 2 (sST2) are indicators of pulmonary hypertension in acute exacerbation of chronic obstructive pulmonary disease (AECOPD-PH).
Methods: All patients admitted to the hospital with AECOPD between July 2020 and October 2021 were enrolled. The patients were then categorized into AECOPD and AECOPD-PH groups according to PH probability, and the differences in GDF-15 and sST2 serum levels in the AECOPD and AECOPD-PH groups were compared. Correlation analysis was carried out to explore the association between GDF-15 and sST2 serum levels and the length of hospital stay of patients with AECOPD-PH. Receiver operating characteristic curve analysis was used to assess the clinical significance of GDF-15 and sST2 in predicting patients with AECOPD-PH.
Results: Included in this study were 126 patients with AECOPD, including 69 with AECOPD and 57 with AECOPD-PH. The serum levels of GDF-15 and sST2 in the AECOPD-PH group were significantly higher than those in the AECOPD group (P < 0.05). There was no significant correlation between the length of hospital stay in AECOPD-PH patients and GDF-15 and sST2 serum levels (P > 0.05). The area under the curves of GDF-15, sST2, and GDF-15 + sST2 for predicting AECOPD-PH and AECOPD-PH patients with poor prognosis were > 0.60 and 0.70, respectively. The optimal cutoff values of GDF-15 and sST2 for predicting AECOPD-PH were 1125.33 pg/mL and 80.68 ng/mL and 1309.72 pg/mL and 59.10 ng/mL for predicting AECOPD-PH patients with poor prognosis, respectively.
Conclusion: GDF-15 and sST2 levels may be useful in the prediction of AECOPD-PH.
Keywords: acute exacerbation of chronic obstructive pulmonary disease, pulmonary hypertension, soluble suppression of tumorigenicity 2, growth differentiation factor-15
Introduction
Chronic obstructive pulmonary disease (COPD), which has the highest incidence and mortality worldwide, is a frequent, treatable, and preventable chronic respiratory disease characterized by persistent airflow restriction.1 COPD has become a global epidemic and is the third leading cause of death worldwide and is also an important cause of disability and death in developing countries, and the latest World Health Organization projections on morbidity and mortality indicate that 5.4 million people could die annually from COPD and related conditions by 2060.2 Acute exacerbation of chronic obstructive pulmonary disease (AECOPD) means that patients with COPD need additional treatment owing to the aggravation of symptoms in a short period of time and have a higher mortality rate.3 Pulmonary hypertension (PH) is defined as a mean pulmonary arterial pressure ≥25 mmHg, which is a common condition in patients with AECOPD. Gupta et al found that the incidence rate of PH increased in patients with mild (16.67%), moderate (54.55%), severe (60.0%), and extremely severe (83.33%) COPD.4 Previous studies have shown that the prevalence of COPD-PH is 20–91%, and up to 90% of patients have a mean PH >20 mmHg at rest.5,6 Patients with COPD-PH lack specificity in their clinical presentation and are more prone to right-sided heart failure and death.7 Although right heart catheterization, echocardiography, and cardiovascular magnetic resonance imaging can be used clinically,8 there are still patients with AECOPD-PH who delay diagnosis due to hidden symptoms and lack of detection methods.
Serum biomarker testing is a valuable and important auxiliary diagnostic method that is relatively simple, convenient, and easy to perform in clinical practice. Soluble suppression of tumorigenicity 2 (sST2) is a reliable biomarker for worse clinical outcomes in patients with cardiovascular disease.9 Mirna et al showed that sST2 is a biomarker of PH.10 Growth differentiation factor-15 (GDF-15) is a stress protein expressed in multiple tissues and organs of the body that exerts cardioprotective effects through anti-inflammation and inhibition of myocardial apoptosis.11 Tantawy et al revealed that GDF-15 can predict adverse outcomes in clinical AECOPD patients.12 Also, it is reported that high levels of GDF-15 in serum are associated with lung transplantation and mortality risk in PH patients, and it can be used to predict low-risk PH patients due to the non-specific characteristics of GDF-15.13 Therefore, we speculate that serum GDF-15 and sST2 levels may be predictive factors for poor prognosis in various types of PH patients. However, studies on the prognostic evaluation of GDF-15 and sST2 in AECOPD-PH remain poorly reported and their clinical value needs to be explored.
Thus, the present study was conducted to confirm whether GDF-15 and sST2 could be used as early indicators of AECOPD-PH and offers useful predictive biomarkers for AECOPD-PH.
Materials and Methods
Study Population
All patients diagnosed with AECOPD who were admitted to the Respiratory Department of the Third Clinical Medical College of Shanxi Medical University between July 2020 and October 2021 were enrolled in this study. The inclusion criteria were as follows: (a) patients diagnosed with COPD were in line with the Global Initiative for Chronic Obstructive Lung Disease (GOLD2020);14 (b) each participant’s COPD diagnosis was based on the Global Initiative for Chronic Obstructive Lung Disease guideline standards (forced expiratory volume during the first second of forced breath [FEV1] to forced vital capacity [FVC] ratio after bronchodilation <0.70). The exclusion criteria were as follows: (a) serious nervous system disease and mental disorder; (b) bronchiectasis, pulmonary tuberculosis, interstitial lung, and other lung diseases; (c) received lung surgery; (d) chronic cardiovascular and cerebrovascular diseases, such as hypertension, diabetes, or coronary heart disease; (e) autoimmune disease; (f) tumors and blood system diseases; (g) systemic infectious diseases; (h) left cardiac insufficiency; (i) idiopathic pulmonary arterial hypertension (IPAH); (j) pulmonary thromboembolism and other diseases; (k) severe liver and kidney insufficiency; (l) undergoing hemodialysis within the last three months; (m) undergoing blood transfusion in recent two weeks; (n) unable or unwilling to cooperate with researchers or have no available spirometry data; and (o) incomplete follow-up data. All enrolled patients signed informed consent forms prior to their participation in this study. This study was approved by the Ethics Committee of the Third Hospital of Shanxi Medical University (approval no. YXLL-2020-056) and complied with the Declaration of Helsinki.
Data Collection
The following clinical pathological data of all enrolled patients were recorded: age, sex, height, weight, body mass index (BMI), smoking index (SI), Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification (based on the determination of pulmonary function after the use of bronchodilators in stable COPD, pulmonary function of patients was divided into 1–4 grades), vital signs, and arterial blood gas analysis indexes within 30 min after admission (pH, PaO2, oxygenation index [OI], and PaCO2). On the morning of the day after admission, 2–3 mL of fasting venous blood was drawn from the enrolled patients, and the GDF-15 and sST2 serum levels were tested using an ELISA kit (Bo’ao Xinyuan Biotechnology Co., Ltd, Shanxi, China). The length of hospital stay was recorded by the attending physician or above who did not participate in this study.
After admission, transthoracic echocardiography was performed, and the peak tricuspid regurgitation velocity, right ventricular parameters, and pulmonary artery systolic pressure were measured and recorded by a senior professional physician to assess the possibility of PH. According to the 2021 Chinese Guidelines for the treatment and diagnosis of PH, the low, intermediate, and high probabilities of PH were evaluated according to the results of transthoracic echocardiography, as shown in Table 1. Patients with a low or intermediate probability of PH were included in the AECOPD group, and those with a high probability of PH were included in the AECOPD-PH group. Differences in general clinical baseline characteristics and serum levels of GDF-15 and sST2 between the AECOPD and AECOPD-PH groups were compared. The association between the levels of GDF-15 and sST2 and PH in AECOPD patients was analyzed. Receiver operating characteristic (ROC) curve analysis was conducted, and the area under the curve (AUC) was evaluated to analyze the predictive probabilities of GDF-15 and sST2 levels in AECOPD-PH patients. Additionally, the association between the levels of GDF-15 and sST2 in AECOPD-PH patients and length of hospital stay was analyzed.
 |
Table 1 Transthoracic Echocardiography (TTE) Examination Performed to Assess the Possibility of PH |
In addition, follow-up was extended until 90 days after the initial hospital admission, and the life state of AECOPD-PH group patients and the event of AECOPD-PH group patients hospitalized again due to new AECOPD (defined as requiring antibiotics and/or oral corticosteroids, emergency and/or hospitalization) within 90 days were recorded. According to whether the patients in the AECOPD-PH group were readmitted within 90 days due to acute exacerbation of COPD, the 57 AECOPD-PH were categorized into poor prognosis and control groups, and the GDF-15 and sST2 serum levels of the two groups were compared. The optimum cutoff values of the GDF-15 and sST2 serum level predictive parameters in AECOPD-PH patients with poor prognosis were analyzed using ROC curve analysis.
Statistical Analysis
All data were processed utilizing SPSS ver. 26.0 software (SPSS Inc., Chicago, IL, USA). The normality of the continuous variable data was analyzed using the Shapiro–Wilk method. Normally distributed numerical variables are shown as mean ± SD, and the parameters that showed a non-normal distribution are presented as median-interquartile range. Normally distributed numerical variables were contrasted using the two independent sample t-test, and a rank sum test of two independent samples was utilized for the comparison of non-normally distributed numerical variables. Categorical variables are shown as number of cases and percentages, and the chi-squared test was used to compare between the groups. Spearman correlation test was used to perform the correlation analysis. Statistical significance was defined as P < 0.05.
Results
Characteristics of Patients
Included in the current study were 126 patients (aged 62.5–83.0 years) with AECOPD, including 104 males and 22 females, with 69 patients in the AECOPD group and 57 in the AECOPD-PH group. Basic clinical information of the patients is presented in Table 2. The age, sex, BMI, SI, GOLD classification, and OI did not differ remarkably between the AECOPD and AECOPD-PH groups (mean age: 70.00 [62.50 and 79.00] vs 73.00 [65.50 and 83.00], respectively, P = 0.269; sex [male/female]: [59/10] vs [45/12], respectively, P = 0.334; BMI: 23.2 [21.70 and 24.60] vs 23.00 [22.50 and 24.20], respectively, P = 0.984; SI: 800.00 [600.00 and 900.00] vs 700 [600.00 and 800.00], respectively, P = 0.336; GOLD classification [1–2/3/4 stage]: [10/17/42] vs [7/17/33], respectively, P = 0.790; and OI: 288.22 ± 72.31 vs 270.92 ± 69.68, respectively, P = 0. 175).
 |
Table 2 Baseline Characteristics of Patients with AECOPD |
Differences of GDF-15 and sST2 Serum Levels in AECOPD and AECOPD-PH Groups
The GDF-15 and sST2 serum levels in the AECOPD-PH group were remarkably higher than those in the AECOPD group (GDF-15: 1146.01 [904.45 and 1448.94] vs 1041.16 [798.88 and 1170.23], respectively, P = 0.004; and sST2: 71.10 [55.62 and 83.38] vs 66.75 [35.28 and 77.67], respectively, P = 0.027) (Table 3).
 |
Table 3 Differences of GDF-15 and sST2 Levels Between AECOPD Group and AECOPD-PH Group |
Association Between GDF-15 and sST2 Serum Levels and Length of Hospital Stay of AECOPD-PH Patients
There was no significant correlation between the length of hospital stay of AECOPD-PH patients and GDF-15 or sST2 serum levels (P > 0.05; Table 4).
 |
Table 4 Correlation Between GDF-15 and sST2 Levels and Length of Hospital Stay of AECOPD-PH Patients |
ROC Curve Analysis of GDF-15, sST2, and GDF-15 + sST2 in AECOPD-PH Patients
The optimal cutoff values of GDF-15 and sST2 were 1125.33 pg/mL and 80.68 ng/mL, respectively. The AUCs of GDF-15, sST2, and GDF-15 + sST2 were >0.60 (Table 5), and no significant differences in AUC were found between GDF-15 and sST2 (Z = 0.561, P = 0.575), GDF-15 and GDF-15 + sST2 (Z = 0.509, P = 0.611), or sST2 and GDF-15 + sST2 (Z = 1.128, P = 0.259) (Figure 1A).
 |
Table 5 ROC Curve Analysis of GDF-15, sST2 and GDF-15 + sST2 in AECOPD-PH Patients |
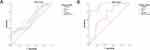 |
Figure 1 Receiver operating characteristic curve (ROC) analysis of GDF-15, sST2, and GDF-15 + sST2. (A) AECOPD-PH; (B) AECOPD-PH patients with poor prognosis. |
In addition, according to whether the AECOPD-PH group patients were readmitted within 90 days due to acute exacerbation of COPD, they were categorized into the AECOPD-PH patients with poor prognosis group (n = 36) and the control group (n = 21). The serum levels of GDF-15 and sST2 in the poor prognosis group were remarkably higher than those in the control group (P < 0.05; Table 6). In AECOPD-PH patients with poor prognosis, the optimal cutoff values of GDF-15 and sST2 were 1309.72 pg/mL and 59.10 ng/mL, respectively. In addition, the sensitivity and specificity of GDF-15, sST2, and GDF-15 + sST2 were >81.0, 41.7, and 81.0%, respectively. The AUCs of GDF-15, sST2, and GDF-15 + sST2 were >0.70 (Table 7). Significant differences in AUC were found between GDF-15 and sST2 (Z = 3.771, P = 0.0002) and between sST2 and GDF-15 + sST2 (Z = 3.790, P = 0.0002) (Figure 1B).
 |
Table 6 The Differences of the Serum GDF-15 and sST2 Levels in AECOPD-PH Patients with Poor Prognosis Group and Control Group |
 |
Table 7 ROC Curve Analysis of GDF-15, sST2 and GDF-15 + sST2 in AECOPD-PH Patients with Poor Prognosis |
Discussion
Patients with AECOPD-PH may develop pulmonary heart disease, leading to disease progression and increased risk of death.7 Early screening and prevention of PH in patients with COPD can improve their survival and quality of life. Serum biomarkers can be effectively measured in a non-invasive manner, which is an ideal disease screening tool.15,16 D’Ascanio et al showed that SpB might be considered as a useful marker in COPD assessment and provided prognostic information on lung functional decline.17 Therefore, the present study aimed to assess whether GDF-15 and sST2 could be early indicators of AECOPD-PH.
It has been reported that GDF-15 can be used as a signature in some diseases. High levels of GDF-15 are mainly related to pathological states, such as myocardial ischemia, inflammation, and cancer.18–20 Verhamme et al revealed that an increase in GDF-15 levels in the lungs of smokers and COPD patients contributed to lung inflammation caused by cigarette smoke.19 Mutlu et al have uncovered that GDF-15 is a novel biomarker predicting acute exacerbation of COPD.21 Kim et al showed that elevated serum GDF-15 level and PaCO2>37 mm Hg were found to predict an adverse outcome independently in patients with COPD exacerbation.22 Hirano et al showed that patients with COPD have higher serum GDF-15 levels than healthy controls.23 In the current study, GDF-15 levels in the AECOPD-PH group were remarkably higher than those in the AECOPD group, and the AUCs of GDF-15 were >0.60. In addition, GDF-15 has been widely studied as a signature of patient outcomes.24–26 Serum GDF-15 levels in AECOPD-PH patients in the poor prognosis group were remarkably higher than those in the control group. Moreover, the specificity and sensitivity of GDF-15 in AECOPD-PH patients with poor prognosis were > 81.0%, and the AUCs of GDF-15 were >0.90. These results suggest that GDF-15 could be a better predictor of AECOPD-PH and prognosis. Therefore, the baseline measurement of GDF-15 should be included in the clinical trial design of the Department of Respiratory and Critical Care Medicine, which considers the prognosis of diseases such as risk stratification as the endpoint of the study, especially in the relevant studies of pulmonary vascular diseases.
sST2 participates in the homeostasis and pathogenesis of some diseases and is triggered and expressed in the process of fibrosis, tissue injury, inflammation, and remodeling as the balance/response of the IL-33/ST2L axis activation.27 Higher levels of sST2 can be detected in the serum of patients with PH, and sST2 expression levels are related to the prognosis and severity of the disease.28 Ye et al showed that sST2 levels were remarkably elevated in patients with connective tissue disease associated with PH compared to that in the control group.29 Plácido et al showed that sST2 is significantly associated with mortality and poor clinical outcomes in PH, and sST2 levels in non-survivors with PH were significantly higher than those in survivors.30 Watanabe et al illustrated that erum sST2 levels correlated positively with asthma severity (treatment step), airway H2O2 levels, and serum IL-8 levels.31 Alladina et al uncovered that plasma sST2 associated with ventilator liberation in acute hypoxemic respiratory failure.21 Urban et al have found that sST2 concentrations are associated with severity of disease and long-term outcome in patients with COPD.32 In the present study, sST2 levels in the AECOPD-PH group and AECOPD-PH patients in the poor prognosis group were remarkably higher than those in the AECOPD and control groups, respectively, which is in line with the results of the above studies. The AUCs for sST2 were >0.60. In addition, the sensitivity of sST2 in AECOPD-PH patients with poor prognosis was >95.0%, which confirmed that sST2 may be a predictor of poor prognosis of AECOPD-PH.
This study had numerous limitations. First, it was conducted with a small sample size; thus, the results may have some errors. Large-scale clinical studies that contain treatment information during hospitalization and after discharge are required. Second, baseline characteristics should include as many factors as possible. Third, the follow-up time of the patients was relatively short, and long-term predicted results should be observed. In addition, no severity grading was conducted during data collection, and multicollinearity/variance inflation factor was not explored in this study. Also, right heart catheterization was not performed during diagnosis and treatment, and false positives and false negatives of PH in acute exacerbation of COPD were not conducted. Besides, the effect of COVID-19 on this study should be further explored. Lastly, all hospitalized patients were patients with acute exacerbation of COPD; thus, stable COPD patients will be included to further investigate the significance and value of GDF-15 in stable COPD patients with PH in future studies.
Conclusion
The current study confirmed that GDF-15 and sST2 levels may be better predictors of AECOPD-PH and its prognosis. Accurate classification utilizing GDF-15 and sST2 may help treat patients with AECOPD-PH. Despite its usefulness, further prospective studies are needed to define its reliability as biomarkers.
Abbreviations
AECOPD, acute exacerbation of chronic obstructive pulmonary disease; AECOPD-PH, pulmonary hypertension in AECOPD; AUC, area under the curve; BMI, body mass index; COPD, chronic obstructive pulmonary disease; GDF-15, growth differentiation factor-15; OI, oxygenation index; ROC, receiver operating characteristic curve; SI, smoking index; sST2, soluble suppression of tumorigenicity 2.
Highlights
- GDF-15 and sST2 serum levels in AECOPD-PH group were remarkably higher than those in AECOPD group.
- The AUCs of GDF-15, sST2, and GDF-15 + sST2 for predicting AECOPD-PH were >0.60.
- The AUCs of GDF-15, sST2, and GDF-15 + sST2 for predicting poor prognosis in AECOPD-PH patients were >0.70.
Ethics Approval and Informed Consent
The research was approved from the Ethics Committee of the Third Hospital of Shanxi Medical University. Written informed consents were obtained from all participants. This study was conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Funding
Funding was provided by the Natural Science Foundation of Shanxi Province (No. 201901D111414). Funding was provided by the Research Project Supported by Shanxi Scholarship Council of China (No. 2020-178). Funding was provided by the Fund Program for the Scientific Activities of Selected Returned Overseas Professionals in Shanxi Province (No. 20200029).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Weitzenblum E, Chaouat A, Kessler R. Pulmonary hypertension in chronic obstructive pulmonary disease. Pneumonol Alergol Pol. 2013;81:390–398.
2. Halpin DMG, Criner GJ, Papi A, et al. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. the 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;203:24–36. doi:10.1164/rccm.202009-3533SO
3. MacLeod M, Papi A, Contoli M, et al. Chronic obstructive pulmonary disease exacerbation fundamentals: diagnosis, treatment, prevention and disease impact. Respirology. 2021;26:532–551. doi:10.1111/resp.14041
4. Gupta NK, Agrawal RK, Srivastav AB, Ved ML. Echocardiographic evaluation of heart in chronic obstructive pulmonary disease patient and its co-relation with the severity of disease. Lung India. 2011;28:105–109. doi:10.4103/0970-2113.80321
5. Oswald-Mammosser M, Apprill M, Bachez P, Ehrhart M, Weitzenblum E. Pulmonary hemodynamics in chronic obstructive pulmonary disease of the emphysematous type. Respiration. 1991;58:304–310. doi:10.1159/000195950
6. Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127:1531–1536. doi:10.1378/chest.127.5.1531
7. Orr R, Smith LJ, Cuttica MJ. Pulmonary hypertension in advanced chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2012;18:138–143. doi:10.1097/MCP.0b013e32834f2093
8. Riley CM, Sciurba FC. Diagnosis and outpatient management of chronic obstructive pulmonary disease: a review. JAMA. 2019;321:786–797. doi:10.1001/jama.2019.0131
9. Ip C, Luk KS, Yuen VLC, et al. Soluble suppression of tumorigenicity 2 (sST2) for predicting disease severity or mortality outcomes in cardiovascular diseases: a systematic review and meta-analysis. IJC Heart Vasc. 2021;37:100887. doi:10.1016/j.ijcha.2021.100887
10. Mirna M, Rohm I, Jirak P, et al. Analysis of novel cardiovascular biomarkers in patients with pulmonary hypertension (PH). Heart Lung Circ. 2020;29:337–344. doi:10.1016/j.hlc.2019.03.004
11. Dea A, Ez C, Es B, Az A. Plasma growth differentiation factor-15 in children with pulmonary hypertension associated with congenital heart disease: a canary in the mine? - ScienceDirect. Prog Pediatr Cardiol. 2020;2020:1.
12. Tantawy AA, Adly AA, Ismail EA, Youssef OI, Ali ME. Growth differentiation factor-15 in children and adolescents with thalassemia intermedia: relation to subclinical atherosclerosis and pulmonary vasculopathy. Blood Cells Mol Dis. 2015;55:144–150. doi:10.1016/j.bcmd.2015.06.001
13. Geenen LW, Baggen VJM, Kauling RM, et al. Growth differentiation factor-15 as candidate predictor for mortality in adults with pulmonary hypertension. Heart. 2020;106:467–473. doi:10.1136/heartjnl-2019-315111
14. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. NHLB/WHO workshop report, Bethesda, National Heart, Lung and Blood Institute. Www.goldcopd.com.
15. Gómez Gómez E, Puche Sanz I, Valero Rosa J, Carrasco Valiente J, Campos Hernandez JP, Anglada Curado FJ. Serum biomarkers for diagnosis and characterization of prostate cancer. Arch Esp Urol. 2022;75:156–164.
16. Masuda Y, Matsuda S, Kotani T, et al. Association between serum biomarkers and peripheral neuropathy in microscopic polyangiitis. Int J Mol Sci. 2022;23:13374. doi:10.3390/ijms232113374
17. D’Ascanio M, Viccaro F, Pizzirusso D, et al. Surfactant protein B plasma levels: reliability as a biomarker in COPD patients. Biomedicines. 2023;2023:11.
18. Andersson J, Fall T, Delicano R, Wennberg P, Jansson JH. GDF-15 is associated with sudden cardiac death due to incident myocardial infarction. Resuscitation. 2020;152:165–169. doi:10.1016/j.resuscitation.2020.05.001
19. Verhamme FM, Seys LJM, De Smet EG, et al. Elevated GDF-15 contributes to pulmonary inflammation upon cigarette smoke exposure. Mucosal Immunol. 2017;10:1400–1411. doi:10.1038/mi.2017.3
20. Mielcarska S, Stopińska K, Dawidowicz M, et al. GDF-15 Level Correlates with CMKLR1 and VEGF-A in tumor-free margin in colorectal cancer. Current Med Sci. 2021;41:522–528. doi:10.1007/s11596-021-2335-0
21. Alladina J, Levy SD, Cho JL, et al. Plasma soluble suppression of tumorigenicity-2 associates with ventilator liberation in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2021;203:1257–1265. doi:10.1164/rccm.202005-1951OC
22. Kim M, Cha SI, Choi KJ, et al. Prognostic value of serum growth differentiation factor-15 in patients with chronic obstructive pulmonary disease exacerbation. Tuberc Respir Dis. 2014;77:243–250. doi:10.4046/trd.2014.77.6.243
23. Hirano T, Doi K, Matsunaga K, et al. A novel role of growth differentiation factor (GDF)-15 in overlap with sedentary lifestyle and cognitive risk in COPD. J Clin Med. 2020;9. doi:10.3390/jcm9092737
24. Havránek Š, Marek J. Biomarker GDF-15 in cardiology. Vnitr Lek. 2021;67:11–14. doi:10.36290/vnl.2021.045
25. Luo JW, Duan WH, Song L, Yu YQ, Shi DZ. A meta-analysis of growth differentiation factor-15 and prognosis in chronic heart failure. Front Cardiovasc Med. 2021;8:630818. doi:10.3389/fcvm.2021.630818
26. Rueda F, Cediel G, García-García C, et al. Growth differentiation factor 15 and early prognosis after out-of-hospital cardiac arrest. Ann Intensive Care. 2019;9:119. doi:10.1186/s13613-019-0593-9
27. Homsak E, Gruson D. Soluble ST2: a complex and diverse role in several diseases. Clin Chim Acta. 2020;507:75–87. doi:10.1016/j.cca.2020.04.011
28. Carlomagno G, Fazio S. Response to letter by Wang Q et al. regarding article “Serum soluble ST2 and interleukin-33 levels in patients with pulmonary arterial hypertension”. Int J Cardiol. 2013;168:3122. doi:10.1016/j.ijcard.2013.04.051
29. Ye H, Wu Q, Zhang N, et al. The prognostic value of sST2 in connective tissue disease patients with pulmonary hypertension. Rheumatology. 2022;61:3989–3996. doi:10.1093/rheumatology/keac055
30. Plácido R, Cortez-Dias N, Robalo Martins S, et al. Prognostic stratification in pulmonary hypertension: a multi-biomarker approach. Rev Port Cardiol. 2017;36:111–125.
31. Watanabe M, Nakamoto K, Inui T, et al. Serum sST2 levels predict severe exacerbation of asthma. Respir Res. 2018;19:169. doi:10.1186/s12931-018-0872-2
32. Urban MH, Stojkovic S, Demyanets S, et al. Soluble ST2 and all-cause mortality in patients with chronic obstructive pulmonary disease-a 10-year cohort study. J Clin Med. 2021;11. doi:10.3390/jcm11010011
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
