Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Predictive Value of Combining Inflammatory Biomarkers and Rapid Decline of FEV1 for COPD in Chinese Population: A Prospective Cohort Study
Authors Wang Y, Liao J, Zhong Y, Zhang C, Li X, Wang G
Received 19 July 2019
Accepted for publication 26 November 2019
Published 5 December 2019 Volume 2019:14 Pages 2825—2833
DOI https://doi.org/10.2147/COPD.S223869
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chunxue Bai
Yunxia Wang,1 Jiping Liao,1 Yijue Zhong,1 Cheng Zhang,1 Xueying Li,2 Guangfa Wang1
1Department of Respiratory and Critical Care Medicine, Peking University First Hospital, Beijing, People’s Republic of China; 2Department of Medical Statistics, Peking University First Hospital, Beijing, People’s Republic of China
Correspondence: Guangfa Wang
Department of Respiratory and Critical Care Medicine, Peking University First Hospital, No.8 Xishiku Street, Xicheng District, Beijing 100034, People’s Republic of China
Email [email protected]
Background: In China, the high prevalence and mortality rate of Chronic Obstructive Pulmonary Disease (COPD) and the poor intervention effect makes it into a heavy social burden. The main reason is that the current diagnosis of COPD mainly based on the static lung function, which is difficult for early intervention. Through matching a predictive model for high-risk groups of COPD that rewards FEV1 rapid decline as the core, we will establish the early warning model and prove its validity and socio-economic value.
Methods: This is a multi-center, prospective, cohort study. A total of 10,000 people aged 40∼75 without lung disease will be recruited and followed for 3 years. Some questionnaires such as St George’s Respiratory Questionnaire (SGRQ), income class, educational level, comorbidity, smoking habit, and biomass smoke exposure history will be collected. The baseline level of Interleukin 6 (IL-6), high-sensitivity C-reactive Protein (hs-CRP), microRNAs-23a (miR-23a) in peripheral blood and pH value in exhaled breath condensate (EBC) will be measured, lung spirometry will be tested in the first, second, and fourth years. Primary outcome is the incidence of COPD, multivariate regression analysis will be used to establish the predictive model for COPD in China.
Discussion: With the rapid decline of lung function as the core and the baseline inflammatory biomarkers in peripheral blood and pH of the exhaled breath condensate as affecting factors, a predictive model to achieve early detection of high-risk COPD groups will be established and promoted.
Trial registration: This study has been registered at www.ClinicalTrials.gov (registration identifier: NCT03532893) on 21 May 2018, https://register.clinicaltrials.gov.
Keywords: COPD, decline in FEV1, inflammatory biomarkers, EBC, microRNA-23a
Background
COPD is characterized by persistent airflow limitation due to airway and/or alveolar abnormalities that are usually caused by exogenous exposure to noxious particles or gases.1,2 COPD is the fourth leading cause of death worldwide in 2010, but it is predicted to be the third by 2020.3,4 More than three million people died of COPD in 2012 accounting for 6% of all deaths globally; many people suffer from it for decades and die prematurely from it or its complications. Globally, COPD is associated with significant economic burden, which is projected to increase in coming decades because of continued exposure to COPD risk factors and aging of the population.5 And in China, the overall incidence of COPD estimated was about 8.2% in 2007,6 and then increased to 8.6% in 2015 as spirometry-defined COPD.7 COPD represents an important public health challenge that needs continuous treatment. Hence, it is particularly important to establish early warning model of COPD, which can help us identify high-risk groups of COPD as early as possible and give early intervention, so as to prevent the occurrence and development of COPD and reduce the burden of social diseases.
It should start with the pathophysiological mechanism of COPD to establish the early warning model including reduced lung function due to small-airway obstruction, alveolar damage caused by inflammatory factors. Then, it needs to combine Chinese special circumstances to establish a special warning model. The special circumstances contain the special dietary and lifestyle habits of drinking tea, high levels of air pollution exposure, biofuels, smoking, cultural, economic and other characteristics.
The presence of poorly reversible airflow limitation, as measured by the forced expiratory volume in 1s (FEV1)/forced vital capacity (FVC) ratio, is the core pathophysiological characteristic of COPD definition8. COPD should be considered in any patient who has dyspnea, chronic cough or sputum production, and/or a history of exposure to risk factors for the disease, while the presence of a post-bronchodilator FEV1/FVC <0.70 confirms the presence of persistent airflow limitation to make a definite diagnosis. Fletcher and Peto first described the progressive decline in lung function considered a hallmark feature of COPD,9 and the accelerated decline in FEV1 is the characteristic of COPD,10 so that measuring lung function by spirometry to find the FEV1 rapid decline population and giving regular follow-up is the key to early diagnosis of COPD.
It is clear that airway and lung inflammation is a primary feature of COPD, which leads to the progressive chronic airflow limitation.11 The inflammatory process of COPD is driven by classical cytokines and chemokines, such as IL-1, IL-6,12 IL-8. Among the cytokines, IL-6 is known as a key factor of increased CRP level in COPD patients13 and play a particular role in COPD: elevated concentrations of it correlate with a decrease in FEV1 sec and are related to high frequencies of COPD exacerbations.14
Just like the inflammatory factors of peripheral blood, the biological indicator in exhaled breath condensate is also the focus of COPD study. Exhaled breath condensate (EBC), the liquid that can be collected by breathing into a condensing unit has been proposed to represent information about airway lining fluid (ALF).15 EBC analysis has been considered as a promising non-invasive way for investigating respiratory diseases, as well as the pH of the condensate is the characteristic that can be determined with the highest reproducibility.16 Meanwhile, the pH of EBC indicates different physiological and pathological states of airway, while acidification of it was first described in asthma exacerbation,17 then in COPD.18 Then, the pH of EBC may reflect the Inflammation or allergies state of the airway. The EBC approach is useful not only due to its low risk but also because it is sensitive enough to detect subclinical inflammation.
MicroRNAs (miRNAs) are endogenous, approximately 20–25nt, noncoding RNAs that can target mRNAs for the cleavage of translational repression.19 MiRNAs have been demonstrated to participate in the pathogenesis and development of COPD. Among the MicroRNAs, miR-23a was downregulated in COPD patients indicating its potential role in the development of COPD, including inflammation, oxidative stress, immune imbalance, EMT, cell proliferation, apoptosis, and lipid metabolism.20 And miR-23a was reported to be associated with the loss of muscle force during the AECOPD,21 similarly, miR-23a was the potential and promising biomarker for discriminating frequent exacerbates from non-frequent exacerbates of COPD patients.20
As mentioned above, the accelerated decline in FEV1 is the core characteristic of COPD, inflammatory biomarkers including IL-6 and high-sensitivity C-reactive protein (hs-CRP) and miR-23a are involved in many facets of COPD pathogenesis while the pH of EBC may reflect the Inflammation or allergies state of the airway. Primary care should play the critical role in prevention, early diagnosis, and management of COPD. Hence, by combining the rapid decline in FEV1, serum IL-6 and hs-CRP level, peripheral blood miR-23a level and the pH of EBC, we aim to explore the predictive model for COPD, to prove its validity and promote it to primary care.
Methods/Design
Study Design
This is a multi-center, prospective, cohort study. A total of 10,000 subjects aged 40~75 without lung disease will be recruited from 10 provinces in China and followed for the second and fourth year. Baseline information collection, pH of EBC, blood sample collection, and inflammatory factor levels detection will be completed in the first year, spirometry will be tested in the first, second, and fourth year. In the second year, according to FEV1, the subjects will be divided into rapid decline group (RD group) and non-rapid decline group (NRD group).
The Department of Respiratory and Critical Care of Peking University First Hospital is responsible for this research. Other nine units participating in the study include the Second Hospital of Hebei Medical University, Henan Provincial People’s Hospital, the Second Hospital of Jilin University, the First Affiliated Hospital of Xi’an Jiaotong University, Shandong Provincial Hospital Affiliated to Shandong University, Shanxi Dayi Hospital, Tianjin Medical University General Hospital, the Affiliated Hospital of Inner Mongolia Medical University, the First Hospital of Qinhuangdao. The 10 units are in areas of northern China where air pollution is high, but the specific levels vary. The education and income level of participants in each unit are also different. In a word, the 10 units of the study could reflect the inclusion and diversity of the study.
Study Population
Investigators or physicians of each center will explain the study in detail to potential participants from inferior communities of each center. Total of 10,000 subjects will be enrolled after signing consent forms.
The inclusion criteria are 1) 40–75 years old; 2) FEV1/FVC>70% after inhaled bronchodilator; 3) have willing to participate in this study, follow the research program and have the ability to sign the informed consent; 4) lived in the screening community for more than 1 years and has no plans to move out in the next 4 years; 5) can be contacted.
The exclusion criteria are 1) history of asthma, COPD, lung cancer, active pulmonary tuberculosis, bronchiectasis, diffuse lung disease (interstitial pneumonia, pulmonary sarcoidosis, occupational lung disease, sarcoidosis et al) and pleural disease; 2) history of lobectomy and/or lung transplantation; 3) predicted life expectancy less than 3 years; 4) history of severe psychiatric illnesses, mental disorders, neurological disorders, malignant tumors, chronic liver disease, heart failure, autoimmune diseases, chronic kidney disease; 5) alcoholism, drug abuse or abuse of toxic solvents; 6) Cannot finish long term follow-up or poor compliance.
Baseline Data Collection
After carefully reading and signing the informed consent, the subject should fill in the detailed screening questionnaire for further determining whether the subjects met the criteria of this study. The information of demographic data, medical and medication history, questionnaire such as St George’s Respiratory Questionnaire (SGRQ), income class, educational level, comorbidity, smoking habit and biomass smoke exposure history, family history and main results of physical, laboratory, and lung function test will be collected. The flowchart of this study is shown in Figure 1.
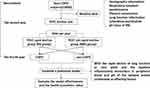 |
Figure 1 The study flowchart. |
The baseline level of laboratory detection including Interleukin 6 (IL-6), high-sensitivity C-reactive protein (hs-CRP), microRNAs-23a (miR-23a) in peripheral blood and the pH of EBC will be measured. Il-6 is detected by enzyme-linked immuno-sorbent assay (ELISA). miRNA-23a is detected by real-time quantification polymerase chain reaction (RT-QPCR).
Pulmonary function is monitored by portable lung function instrument COPD-6, UK Ireland. The preparation process includes the following: first, install two batteries into COPD-6 and start-up; second, enter the subject’s age, height, and gender; third, insert the disposable nozzle. Next, is the monitoring process: subjects inhale in hardly to maximum state, pause for a second and pinch the nose at the same time, and then bite and wrap the disposable nozzle to blow with the maximum power in the fastest speed until hearing two cue sounds which mean 6 s. The above blowing process needs to be repeated three times. If the screen says “!”, it means a bad air blowing that needs a reblown. Last, project investigators can press “enter” to display the best results of three blows and transfer data to a computer.
EBC will be collected using a condensing device (Huitongboyu technology co. LTD, China). The machine will be switched on at least 30 mins before collection to allow the cooling cuff to reach operating temperature (−35°C). The sealing cap will be applied to the cuff to insulate the internal cooling area and to avoid condensation of ambient moisture (which would freeze the lamellar condenser onto the cuff when it is inserted). EBC will be collected for at least 15 mins, during which time subjects are seated, wearing a nose clip and instructed to breathe tidally. Approximately 2 mL of condensate will be obtained per collection. After collection, the interface will be removed from the cooling cuff, and the sealing cap re-placed. With the condenser held upright, the sample collection vessel will be removed and its contents defrosted. Samples will be deaerated with inert argon gas (350 mL/min for 10 mins). EBC pH will then measured using an electronic pH meter (Leici, China). The pH meter, with a resolution of 0.01 and a working range from −2.00 to 16.00, will be calibrated using standard pH buffer solutions daily before usage. Then, exhaled breath condensate will be stored in labeled cryotubes. Samples are immediately stored at −80°C for later analysis. The reusable collection interface will be sterilized according to the manufacturers’ recommendation. All components will then be thoroughly rinsed in double distilled and de-ionized water to avoid sample contamination.
Follow-Up Schedule
Subjects will be formally visited in the second year and the fourth year. Questionnaires including SGRQ will be filled out and spirometry will be tested. The continuous monitoring data of air pollution (daily PM2.5, NO2, SO2, O3, etc.) in the cities where subjects living in will be recorded. The details needed during the study are listed in Table 1.
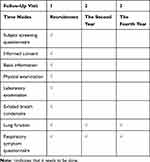 |
Table 1 Follow-Up Schedule and Data Needed |
Outcomes
Primary outcome is the incidence of COPD in the fourth year diagnosed according to GOLD2017.
Secondary outcomes include 1) the decline rate of FEV1; 2) The baseline indexes of subjects, including IL-6, hs-CRP, microRNA-23a, pH of EBC, ambient air pollution level.
Data Collection and Management
All data will be recorded on an electronic data capture (EDC) system. And the data will be uploaded through the smart way to avoid errors caused by human input, such as the laboratory test results will be exported through excel and the pulmonary function will be exported through PDF, then the data entry team will extract the data from them. Project investigators of each center, study coordinators, investigators for follow-up visits and data managers will have accordingly authorized access to this system. Complete online data will be exported into the electronic data capture. Online data will be monitored carefully by two data managers every month, the main content of data quality control is data integrity and lung function data monitored by COPD-6.
Data inspectors will browse the electronic data system to verify the integrity of the subject’s information, and determine the accuracy of pulmonary function reports. About pulmonary function reports, data inspectors need focus on two key aspects, firstly, the subjects must blow out ≥3 times of pulmonary function test to ensure 3 times of good blows; secondly, the variation of FEV1 and FEV6 in the three results of good blows should stay within 5% and the value should not exceed 150 mL.
Sample Size
According to the pre-experiment and related literatures, the prevalence of COPD in the natural population was 8.2%,6 and the rate of lung function rapid decline in non-COPD subjects was approximately 20%. Wang et al showed that approximately 32% of ever smokers exhibited FEV1 rapid decline. Among ever smokers without a baseline spirometric abnormality, rapid decline was associated with an increased risk for incident COPD (OR, 1.88; P=0.003).22 Through long-term follow-up observations, short-term monitoring (1–5 years follow-up) of rapid FEV1 decline people, they continued showing accelerated FEV1 decline even appeared risk of airway obstruction and airflow limitation, and the ratio was about 3–18 times higher than normal people.23
Assuming the difference of FEV1 decline between the FEV1 rapid decline group (RD group) and FEV1 not-rapid decline group (NRD group) is 15 mL,24 with the help of PASS11.0 software to sample size estimation, a total of 680 COPD subjects are required after 4 years to make an 80% power with a two-sided significance level of 0.05. Considering a dropout rate of approximately 20% and subject compliance, a total of 10,000 participants will be enrolled in this study.
Statistical Analysis
Two-tailed tests will be used in all statistical analysis, and p values < 0.05 will be considered to have statistical significance (unless otherwise specified). Numeric variables will be presented as mean (standard deviation) or median (minimum, maximum; or interquartile range) and categorical variables will be presented as number of cases (percentage).
Accordingly, data will be analyzed according to data characteristics with independent sample t test, Wilcoxon rank-sum test, chi-square test, continuity correction Chi-squared test or Fisher’s exact test. Pearson correlation analysis or spearman correlation analysis will be adopted for correlation analysis according to data characteristics.
About the primary outcome of COPD incidence in the fourth year diagnosed according to GOLD2017. Multivariate logistic regression model will be used for multivariate analysis to establish the predictive model for COPD in China, the variable factors including FEV1 rapid decline, serum IL-6 and hs-CRP level, peripheral blood miR-23a level, the pH value of EBC, ambient air pollution level, smoking habit, biomass exposure history, and demographic data. And survival analysis will be used to estimate the correlation between covariates and COPD incidence.
About the secondary outcome of correlation between the rate of FEV1 decline and other parameters, such as inflammatory biomarkers, microRNA-23a, smoking habit, biomass exposure history, eating habit, incoming class, and occupation of subjects, we should first assess which covariates could include in the final prediction model based on the R2 or AIC, or p-value, then consider whether if the indicator will be finally incorporated into the prediction model equation.
The influences associated with different centers or baseline data will be considered.
Characteristics of baseline will be summarized with equilibrium test. Inflammatory biomarkers, microRNA-23a, smoking habit, biomass exposure history, eating habit, incoming class and occupation of subjects will be compared between the RD and NRD group.
For a quick directory of all files, you could find in Supplementary Table 1: SPIRIT-checklist
Discussion
Chronic obstructive pulmonary disease (COPD) is currently defined as “a preventable and treatable disease” with some significant extrapulmonary effects that may contribute to the severity in patients. Prevalence surveys indicate that up to almost 15% adults aged ≥40 years old have mild airflow obstruction.25,26 In the past few decades, there were much important progress in the understanding of the epidemiology, pathophysiology, diagnosis, and treatment of COPD, but important issues remain unresolved including the early warning and diagnosis of COPD.4 Among COPD patients, the intervention (including smoking cessation, reduction in exposure to environmental and occupational risk factors, and yearly influenza vaccinations) lessens the decline of FEV1 by about 35 mL per year,27 which slows disease progression and lowers mortality by 18%28 Hence, the predictive model to achieve early detection of high-risk COPD groups so that providing timely intervention to slow the occurrence and progression of COPD is of great significance for the people ≥40 years old all over the world.
In clinical practice, clinician use the GOLD definition of chronic airflow obstruction, for which the threshold is a postbronchodilator ratio of forced expiratory volume in 1s (FEV1) to forced vital capacity of 0.7. The rapid rate of FEV1 decline is a rare feature of airflow limitation, especially for biomass-induced ones.29 Among the COPD patients, most of them are exposed to the cigarette smoke or tobacco smoke, so that the rapid decline of FEV1 is of great concern in the predictive model for early detection of high-risk COPD groups. How to choose the cut off criterion of decline in lung function is crucial. In addition to the normal physiological decline in lung function with age and gender, patients with COPD typically experience progressively and rapidly declining pulmonary function. Studies have confirmed that the average rate of FEV1 decline in patients with COPD is approximately double that of subjects without the disease.30,31 A review article showed the mean rate of FEV1 decline ranged from 40mL/year to 65mL/year observed in large COPD studies in patients with mild-moderate degrees of airflow limitation.32 Along with a study had defined annual FEV, decline of 30 mL/year as a rapid decline,22 we choose 30mL/year of FEV1 decline to be the group standard to divide subjects into groups of RD group/NRD group in the second year of research.
There are many predictive models of COPD disease before, including the Peabody model,33 which estimates of COPD prevalence; the Atsou model34 which gave the life expectancy gains of individual smokers who quit smoking and associated costs; and the Pichon-Riviere model35 which gave the costs and cost-effectiveness of smoking quit programmes. However, in China, early diagnosis, prevention, and treatment of COPD are deficient. Ethnic, lifestyle, air pollution levels, education, cultural between Chinese and western countries is different; therefore, the prediction model of COPD in China is urgent needs to be established.
In addition to lung function, the serum inflammatory markers are also critical in the development of COPD which is characterized by chronic inflammation of the respiratory tract. Among previous studies, IL-6 and CRP were core inflammatory molecules in COPD. IL-6 was a cytokine secreted by monocytes/macrophages, T cells, B cells, fibroblasts, bone marrow stromal cells, keratinocytes, and endothelial cells, CRP was almost secreted by hepatocytes.36 Some studies have shown that increased interleukin-6 (IL-6) levels may be associated with the progression of COPD.15,37 Along with IL-6, CRP is a commonly used biomarker of systemic inflammation in patients with COPD.14 Plasma CRP levels are elevated in COPD38 and are associated with increased mobility and mortality.39 Except the mobility and mortality, CRP levels are associated with the clinical status of COPD patients, such as exercise tolerance and muscle strength. IL-6 and CRP levels are both critical in the progression of COPD, and CRP is regulated by IL-6 in hepatocytes, means IL-6 and CRP levels correlate in COPD patients.40 In a word, IL-6 and CRP are identified as important factors in the prediction model.
Not only in serum but inflammation response has also happened in pulmonary microenvironment. Exhaled breath condensate (EBC) has been proposed to represent information about ALF, and it is assumed that airway surface liquid becomes aerosolised during turbulent airflow and that the content of the condensate reflects the composition of airway surface liquid. Among the huge number of indicators of EBC, the pH of the expired breath condensate might be a simple, inexpensive, and easily repeatable procedure for the evaluation of the inflammatory process in airway diseases. Previous study shows that endogenous airway acidification (assessed by pH in expired breath condensate) has been implicated in asthma pathophysiology.17 In patients with COPD and bronchiectasis, the values of pH were significantly correlated with both sputum neutrophilia and oxidative stress.18 In consideration of the EBC could reflect the level of airway inflammation, and is a non-invasive and repeatable operation, we choose it as the factor of the prediction model.
MicroRNAs (miRNAs) are small endogenously expressed noncoding RNAs that regulate gene expression at the posttranscriptional level, inhibiting or degrading their target RNAs.41 These molecules secreted by different cells could circulate freely in mammalian blood, and several studies have proposed that they can serve as biomarkers for different diseases, such as early myocardial infarction and heart failure. MiRNAs comprise one of the more abundant classes of gene regulatory molecules in multicellular organisms and likely influence the output of many protein-coding genes. Previous studies have identified a number of microRNAs that may have significant regulatory functions in the progression of COPD, such as miR-210 contributed to abnormal airway remodeling,42 miR-146a-5p involved in the epithelial-fibroblast cross talk,43 the up-regulation of miR-7 might provide potential biomarkers for therapeutic strategy44 and miR-20a to miR-181a was associated with the early stage of COPD in asymptomatic heavy smokers.45 Among the MicroRNAs, miR−23a was the critical player in the development of COPD involving into inflammation, oxidative stress, immune imbalance, EMT, cell proliferation, apoptosis and lipid metabolism20 As important role as miR-23a in progression of COPD, it has been chosen as a critical factor in the predictive model focusing on achieving early detection of high-risk COPD groups.
In conclusion, many inflammatory factors are significantly correlated with the occurrence and development of COPD, but unfortunately, there are no COPD-specific biomarkers. And as early as possible in diagnosing COPD and providing timely intervention can alleviate the enormous socio-economic pressures of COPD. Therefore, the predictive model received by multivariate regression analysis, which is composed of FEV1 rapid decline, serum IL-6 and hs-CRP level, peripheral blood miR-23a level and pH value of EBC is of importance. The predictive model for screening high-risk groups of COPD is suitable for primary care prevention. We hoped to use the predictive model to achieve early detection of high-risk COPD people so that providing timely intervention to slow the occurrence and progression of COPD, eventually reducing socio-economic pressure and improving the living quality of COPD patients.
Abbreviations
COPD, chronic obstructive pulmonary disease; SGRQ, St George’s Respiratory Questionnaire; IL-6, Interleukin 6; hs-CRP, high-sensitivity C-reactive protein; miR, microRNAs; EBC, exhaled breath condensate; FEV1, forced expiratory volume in 1s; FEC, forced vital capacity; ALF, airway lining fluid; EMT, epithelial–mesenchymal transition.
Trial Status
The trial is currently at the stage of patient recruitment and data collection.
Ethics Approval and Consent to Participate
The first version study protocol has been approved by the Peking University First Hospital Institutional Review Board (IRB) (2018[31]) on 7 March 2018. Any protocol modifications will be submitted for the IRB review and approval. The study will be conducted in accordance with Good Clinical Practice (GCP) requirements and ethical principles of the Declaration of Helsinki. The purposes, procedures, as well as potential benefits and risks of the study, will be explained carefully by investigators with a written informed consent. Written informed consent will be obtained from each participant or from the surrogate of the participant who cannot provide informed consent. Personal information and related documents of all participants will be kept strictly. Every participant will be identified by a subject number and a name acronym in the Case Report Form. Results of the study will be submitted to peer-reviewed journals and academic conferences and until now not any result of this study has already been published or been submitted to any journal.
Data Sharing Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Acknowledgments
The authors thank Dr Chengli Que, Dr Shixuan Wang, Dr Haiyan Tang, as well as nurse Liping Feng from the Department of Respiratory and Critical Care Medicine and Lina Zhang from Shichahai Community Health Service Center for their help in investigator training. The authors feel really appreciative for the cooperation in enrolling the subjects and advancing the research to Dr Ruiying Wang from Department of Respiratory Medicine, Shanxi Dayi Hospital, Dr Jinzhi Yin from Department of Respiratory and Critical Care Medicine, the Second Hospital of Jilin University, Dr Xixin Yan from Department of Respiratory Medicine, the Second Hospital of Hebei Medical University, Dr Xiaomin Dang from Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Xi’an Jiaotong University, Dr Limin Zhao from Department of Respiratory Medicine, Henan Provincial People’s Hospital, Dr Xiuhua Fu from Department of Respiratory Medicine, the Affiliated Hospital of Inner Mongolia Medical University, Dr Lixia Dong from Department of Respiratory Medicine, Tianjin Medical University General Hospital, Dr Shujuan Jiang from Department of Respiratory Medicine, Shandong Provincial Hospital Affiliated to Shandong University, Dr Hua Qiao from Department of Respiratory Medicine, The First Hospital of Qinhuangdao.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Dr Guangfa Wang reports grants from the National Science and Technology Ministry Project of Chronic Diseases, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS; GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:1256–1276. doi:10.1164/ajrccm.163.5.2101039
2. Yao W, Wang C, Zhong N, et al. Effect of once-daily indacaterol in a predominantly Chinese population with chronic obstructive pulmonary disease: a 26-week Asia-Pacific study. Respirology. 2014;19:231–238. doi:10.1111/resp.12211
3. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi:10.1016/S0140-6736(12)61728-0
4. Marc Decramer WJMM. Chronic obstructive pulmonary disease. Lancet. 2012;1341–1351. doi:10.1016/S0140-6736(11)60968-9
5. Han MK, Muellerova H, Curran-Everett D, et al. GOLD 2011 disease severity classification in COPDGene: a prospective cohort study. Lancet Respir Med. 2013;1:43–50. doi:10.1016/S2213-2600(12)70044-9
6. Zhong N, Wang C, Yao W, et al. Prevalence of chronic obstructive pulmonary disease in China. Am J Respir Crit Care Med. 2007;176:753–760. doi:10.1164/rccm.200612-1749OC
7. Wang C, Xu J, Yang L. Prevalence and risk factors of chronic obstructive pulmonary disease in China (The China Pulmonary Health [CPH] study): a national cross-sectional study. The Lancet. 2018;391:1706–1717. doi:10.1016/S0140-6736(18)30841-9
8. Pauwels RA, Buist AS, Calverley PM et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276.
9. Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1.
10. Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi:10.1056/NEJMoa032158
11. Chung KF, Adcock IM. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur Respir J. 2008;31:1334–1356. doi:10.1183/09031936.00018908
12. Bucchioni E, Kharitonov SA, Allegra L, Barnes PJ. High levels of interleukin-6 in the exhaled breath condensate of patients with COPD. Respir Med. 2003;97:1299–1302. doi:10.1016/j.rmed.2003.07.008
13. Kolsum U, Roy K, Starkey C, et al. The repeatability of interleukin-6, tumor necrosis factor- α, and C-reactive protein in COPD patients over one year. Int J Chron Obstruct Pulmon Dis. 2009;4:149–156. doi:10.2147/COPD.S5018
14. Bhowmik A. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax. 2000;55:114–120. doi:10.1136/thorax.55.2.114
15. Horváth I, Hunt J, Barnes PJ. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J. 2005;26:523–548. doi:10.1183/09031936.05.00029705
16. Kullmann T, Barta I, Lazar Z, et al. Exhaled breath condensate pH standardised for CO2 partial pressure. Eur Respir J. 2007;29:496–501. doi:10.1183/09031936.00084006
17. HUNT JF, Fang K, Malik R. Endogenous airway acidification: implications for asthma pathology. Am J Resp Crit Care Med. 2000;161:694–699. doi:10.1164/ajrccm.161.3.9911005
18. Kostikas K, Papatheodorou G, Ganas K, Psathakis K, Panagou P, Loukides S. pH in expired breath condensate of patients with inflammatory airway diseases. Am J Respir Crit Care Med. 2002;165:1364–1370. doi:10.1164/rccm.200111-068OC
19. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi:10.1016/S0092-8674(04)00045-5
20. Liu X, Qu J, Xue W, et al. Bioinformatics-based identification of potential microRNA biomarkers in frequent and non-frequent exacerbators of COPD. Int J Chron Obstructive Pulm Dis. 2018;13:1217–1228.
21. Duan Y, Zhou M, Xiao J, et al. Prediction of key genes and miRNAs responsible for loss of muscle force in patients during an acute exacerbation of chronic obstructive pulmonary disease. Int J Mol Med. 2016;38:1450–1462. doi:10.3892/ijmm.2016.2761
22. Petersen H, Sood A, Meek PM, et al. Rapid lung function decline in smokers is a risk factor for COPD and is attenuated by angiotensin-converting enzyme inhibitor use. Chest. 2014;145.
23. Wang ML, Avashia BH, Petsonk EL. Interpreting periodic lung function tests in individuals: the relationship between 1- to 5-year and long-term FEV1 changes. Chest. 2006;130.
24. Decramer M, Celli B, Tashkin DP, et al. Clinical trial design considerations in assessing long-term functional impacts of tiotropium in COPD: the UPLIFT trial. COPD. 2004;1.
25. Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (The BOLD Study): a population-based prevalence study. Lancet. 2007;370:741–750. doi:10.1016/S0140-6736(07)61377-4
26. Menezes AM, Perez-Padilla R, Jardim JR, et al.; PLATINO Team. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet (London, England). 2005:366.
27. Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of lung health study participants after 11 years. Am J Respir Crit Care Med. 2002;166.
28. Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE, Lung Health Study Research Group. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;14:614–615.
29. Ramírez-Venegas A, Sansores RH, Quintana-Carrillo RH, et al. FEV1 decline in patients with chronic obstructive pulmonary disease associated with biomass exposure. Am J Respir Crit Care Med. 2014;190:996–1002. doi:10.1164/rccm.201404-0720OC
30. Vestbo J, Sørensen T, Lange P, Brix A, Torre P, Viskum K. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet (London, England). 1999;353.
31. Pauwels RA, Löfdahl CG, Laitinen LA, et al. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. N Engl J Med. 1999;340.
32. Welte T, Vogelmeier C, Papi A. COPD: early diagnosis and treatment to slow disease progression. Int J Clin Pract. 2015;69:336–349. doi:10.1111/ijcp.2015.69.issue-3
33. Peabody JW, Schau B, Lopez-Vidriero M, Vestbo J, Wade S, Iqbal A. COPD: a prevalence estimation model. Respirology. 2005;10.
34. Atsou K, Chouaid C, Hejblum G. Simulation-based estimates of effectiveness and cost-effectiveness of smoking cessation in patients with chronic obstructive pulmonary disease. PLoS ONE. 2011;6.
35. McLean S, Barbour V, Wild S, Simpson C, Sheikh A. Models for estimating projections for disease prevalence and burden: a systematic review focusing on chronic obstructive pulmonary disease. J Health Serv Res Policy. 2015;20:246–253. doi:10.1177/1355819615579232
36. Kishimoto T. The Biology of Interleukin-6. Blood. 1989;74:1–10. doi:10.1182/blood.V74.1.1.1
37. Yasuda N, Gotoh K, Minatoguchi S, et al. An increase of soluble Fas, an inhibitor of apoptosis, associated with progression of COPD. Respir Med. 1998;92:993–999. doi:10.1016/S0954-6111(98)90343-2
38. Broekhuizen R, Wouters EF, Creutzberg EC, et al. Raised CRP levels mark metabolic and functional impairment in advanced COPD. Thorax. 2006;61.
39. Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175.
40. Garrod R, Marshall J, Barley E, Fredericks S, Hagan G. The relationship between inflammatory markers and disability in chronic obstructive pulmonary disease (COPD). Prim Care Respir J. 2007;16:236–240. doi:10.3132/pcrj.2007.00047
41. Schwarz DS, Hutvágner G, Du T, Xu Z, Aronin N, Zamore PD. Asymmetry in the assembly of the RNAi enzyme complex. Cell. 2003;115.
42. Fujita Y, Araya J, Ito S, et al. Suppression of autophagy by extracellular vesicles promotes myofibroblast differentiation in COPD pathogenesis. J Extracell Vesicles. 2015;4:28388. doi:10.3402/jev.v4.28388
43. Osei ET, Florez-Sampedro L, Tasena H, et al. miR-146a plays an essential role in the aberrant epithelial-fibroblast crosstalk in COPD. Eur Respir J. 2017;49:1602538. doi:10.1183/13993003.02538-2016
44. Akbas F, Coskunpinar E, Aynacı E, Müsteri Oltulu Y, Yildiz P. Analysis of serum micro-RNAs as potential biomarker in chronic obstructive pulmonary disease. Exp Lung Res. 2012;38:286–294. doi:10.3109/01902148.2012.689088
45. Xie L, Wu M, Lin H, et al. An increased ratio of serum miR-21 to miR-181a levels is associated with the early pathogenic process of chronic obstructive pulmonary disease in asymptomatic heavy smokers. Mol Biosyst. 2014;10:1072–1081. doi:10.1039/C3MB70564A
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
