Back to Journals » International Journal of General Medicine » Volume 16
Predictive Value of COPD History on In-Stent Restenosis in Coronary Arteries Following Percutaneous Coronary Intervention
Authors Hou L , Su K, Zhao J, Li Y
Received 15 July 2023
Accepted for publication 29 August 2023
Published 31 August 2023 Volume 2023:16 Pages 3977—3984
DOI https://doi.org/10.2147/IJGM.S427425
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Yuriy Sirenko
Ling Hou,1 Ke Su,2 Jinbo Zhao,2 Yuanhong Li2
1Department of Central Hospital of Tujia and Miao Autonomous Prefecture, Hubei University of Medicine, Shiyan, Hubei Province, People’s Republic of China; 2Cardiovascular Disease Center, Central Hospital of Tujia and Miao Autonomous Prefecture, Hubei University of Medicine, Enshi, Hubei Province, People’s Republic of China
Correspondence: Yuanhong Li, Email [email protected]
Objective: Chronic obstructive pulmonary disease (COPD) is a prevalent chronic respiratory disease that poses a significant health risk to individuals. Patients with COPD are predisposed to a higher incidence of coronary artery disease (CAD) than the general population. This study aims to investigate the correlation between COPD and the incidence of in-stent restenosis (ISR) after percutaneous coronary intervention (PCI).
Methods: This study retrospectively analyzed the clinical data and laboratory test results of patients who underwent PCI at our hospital between January 2018 and December 2021 to investigate the relationship between COPD and drug-Eluting Stents (DES) postoperative ISR. We employed the best subset method to select the most suitable combination of predictive factors, utilizing the data, and verified the precision of the model by means of internal validation. We ultimately assessed the performance of the prediction model using an ROC curve.
Results: The research indicates that COPD is an independent risk factor for ISR after PCI (OR=2.437, 95% CI [1.336, 4.495], P=0.004). The analysis revealed an area under the receiver operating characteristic (ROC) curve of 0.783 for the training group and 0.705 for the testing group, indicating a model fitting for both groups (both > 0.5).
Conclusion: COPD history is a dependable predictor of stent restenosis post percutaneous coronary intervention.
Keywords: percutaneous coronary intervention, PCI, chronic obstructive pulmonary disease, COPD, in-stent restenosis, ISR, drug-eluting stents, DES, optimal subset regression
Background
Cardiovascular disease is still one of the most common and deadly chronic diseases globally.1 In the past 40 years, the intravascular coronary artery stent implantation has become a main treatment for CAD, which can significantly improve blood flow dynamics.2 PCI reduces the mortality rate of Acute Coronary Syndrome (ACS) and enhances patients’ quality of life who are diagnosed with stable angina.3 Following the emergence of DES in the 21st century, the incidence rate of ISR decreased to about 5% to 10% per year.4 However, considering the millions of DES implants each year, ISR remains a huge challenge.5
COPD is a slowly progressing lung disease that trigger systemic inflammation. The mortality rate of this disease is high. It is estimated that by 2030, COPD will rank seventh on the world’s disease burden list. Among patients diagnosed with COPD, incidence of CAD ranges between 2.4% and 23.3%.6 COPD results in varying degrees of decline in lung function and systemic inflammation.7 Literature has reported that these effects can have an impact on the prognosis of cardiovascular disease.8 Therefore, we believe that the severity and prognosis of COPD are related to cardiovascular disease, including ISR.
Research indicates that complex vascular lesions, suboptimal stent expansion, and long-term chronic diseases are all predictive factors for ISR.9–12 Several studies demonstrate that COPD patients have a higher risk of mortality after undergoing PCI surgery.13 However, other clinical outcomes related to adverse events have yet to be reported. Currently, there is still insufficient research data on the correlation between COPD and in-stent restenosis. Therefore, this study adopts a retrospective research method aimed at exploring the relationship between COPD and ISR.
Methods
This study investigated who underwent PCI treatment at the central hospital of the Tujia and Miao in Enshi Autonomous Prefecture from January 2018 to December 2021. A total of 983 patients underwent DES PCI. The following are the exclusion criteria for this study: 1. Patients with congenital heart disease, heart failure, or other cardiac conditions were not included. 2. Patients with liver or kidney dysfunction, immune disorders, or cancer were not included. 3. Patients who had undergone major trauma surgery in the recent past were not included. 4. Patients with incomplete follow-up data or incomplete clinical data were not included. All patients were continuously taking antiplatelet and statin medications during the follow-up period (Figure 1).
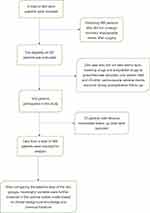 |
Figure 1 The selection procession of selected subjects. |
Patients were classified into ISR and non-ISR groups based on the results of the first follow-up coronary angiography performed within 12 months after PCI. The ISR group consists of patients who experienced a late lumen loss ≥50% within a 5mm range from the proximal to distal end of either the main branch or side branch stent.14 This study was approved by the Ethics Committee of Enshi Autonomous Prefecture Central Hospital in accordance with the Helsinki Declaration. As the committee did not require patient consent for the review of their medical records, obtaining informed consent from patients is not necessary for this retrospective study. Nonetheless, in order to ensure ethical compliance, we have supplied the editor with the approval certificate issued by the ethics committee. Firstly, this study solely collected clinical case data without causing adverse reactions or harm to patients. Secondly, the patient data utilized in this paper has been anonymized, with anonymization performed solely prior to data access and analysis. Thus, patients’ medical records can be examined without their prior consent.
Collect comprehensive demographic information from all patients including their age and gender. Perform a series of blood tests including a complete blood count as well as assessments of liver and kidney function. Furthermore, collect a comprehensive medical history from all patients, encompassing conditions like hypertension, diabetes, COPD, smoking history, the presence of stents, and details of post-operative medication usage. COPD is defined as FEV1/FVC < 0.7, with measurement conducted after the patient inhales a bronchodilator.15
The R Studio application software was utilized to scrutinize the data and compare differences between the baseline data groups. Mean (standard deviation, SD) was calculated for continuous variables, thereafter a t-test was performed or non-parametric rank test (Mann–Whitney U-test or Wilcoxon signed-rank test), depending on whether or not the data satisfied normal distribution and homogeneity of variance. Categorical variables were represented by the median (25th and 75th percentile: P25, P75). To compare differences between groups, a chi-square test was administered in case the data were in line with normal distribution and, otherwise, Wilcoxon test was utilized. The logistic regression model initially included all variables. The optimal subset method was employed to determine the most salient predictor variables with the lowest Akaike’s Information Criterion (AIC) value. Using odds ratio (OR) and P-values with a 95% confidence interval, a thorough examination of predictor variables was conducted. The sensitivity of the model was evaluated using ROC analysis. A statistically significant difference was confirmed by a P-value of less than 0.05.
Results
Baseline Information
Among the 469 patients who met the inclusion criteria. Table 1 shows the comparison of baseline characteristics between 380 patients without in-stent restenosis and 89 patients with in-stent restenosis.
 |
Table 1 The Baseline Characteristics of the Subjects Between ISR Group and Non-ISR Group |
Table 1 indicates none of the factors considered including underlying diseases (hypertension, diabetes, and stroke), N, L, M, LMR, PLT, MPV, RDW, and PCT demonstrated statistical significance. Conversely, notable statistical differences were observed in terms of age, gender, PDW, NLR, and underlying diseases such as COPD.
Patients with multi-vessel coronary artery disease undergoing PCI are at high risk of developing in-stent restenosis. β-Blockers and angiotensin-converting enzyme inhibitors used for post-PCI treatment have shown no significant statistical difference in the prevention of in-stent restenosis. There are significant differences in ALT, DBIL, and LDL-C between the two groups in terms of liver and kidney function and lipid metabolism, while there are no significant differences in AST, TBIL, TC, TG, HDL-C, Cr, and UA.
Optimal Subset Regression Models
Include all variables from the baseline data into the initial logistic regression model to select the best subset model. Use the principle of selecting the model with the lowest AIC value as the best model, and use backward stepwise multiple logistic regression to select the ideal subset. We found that age, gender, COPD, multiple vessel disease, white blood cells are closely related to the occurrence of ISR (Table 2).
 |
Table 2 Regression Results for the Optimal Subset |
The area under the ROC curve was 0.783 (Figure 2) and 0.705 (Figure 3) for each respective optimal subset group. This indicates that COPD history can serve as an independent predictor for ISR, both values are greater than 0.5.
 |
Figure 2 The receiver operator characteristic curve for the train group. |
 |
Figure 3 The receiver operator characteristic curve for the test group. |
Discussion
Research has demonstrated that individuals diagnosed with COPD are at a higher risk of developing adverse cardiovascular outcomes.16 An increasing amount of evidence indicates that patients with COPD experience both local and systemic potential inflammation.17,18 Studies indicate that 16% of COPD patients exhibit persistent chronic inflammation.19 Patients with COPD display elevated levels of inflammatory markers, particularly C-reactive protein and fibrinogen, which have been identified as independent risk factors for a higher incidence of myocardial infarction and mortality.20–22 Even when the risk factors are removed, the inflammation characteristic of COPD persists and continues to progress.23 Chronic inflammation in COPD has been found to involve both innate and adaptive immunity.24 Our research has confirmed that elevated white blood cell count is an independent risk factor for restenosis within the stent, thus providing further validation of the role of inflammation.
The deterioration of lung function is the main cause of adverse cardiovascular events in COPD patients.25 The FEV1/FVC ratio is an independent predictive factor negatively correlated with cardiovascular risk.26 In patients with coronary heart disease, there is an equivalent effect of lung function parameter FEV1 and blood lipid level.27 Research reveals that the reduction of FEV1 over a period is closely related to death and other cardiovascular diseases in patients with coronary heart ailment. Patients with COPD have a higher number and severity of coronary artery lesions after PCI treatment, which significantly augments the risk of revascularization and postoperative death.28 This observation aligns with our research findings. In comparison to healthy individuals, COPD leads to variations in coagulation factors dynamics and elevated levels of FVIII, potentially resulting in diverse thrombotic characteristics in COPD.29 In summary, the medical history of COPD can be utilized for evaluating the progression of cardiovascular diseases.
DES represent a common therapeutic approach in coronary artery intervention therapy. Despite their ability to suppress neointimal hyperplasia, DES implantation still poses a risk of restenosis occurrence.30 Although numerous treatments have been implemented to address restenosis within stents, their efficacy in terms of long-term coronary angiography and clinical prognosis of DES-ISR patients remains inadequate.31 The missed diagnosis rate of COPD among patients with coronary heart disease is at least 80%.32 Although the incidence of COPD is high among patients with coronary artery disease, its diagnostic rate is low. Although studies indicate that there is an increase in adverse events in COPD patients undergoing percutaneous coronary intervention, we conducted this study to better understand the development of In-Stent Restenosis after DES surgery and improve patient prognosis.
Our research revealed that age, gender, the history of COPD, multi-vessel coronary artery disease, and white blood cell count are significant risk factors for ISR. Previous studies have provided confirmation that both age and the presence of multi-vessel coronary artery disease serve as predictive factors for restenosis occurring within the stent.33–36 The study utilized the best subset regression method to construct a model. Our findings indicated that a history of COPD was effective predictors of restenosis. In comparison with previous models, our model demonstrated a better fit and diagnostic value.
This study has several limitations. Firstly, when investigating the correlation between COPD and ISR, it should be noted that smoking is one of the primary etiological factors of COPD, which can lead to a significant overlap of patients with a history of both COPD and smoking. Consequently, potential confounding factors may arise. Secondly, this study is a single-center retrospective study conducted with a relatively small sample size. As a result, the generalizability and reliability of the results may be limited. In the future, we plan to address these limitations by conducting a larger sample size and a more comprehensive study, thereby obtaining more accurate and reliable results. Despite these limitations, the research findings hold clinical significance and offer valuable hints for further investigations.
Conclusion
In summary, we found that patients with a history of COPD have an increased risk of ISR after receiving drug-eluting stent implantation. Our model, which incorporates factors including age, gender, multi-vessel coronary artery disease, history of COPD, use of beta blockers, platelet distribution width and white blood cell levels, demonstrated satisfactory predictive value.
Funding
This work was funded by the National Natural Science Foundation of China (No.82160072) and the Science and Technology Support Project of Enshi Prefecture Science and Technology Bureau (D20210024).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Collaborators GBDCo D. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–1210. doi:10.1016/S0140-6736(17)32152-9
2. Piccolo R, Giustino G, Mehran R, Windecker S. Stable coronary artery disease: revascularisation and invasive strategies. Lancet. 2015;386(9994):702–713. doi:10.1016/S0140-6736(15)61220-X
3. Pleva L, Kukla P, Hlinomaz O. Treatment of coronary in-stent restenosis: a systematic review. J Geriatr Cardiol. 2018;15(2):173–184. doi:10.11909/j.issn.1671-5411.2018.02.007
4. Moussa ID, Mohananey D, Saucedo J, et al. Trends and Outcomes of Restenosis After Coronary Stent Implantation in the United States. J Am Coll Cardiol. 2020;76(13):1521–1531. doi:10.1016/j.jacc.2020.08.002
5. Oguz M, Akbulut T, Saylik F, Sipal A, Erdal E. Association of coronary artery severity and late in-stent restenosis: an Angiographic Imaging Study. Angiology. 2023. doi:10.1177/00033197221150953
6. Zheng Y, Qi Y, Seery S, et al. Long-term outcomes for Chinese COPD patients after PCI: a Propensity Score Matched, Double-Cohort Study. Front Cardiovasc Med. 2022;9:827635. doi:10.3389/fcvm.2022.827635
7. Chan SMH, Selemidis S, Bozinovski S, Vlahos R. Pathobiological mechanisms underlying metabolic syndrome (MetS) in chronic obstructive pulmonary disease (COPD): clinical significance and therapeutic strategies. Pharmacol Ther. 2019;198:160–188. doi:10.1016/j.pharmthera.2019.02.013
8. Stone IS, Barnes NC, Petersen SE. Chronic obstructive pulmonary disease: a modifiable risk factor for cardiovascular disease? Heart. 2012;98(14):1055–1062. doi:10.1136/heartjnl-2012-301759
9. Hlatky MA, Boothroyd DB, Bravata DM, et al. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Article. Lancet. 2009;373(9670):1190–1197. doi:10.1016/S0140-6736(09)60552-3
10. Stettler C, Allemann S, Wandel S, et al. Drug eluting and bare metal stents in people with and without diabetes: collaborative network meta-analysis. Article. BMJ. 2008;337. doi:10.1136/bmj.a1331
11. Stefanini GG, Holmes DR. Drug-eluting coronary-artery stents. N Engl J Med. 2013;368(3):254–265. doi:10.1056/NEJMra1210816
12. Singh AD, Singal AK, Mian A, et al. Recurrent drug-eluting stent in-stent restenosis: a State-of-the-Art review of pathophysiology, diagnosis, and management. Journal Article: Review. Cardiovasc Revasc Med. 2020;21(9):1157–1163. doi:10.1016/j.carrev.2020.01.005
13. Enriquez JR, Parikh SV, Selzer F, et al. Increased adverse events after percutaneous coronary intervention in patients with COPD: insights from the National Heart, Lung, and Blood Institute dynamic registry. Chest. 2011;140(3):604–610. doi:10.1378/chest.10-2644
14. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;72(18):2231–2264. doi:10.1016/j.jacc.2018.08.1038
15. Agustí A, Celli BR, Criner GJ, et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Eur Respir J. 2023;61(4):2300239. doi:10.1183/13993003.00239-2023
16. Feary JR, Rodrigues LC, Smith CJ, Hubbard RB, Gibson JE. Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: a comprehensive analysis using data from primary care. Thorax. 2010;65(11):956–962. doi:10.1136/thx.2009.128082
17. Fu JJ, McDonald VM, Gibson PG, Simpson JL. Systemic inflammation in older adults with asthma-COPD overlap syndrome. Allergy Asthma Immunol Res. 2014;6(4):316–324. doi:10.4168/aair.2014.6.4.316
18. Magnussen H, Watz H. Systemic inflammation in chronic obstructive pulmonary disease and asthma: relation with comorbidities. Journal Article. Review. Proc Am Thorac Soc. 2009;6(8):648–651. doi:10.1513/pats.200906-053DP
19. Agusti A, Edwards LD, Rennard SI, et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One. 2012;7(5):e37483. doi:10.1371/journal.pone.0037483
20. Chang CL, Robinson SC, Mills GD, et al. Biochemical markers of cardiac dysfunction predict mortality in acute exacerbations of COPD. Thorax. 2011;66(9):764–768. doi:10.1136/thx.2010.155333
21. McAllister DA, Maclay JD, Mills NL, et al. Diagnosis of myocardial infarction following hospitalisation for exacerbation of COPD. Eur Respir J. 2012;39(5):1097–1103. doi:10.1183/09031936.00124811
22. Pelgrim CE, Peterson JD, Gosker HR, et al. Psychological co-morbidities in COPD: targeting systemic inflammation, a benefit for both? Eur J Pharmacol. 2019;842:99–110. doi:10.1016/j.ejphar.2018.10.001
23. Hogg JC, Pare PD, Hackett T-L. The contribution of small airway obstruction to the pathogenesis of chronic obstructive pulmonary disease. Review. Physiol Rev. 2017;97(2):529–552. doi:10.1152/physrev.00025.2015
24. Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138(1):16–27. doi:10.1016/j.jaci.2016.05.011
25. Mannino DM, Watt G, Hole D, et al. The natural history of chronic obstructive pulmonary disease. Journal Article Research Support, Non-U.S. Gov’t. Review. Eur Respir J. 2006;27(3):627–643. doi:10.1183/09031936.06.00024605
26. Sin DD, Man SF. Chronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortality. Proc Am Thorac Soc. 2005;2(1):8–11. doi:10.1513/pats.200404-032MS
27. Wu IH, Sun ZJ, Lu FH, et al. Restrictive spirometry pattern is associated with increased arterial stiffness in men and women. Chest. 2017;152(2):394–401. doi:10.1016/j.chest.2017.03.039
28. Hayıroğlu Mİ, Keskin M, Uzun AO, et al. Predictive value of SYNTAX score II for clinical outcomes in cardiogenic shock underwent primary percutaneous coronary intervention; a pilot study. Int J Cardiovasc Imaging. 2017;34(3):329–336. doi:10.1007/s10554-017-1241-9
29. Undas A, Jankowski M, Kaczmarek P, Sladek K, Brummel-Ziedins K. Thrombin generation in chronic obstructive pulmonary disease: dependence on plasma factor composition. Thromb Res. 2011;128(4):e24–e28. doi:10.1016/j.thromres.2011.05.004
30. Wu BJ, Li Y, Ong KL, et al. Reduction of In-stent restenosis by cholesteryl ester transfer protein inhibition. Arterioscler Thromb Vasc Biol. 2017;37(12):
31. Van den Berg JC. Management of in-stent restenosis in the superficial femoral artery. J Cardiovasc Surg. 2016;57(2):248–256.
32. Almagro P, Lapuente A, Pareja J, et al. Underdiagnosis and prognosis of chronic obstructive pulmonary disease after percutaneous coronary intervention: a prospective study. Int J Chron Obstruct Pulmon Dis. 2015;10:1353–1361. doi:10.2147/COPD.S84482
33. Çinar T, Hayiroğlu Mİ, Şeker M, et al. The predictive value of age, creatinine, ejection fraction score for in-hospital mortality in patients with cardiogenic shock. Coron Artery Dis. 2019;30(8):569–574. doi:10.1097/mca.0000000000000776
34. Hayıroğlu Mİ, Keskin M, Uzun AO, et al. Predictors of In-hospital mortality in patients with ST-Segment elevation myocardial infarction complicated with cardiogenic shock. Heart Lung Circ. 2019;28(2):237–244. doi:10.1016/j.hlc.2017.10.023
35. Çınar T, Şaylık F, Akbulut T, et al. Evaluation of Intermountain risk score for short- and long-term mortality in ST elevation myocardial infarction patients. Angiology. 2022;74(4):357–364. doi:10.1177/00033197221105753
36. Mert Ilker H, Faysal S, Ahmet Çağdaş Y, Murat S, Tufan Ç. Prognostic value of Intermountain Risk Score for short- and long-term mortality in patients with cardiogenic shock. Coron Artery Dis. 2023. doi:10.1097/mca.0000000000001219
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
